Abstract
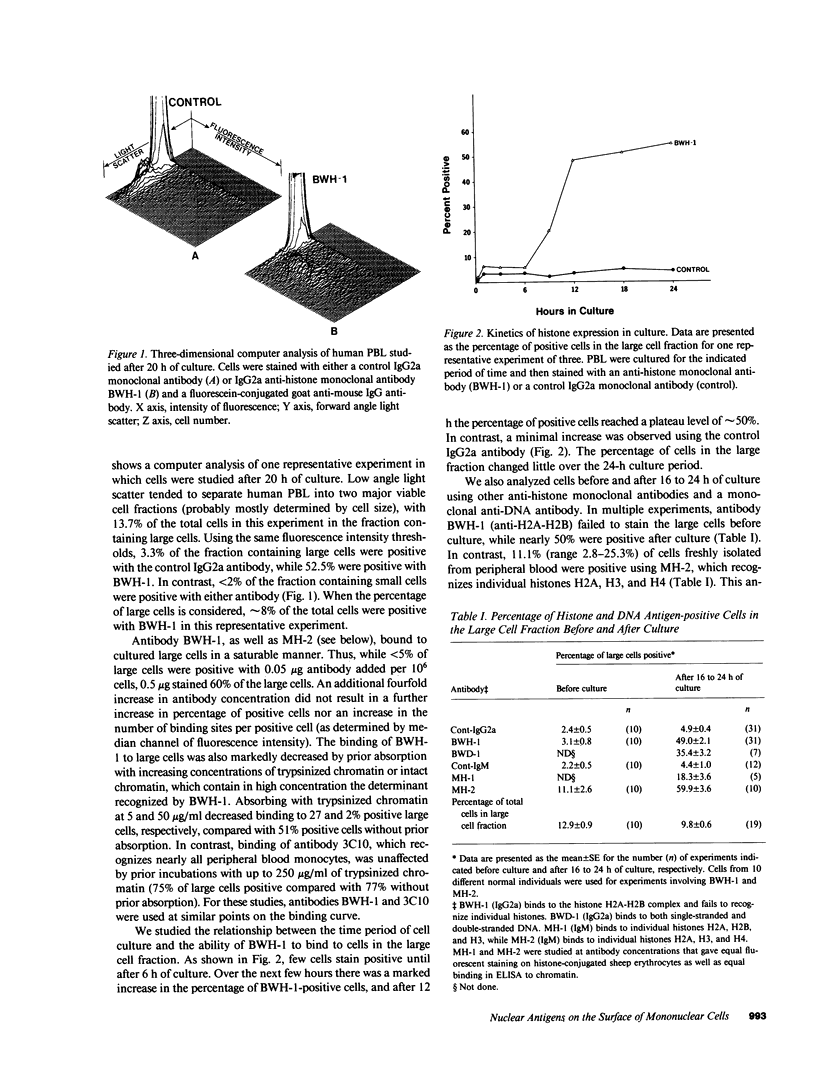
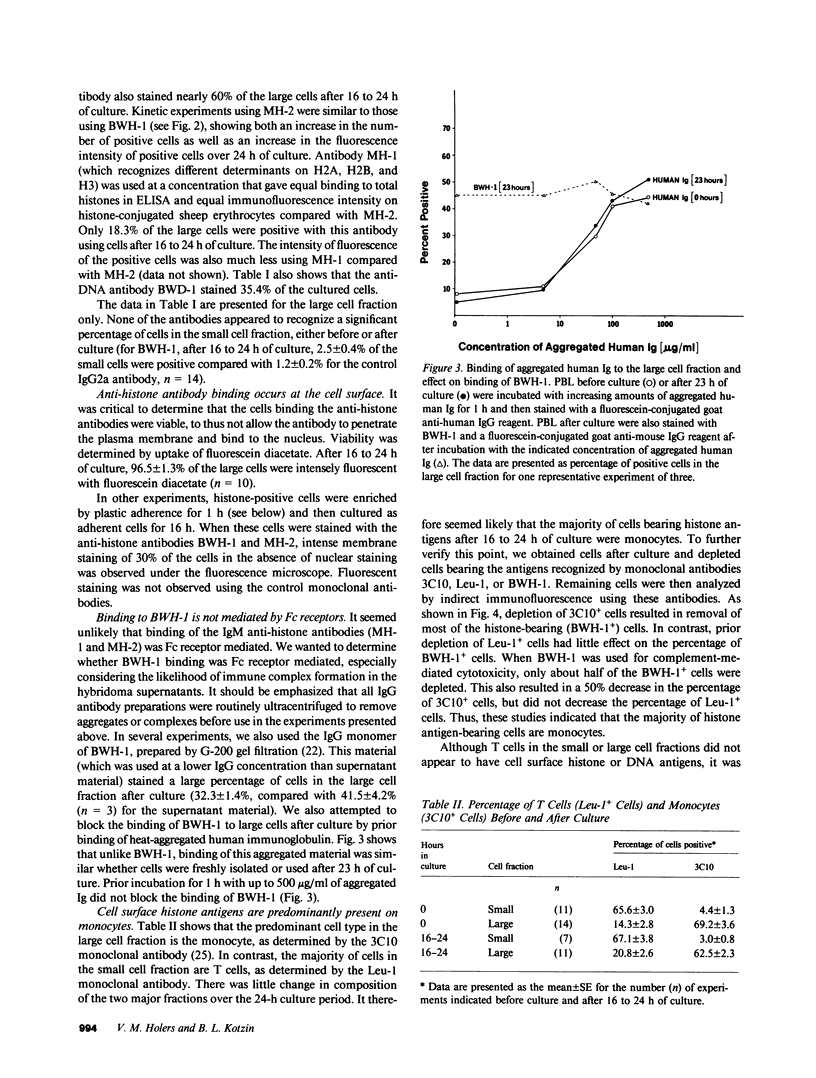
We used monoclonal anti-nuclear autoantibodies and indirect immunofluorescence to examine normal human peripheral blood mononuclear leukocytes for the presence of cell surface nuclear antigens. Only one monoclonal anti-histone antibody (MH-2) was found to bind to freshly isolated PBL, staining approximately 10% of large cells. However, after cells were placed into culture for 16-24 h, a high percentage (up to 60%) of large-sized cells were recognized by an anti-DNA (BWD-1) and several different antihistone monoclonal antibodies (BWH-1, MH-1, and MH-2). These antibodies recognize separate antigenic determinants on chromatin and histones extracted from chromatin. None of the monoclonal autoantibodies appeared to bind to a significant percentage of cells of relatively small cell size, either before or after culture. The histone antigen-positive cells were viable, and the monoclonal antibodies could be shown to be binding to the cell surface and not to the nucleus. Further experiments, including those using aggregated Ig to block antibody binding, strongly indicated that anti-histone antibody binding was not Fc receptor mediated. Using monoclonal antibodies specific for monocytes and T cells, and complement-mediated cytotoxicity, the cells bearing histone antigens were shown to be primarily monocytes. The appearance of histone and DNA antigen-positive cells was nearly completely inhibited by the addition of low concentrations (0.25 micrograms/ml) of cycloheximide at initiation of the cultures. In contrast, little effect on the percentage of positive cells was detected if cells were exposed to high doses of gamma irradiation before culture. These data further support the existence of cell surface nuclear antigens on selected cell subsets, which may provide insight into the immunopathogenesis of systemic lupus erythematosus and related autoimmune diseases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcon-Segovia D., Ruiz-Arguelles A., Fishbein E. Antibody to nuclear ribonucleoprotein penetrates live human mononuclear cells through Fc receptors. Nature. 1978 Jan 5;271(5640):67–69. doi: 10.1038/271067a0. [DOI] [PubMed] [Google Scholar]
- Bennett R. M., Davis J., Campbell S., Portnoff S. Lactoferrin binds to cell membrane DNA. Association of surface DNA with an enriched population of B cells and monocytes. J Clin Invest. 1983 Mar;71(3):611–618. doi: 10.1172/JCI110807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. M., Davis J. Lactoferrin binding to human peripheral blood cells: an interaction with a B-enriched population of lymphocytes and a subpopulation of adherent mononuclear cells. J Immunol. 1981 Sep;127(3):1211–1216. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Engleman E. G., Warnke R., Fox R. I., Dilley J., Benike C. J., Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1791–1795. doi: 10.1073/pnas.78.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzler M. J., Kinsella T. D. The CREST syndrome: a distinct serologic entity with anticentromere antibodies. Am J Med. 1980 Oct;69(4):520–526. doi: 10.1016/0002-9343(80)90462-3. [DOI] [PubMed] [Google Scholar]
- Fritzler M. J., Tan E. M. Antibodies to histones in drug-induced and idiopathic lupus erythematosus. J Clin Invest. 1978 Sep;62(3):560–567. doi: 10.1172/JCI109161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Agnello V., Kimkel H. G. Polynucleotide immune complexes in serum and glomeruli of patients with systemic lupus erythematosus. Am J Pathol. 1974 Jan;74(1):109–124. [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Kunkel H. G. Mechanisms of renal injury in systemic lupus erythematosus. Am J Med. 1968 Aug;45(2):165–169. doi: 10.1016/0002-9343(68)90034-x. [DOI] [PubMed] [Google Scholar]
- Koffler D., Schur P. H., Kunkel H. G. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967 Oct 1;126(4):607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzin B. L., Kansas G. S., Engleman E. G., Hoppe R. T., Kaplan H. S., Strober S. Changes in T-cell subsets in patients with rheumatoid arthritis treated with total lymphoid irradiation. Clin Immunol Immunopathol. 1983 May;27(2):250–260. doi: 10.1016/0090-1229(83)90075-2. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Lafferty J. A., Portanova J. P., Rubin R. L., Tan E. M. Monoclonal anti-histone autoantibodies derived from murine models of lupus. J Immunol. 1984 Nov;133(5):2554–2559. [PubMed] [Google Scholar]
- Kávai M., Lukács K., Sonkoly I., Páloczi K., Szegedi G. Circulating immune complexes and monocyte Fc function in autoimmune diseases. Ann Rheum Dis. 1979 Feb;38(1):79–83. doi: 10.1136/ard.38.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kávai M., Zsindely A., Sonkoly I., Major M., Demján I., Szegedi G. Signals of monocyte activation in patients with SLE. Clin Exp Immunol. 1983 Feb;51(2):255–260. [PMC free article] [PubMed] [Google Scholar]
- Lamoyi E., Nisonoff A. Preparation of F(ab')2 fragments from mouse IgG of various subclasses. J Immunol Methods. 1983 Jan 28;56(2):235–243. doi: 10.1016/0022-1759(83)90415-5. [DOI] [PubMed] [Google Scholar]
- LeFeber W. P., Norris D. A., Ryan S. R., Huff J. C., Lee L. A., Kubo M., Boyce S. T., Kotzin B. L., Weston W. L. Ultraviolet light induces binding of antibodies to selected nuclear antigens on cultured human keratinocytes. J Clin Invest. 1984 Oct;74(4):1545–1551. doi: 10.1172/JCI111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie R. G., Alexander M. D. Cytophilic antibodies. Curr Top Microbiol Immunol. 1979;88:25–104. doi: 10.1007/978-3-642-67331-3_2. [DOI] [PubMed] [Google Scholar]
- Maddison P. J., Provost T. T., Reichlin M. Serological findings in patients with "ANA-negative" systemic lupus erythematosus. Medicine (Baltimore) 1981 Mar;60(2):87–94. doi: 10.1097/00005792-198103000-00002. [DOI] [PubMed] [Google Scholar]
- Meinke W., Hall M. R., Goldstein D. A., Kohne D. E., Lerner R. A. Physical properties of cytoplasmic membrane-associated DNA. J Mol Biol. 1973 Jun 25;78(1):43–56. doi: 10.1016/0022-2836(73)90427-0. [DOI] [PubMed] [Google Scholar]
- Parham P. On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J Immunol. 1983 Dec;131(6):2895–2902. [PubMed] [Google Scholar]
- Perussia B., Dayton E. T., Lazarus R., Fanning V., Trinchieri G. Immune interferon induces the receptor for monomeric IgG1 on human monocytic and myeloid cells. J Exp Med. 1983 Oct 1;158(4):1092–1113. doi: 10.1084/jem.158.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portanova J. P., Kotzin B. L., Coleman E. A., Claman H. N. Distribution of anti-histone-antibody-secreting cells in NZB/NZW mice. Cell Immunol. 1984 Sep;87(2):485–493. doi: 10.1016/0008-8749(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Reid B. L., Charlson A. J. Cytoplasmic and cell surface deoxyribonucleic acids with consideration of their origin. Int Rev Cytol. 1979;60:27–52. doi: 10.1016/s0074-7696(08)61258-9. [DOI] [PubMed] [Google Scholar]
- Rekvig O. P., Hannestad K. Certain polyclonal antinuclear antibodies cross-react with the surface membrane of human lymphocytes and granulocytes. Scand J Immunol. 1977;6(10):1041–1054. doi: 10.1111/j.1365-3083.1977.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Rekvig O. P., Hannestad K. Human autoantibodies that react with both cell nuclei and plasma membranes display specificity for the octamer of histones H2A, H2B, H3, and H4 in high salt. J Exp Med. 1980 Dec 1;152(6):1720–1733. doi: 10.1084/jem.152.6.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekvig O. P., Hannestad K. Properties of antinuclear antibodies that cross-react with plasma membranes. Scand J Immunol. 1979;9(4):325–332. doi: 10.1111/j.1365-3083.1979.tb03170.x. [DOI] [PubMed] [Google Scholar]
- Rekvig O. P., Hannestad K. The specificity of human autoantibodies that react with both cell nuclei and plasma membranes: the nuclear antigen is present on core mononucleosomes. J Immunol. 1979 Dec;123(6):2673–2681. [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., May C. M., Holman H. R., McDuffie F. C., Hess E. V., Schmid F. R. Association of antibodies to ribonucleoprotein and Sm antigens with mixed connective-tissue disease, systematic lupus erythematosus and other rheumatic diseases. N Engl J Med. 1976 Nov 18;295(21):1149–1154. doi: 10.1056/NEJM197611182952101. [DOI] [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., Tan E. M., Gould R. G., Holman H. R. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972 Feb;52(2):148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- Sontheimer R. D., Maddison P. J., Reichlin M., Jordon R. E., Stastny P., Gilliam J. N. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982 Nov;97(5):664–671. doi: 10.7326/0003-4819-97-5-664. [DOI] [PubMed] [Google Scholar]
- Steplewski Z., Lubeck M. D., Koprowski H. Human macrophages armed with murine immunoglobulin G2a antibodies to tumors destroy human cancer cells. Science. 1983 Aug 26;221(4613):865–867. doi: 10.1126/science.6879183. [DOI] [PubMed] [Google Scholar]
- Svensson B. O. Serum factors causing impaired macrophage function in systemic lupus erythematosus. Scand J Immunol. 1975;4(2):145–150. doi: 10.1111/j.1365-3083.1975.tb02611.x. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Rodnan G. P., Garcia I., Moroi Y., Fritzler M. J., Peebles C. Diversity of antinuclear antibodies in progressive systemic sclerosis. Anti-centromere antibody and its relationship to CREST syndrome. Arthritis Rheum. 1980 Jun;23(6):617–625. doi: 10.1002/art.1780230602. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston W. L., Harmon C., Peebles C., Manchester D., Franco H. L., Huff J. C., Norris D. A. A serological marker for neonatal lupus erythematosus. Br J Dermatol. 1982 Oct;107(4):377–382. doi: 10.1111/j.1365-2133.1982.tb00380.x. [DOI] [PubMed] [Google Scholar]