Background: Essential gluconeogenesis pathway in Leishmania parasites is poorly understood.
Results: Glycerol kinase, phosphoenolpyruvate carboxykinase, and pyruvate phosphate dikinase allow the entry of glycerol, aspartate, and alanine into Leishmania mannogen, respectively.
Conclusion: Contribution of these enzymes into gluconeogenesis differs between promastigotes and amastigotes.
Significance: This valuable information may help establish amastigote-specific metabolic models, which could reveal new therapeutic avenues.
Keywords: Enzyme, Gluconeogenesis, Glucose Metabolism, Leishmania, Parasite, Trypanosoma cruzi, Amastigotes, Glycosome
Abstract
Gluconeogenesis is an active pathway in Leishmania amastigotes and is essential for their survival within the mammalian cells. However, our knowledge about this pathway in trypanosomatids is very limited. We investigated the role of glycerol kinase (GK), phosphoenolpyruvate carboxykinase (PEPCK), and pyruvate phosphate dikinase (PPDK) in gluconeogenesis by generating the respective Leishmania mexicana Δgk, Δpepck, and Δppdk null mutants. Our results demonstrated that indeed GK, PEPCK, and PPDK are key players in the gluconeogenesis pathway in Leishmania, although stage-specific differences in their contribution to this pathway were found. GK participates in the entry of glycerol in promastigotes and amastigotes; PEPCK participates in the entry of aspartate in promastigotes, and PPDK is involved in the entry of alanine in amastigotes. Furthermore, the majority of alanine enters into the pathway via decarboxylation of pyruvate in promastigotes, whereas pathway redundancy is suggested for the entry of aspartate in amastigotes. Interestingly, we also found that l-lactate, an abundant glucogenic precursor in mammals, was used by Leishmania amastigotes to synthesize mannogen, entering the pathway through PPDK. On the basis of these new results, we propose a revision in the current model of gluconeogenesis in Leishmania, emphasizing the differences between amastigotes and promastigotes. This work underlines the importance of studying the trypanosomatid intracellular life cycle stages to gain a better understanding of the pathologies caused in humans.
Introduction
Trypanosomatids of the genera Trypanosoma and Leishmania are the causative agents of serious diseases in humans. In particular, Leishmania species are responsible for various manifestations of leishmaniasis in most tropical and subtropical areas of the world (1). During their life cycle, Leishmania parasites develop into extracellular flagellated promastigotes within the alimentary tract of the sand fly vector and into intracellular amastigotes within the acidified macrophage phagolysosome of the mammalian host (2).
Glucose and other hexoses are critical cellular nutrients. Leishmania parasites take up and metabolize glucose and other sugars throughout their life cycle (3, 4). The promastigotes are able to import sugar from the extracellular environment when available (5), and they are able to synthesize sugar de novo, via gluconeogenesis, under sugar starvation (6, 7). In contrast, Leishmania amastigotes actively carry out gluconeogenesis even in the presence of external sugar (7), a condition in which gluconeogenesis is down-regulated in mammalian cells (8), suggesting that amastigotes are adapted to sugar-limiting intracellular environments. Gluconeogenesis is important for amastigote survival (9); however, it is unknown how this pathway occurs in trypanosomatids. Improving our understanding of Leishmania gluconeogenesis could aid in the design of better treatments against amastigotes, which are responsible for the pathologies observed in humans.
It has been previously demonstrated that Leishmania mexicana is able to use glycerol, aspartate, and alanine to synthesize their storage carbohydrate mannogen (7), previously called β-mannan (10). It was proposed that glycerol enters the gluconeogenesis pathway through GK,2 whereas aspartate and alanine enter through PEPCK and PPDK, respectively (7), after their conversion by specific aminotransferases into oxaloacetate (aspartate) and pyruvate (alanine) (supplemental Fig. S1). GK catalyzes the conversion of glycerol into glycerol 3-phosphate; PEPCK catalyzes the conversion of oxaloacetate into phosphoenolpyruvate (PEP), and PPDK catalyzes the conversion of pyruvate into PEP (8). In mammalian cells, PPDK is absent, and alanine enters into the pathway by the combined action of pyruvate carboxylase and PEPCK (supplemental Fig. S1). However, the lack of a bona fide pyruvate carboxylase gene in the trypanosomatid genome databases raises the question about the entry of alanine into the pathway in these parasites. GK, PPDK, and PEPCK have been described in Leishmania or related parasites (11–13) and are localized in the glycosomes (11, 13, 14), peroxisome-like organelles involved in the energy and intermediary metabolism (15). PEPCK plays a role in the maintenance of the glycosomal redox/ATP balance via the glycosomal succinate fermentation (14); GK is important when parasites experience periods of low oxygen tension (16); and PPDK may fulfill a glycolytic role by contributing to the PPi and ATP balance inside the glycosomes (15, 17). However, the role of GK, PEPCK, and PPDK in gluconeogenesis has not been demonstrated.
The objective of this work was to study the contribution of these enzymes to the gluconeogenesis pathway in Leishmania parasites. We demonstrated that GK, PEPCK, and PPDK allow the entry of glycerol, aspartate, and alanine into the pathway, respectively; however, their participations in the entry of such substrates differed between the parasite life cycle stages. Our results also supported previous reports suggesting the importance of PEPCK in the carbohydrate metabolism of the promastigotes (14), although this enzyme was dispensable for the parasite viability. Finally, we describe the use of another glucogenic precursor abundant in mammals, l-lactate, that could be utilized for gluconeogenesis by L. mexicana amastigotes.
EXPERIMENTAL PROCEDURES
Parasite Cell Lines and Culture Growth Conditions
The L. mexicana (strain MNYZ/BZ/62/M379) parasites were generously donated by Dr. Scott Landfear (Oregon Health and Science University). Promastigotes and axenic amastigotes of L. mexicana wild type, Δgk, Δppdk, and Δpepck null mutants and add-back cell lines were cultured as described previously (18). Promastigotes were cultured at 26 °C in glucose-free RPMI 1640 medium, pH 7.4, containing 10% iFBS, with either 10 mm glucose or no sugar (see below). Continuous cultures were maintained by dilution of parasites in logarithmic phase of growth twice a week, and new promastigote cultures were initiated frequently from frozen stocks. Axenic (culture form) amastigotes were cultivated at 32.5 °C in Dulbecco's modified Eagle's medium modified for Leishmania (DME-L), pH 5.5, supplemented with 30 mm MES instead of HEPES, 20% iFBS, and 10 mm glucose (19), and maintained by weekly dilutions. The Δgk[GK] and Δppdk[PPDK] cell lines were grown with 100 μg/ml G418 drug and Δpepck[PEPCK] with 50 μg/ml phleomycin, respectively. Transfections employed to generate null mutants and episomally complemented cell lines were performed as reported (20). In glucose-limited conditions, glucose-free RPMI 1640 medium was only supplemented with 10% iFBS (glucose concentration of this medium was ∼0.5 mm), and for glucose-repleted conditions, 1.8 g/liter glucose (10 mm) was added to this medium (its glucose concentration was ∼10.5 mm). Growth curves were determined by fixing parasites in 2% formaldehyde followed by counting triplicate samples on a hemocytometer grid.
Isolation, Cloning, and Analysis of Nucleic Acids
Genomic DNA samples were prepared using DNAzol® reagent (Invitrogen). PCR products were gel-purified using the QIAquick gel extraction kit (Qiagen, CA) and, if intended, ligated into pCR 2.1-TOPO vectors (Invitrogen) prior to their cloning in the Leishmania expression vectors. Plasmid DNA was purified using the Wizard Plus SV Minipreps DNA purification system (Promega, WI). All plasmids generated were sequenced at the Core Facility at the Oregon Health and Science University to verify accuracy. Southern blots were performed as described previously (21), using 10 μg of genomic DNA per sample. Probes were obtained by PCR amplification from genomic DNA using the oligonucleotides described in supplemental Table S1 and were labeled with [α-32P]dCTP (3000 Ci/mmol) using the Ready-to Go DNA labeling beads kit (GE Healthcare) and purified using the ProbeQuantTM G-50 micro-columns (GE Healthcare). A 0.68-kb region of the LmxPPDK ORF, a 0.77-kb fragment of the LmxGK ORF, a 1.2-kb fragment of the LmxPEPCK ORF, and a 0.68-kb fragment of the LmxGT2 ORF (used as control of loading) were used as probes.
Generation of the Δgk, Δppdk, and Δpepck Null Mutants and Add-back Cell Lines
In L. mexicana, GK and PPDK are encoded by single copy genes (LmxM34.3080 and LmxM11.1000, respectively), whereas PEPCK is encoded by three repeated genes (LmxM27.1810, LmxM27.1807, and LmxM27.1805) organized in tandem. For generation of Δgk, Δppdk, and Δpepck null mutants, recombinant homologous gene replacement constructs were prepared containing the following drug selectable markers: blasticidin and nourseothricin resistances for GK; hygromycin B and phleomycin resistances for PPDK; and puromycin and hygromycin B resistances for PEPCK. The flanking regions used to target each gene replacement were as follows: Δgk, upstream targeting sequence, 0.47 kb extending 110 bp into the ORF, and downstream targeting sequence, 0.79 kb beginning 132 bp before the stop codon; Δppdk, upstream targeting sequence, and 0.82 kb extending 200 bp into the ORF, and downstream targeting sequence, 0.65-kb beginning 450 bp before the stop codon; Δpepck, upstream targeting sequence 0.76-kb finishing 160 bp before the starting ATG codon of gene LmxM27.1810, and downstream targeting sequence, 0.93 bp beginning 71 bp after the stop codon of gene LmxM27.1805 (supplemental Table S1). The knock-out cell lines were obtained and confirmed as previously described (21). Southern blot experiments confirmed that Δgk, Δppdk, and Δpepck null mutants did not retain any wild type copy of the GK, PPDK, and PEPCK genes, respectively (Fig. 1A). For the generation of add-back cell lines, LmxGK ORF, LmxPPDK ORF, and LmxPEPCK ORF were amplified by PCR from L. mexicana genomic DNA with specific oligonucleotides containing flanking BamHI restriction sites (supplemental Table S1) for cloning into the Leishmania expression vectors. LmxGK ORF and LmxPPDK ORF were subcloned into pX63NeoRI, whereas the LmxPEPCK ORF was subcloned into pX63PHLEO expression vectors (21).
FIGURE 1.
Characterization of the Δgk, Δppdk, and Δpepck null mutants. A, Southern blots of genomic DNA from wild type (WT), specific heterozygote null mutants (+/−) and specific homozygote null mutants (−/−) hybridized to ORFs from each specific enzyme gene (GK, PPDK, or PEPCK) and from an unrelated gene (glucose transporter GT2) as control. The genomic DNA in the left panel was digested with HindIII/XhoI, in the middle panel with KpnI/EcoRV, and in right panel with NheI/EcoRV restriction enzymes. The gels are stained with ethidium bromide. B, Western blot from total lysates of wild type (WT), Δppdk, Δpepck, and the respective add-back promastigotes. Immunoblots were probed with the specific antisera against PPDK (top panel), PEPCK (middle panel), or PYK (control; bottom panel). C, growth of promastigotes at 26 °C. Parasites were inoculated at a density of 2 × 105 cells/ml in RPMI 1640 medium and containing 10 mm glucose (left panel) or no sugar (right panel). The add-back cell lines (Δppdk[PPDK], Δgk[GK], and Δpepck[PEPCK]) also showed similar growth rates as wild type parasites in both assayed conditions (data not shown). Values were plotted as the mean ± S.D. for the three replicate measurements of one representative experiment. The y axis was graphed on the log10 scale (large squares) and in linear scale (inset, small squares), for better visualization of data at lower and higher densities, respectively.
Phenotypical Characterization
Assays for glucose uptake were performed by an oil-stop method as reported (22). Promastigotes were grown in the presence of 10 mm glucose, and uptake assays were performed using cells in early/middle logarithmic (0.6–1.4 × 107 cells/ml) or late logarithmic/early stationary (3.5–6.0 × 107 cells/ml) phases of growth. Uptake of 100 μm d-[14C]glucose was carried out between 0 and 60 s, and the data were fitted to a straight line by linear regression (Prism version 5; GraphPad, San Diego). Rate of glucose uptake was normalized to milligrams of total protein. To determine the expression levels of mannogen in wild type and Δpepck cell lines, this carbohydrate was quantified using the phenol-sulfuric acid method (23). Promastigotes in late log/early stationary phases of growth were incubated in RPMI 1640 medium supplemented with 10% dialyzed FBS, in the presence or absence of 10 mm glucose at 26 °C by 24 h. Mannogen fractions were extracted from wild type, Δpepck, and Δpepck[PEPCK] parasites as described below. The sugar concentration of each sample was obtained using a standard curve for d-mannose. Samples were assayed in duplicate, and the experiment was repeated three times. The Δpepck[PEPCK] promastigotes expressed similar levels of PEPCK expression than wild type parasites as was determined by Western blot (WT/add-back ratio was 1.2 ± 0.01 and 1.4 ± 0.2 in both conditions, respectively). Total cell lysates used for Western blot experiments and normalization of several assays were obtained as described previously (7), and samples were stored in small aliquots at −70 °C. Protein determination was performed using the DC protein assay kit (Bio-Rad). In Western blot experiments, cell lysate samples were resolved by electrophoresis, transferred, and developed as described (24). Briefly, cell lysates were diluted 1:1 in 2× Laemmli sample buffer containing 2-mercaptoethanol, heated to 70 °C for 5 min, and separated on a NuPAGE Novex BisTris 4–12% acrylamide gel using the XCell SureLock mini-cell system (Invitrogen). For immunoblotting, separated proteins were transferred to Whatman nitrocellulose membranes using the XCell II blot module (Invitrogen). Membranes were blocked overnight at 4 °C using 5% nonfat dry milk in PBST buffer (PBS containing 0.05% Tween 20). Blots were developed using the SuperSignal West Pico chemiluminescence substrate (Thermo Fisher Scientific, MA) and Kodak BioMax MR films. Quantification of the band intensity was carried out by densitometry. All statistical analyses were carried out using a two-tailed unpaired t test (Prism version 5; GraphPad, San Diego).
Generation of Specific Antibodies Used in Western Blots
The ORF of GK, PPDK, PEPCK, FBPase, and PYK were amplified from L. mexicana genomic DNA by PCR using oligonucleotides designed against the NH2- and COOH-terminal sequences of the corresponding ORF from L. mexicana (LmxM34.3080, LmxM11.1000, LmxM27.1810, LmxM04.1160, and LmxM34.0030, respectively; see supplemental Table S1). Recombinant proteins were purified using the pET200 vector system (Invitrogen), by generating recombinant proteins with an NH2-terminal polyhistidine (His6) tag. Recombinant proteins were purified by cobalt column chromatography using the HisPur Cobalt Resin (Thermo Fisher Scientific) and sent to Cocalico Biologicals, Inc. (Reamstown, PA), to raise specific antisera in rabbits. Specific antisera against the L. mexicana enzymes PPDK, PEPCK, FBPase, and PYK were used at 1:5000 dilution; goat anti-rabbit IgG F(ab2) fraction coupled to horseradish peroxidase (Invitrogen) was used as described by the manufacturer. Two independent attempts to raise specific antiserum against the recombinant L. mexicana GK were made with unsuccessful results.
Metabolic Labeling of Cells and Gluconeogenesis Studies
Extraction of mannogen and phospholipid fractions, their analysis by HPTLC, and metabolic labeling of wild type and null mutant parasites were performed as detailed previously (7). Promastigotes at late logarithmic/early stationary phase of growth were harvested by centrifugation, and 1 × 108 parasites were resuspended in 5 ml of glucose-free RPMI 1640 medium supplemented with 10% dialyzed iFBS. Parasites were metabolically labeled with the glucogenic precursors by adding to the cultures 0.5 mm of the cold substrate plus 5 μCi/ml of the radiolabeled precursors. The following radiolabeled precursors were used: l-[U-14C]aspartic acid (Moravek Biochemicals); l-[U-14C]alanine, l-[1-14C]alanine; [2-14C]alanine; [1,3-14C]glycerol, dl-[1-14C]lactic acid, sodium salt, l-[1-14C]sodium salt lactic acid, and d-[1-14C]lactic acid, sodium salt (American Radiolabeled Chemicals, Inc.); and d-[1-14C]mannose (Moravek Biochemicals). Amastigotes were metabolically labeled as described for promastigotes, using glucose-free DME-L medium, pH 5.5 (19), and supplemented with 20% dialyzed iFBS, and the cells were incubated at 32.5 °C. Cells were washed twice in PBS, and mannogen and phospholipid fractions were purified by extraction of parasites with CHCl3/CH3OH/H2O (1:2:0.8, v/v) as described (10), with modifications (7) as follows: the cell pellet was extracted by vortexing with glass beads for 10 min; insoluble material was removed by centrifugation and re-extracted with CHCl3/CH3OH/H2O twice. The pooled fractions were dried and partitioned by biphasic separation in 1-butanol/water (1:1 v/v). Phospholipids were recovered in the upper organic phase. The lower aqueous phase containing mannogen was extracted twice with water-saturated butanol, desalted using AG501-X8 resin (Bio-Rad), and dried before separating by HPTLC. In some assays, lipophosphoglycan (LPG) fractions were extracted from delipidated pellets from promastigotes using 1-butanol-saturated water (7). Mannogen fractions were analyzed on glass-backed Silica Gel 60 plates (Merck) and developed twice in 1-butanol/ethanol/water (4:3:3 v/v). Radiolabeled bands were detected by fluorography after spraying HPTLC plates with EN3HANCE spray (PerkinElmer Life Sciences) and exposing them to Kodak BioMax MR film at −70 °C. Films were obtained for each plate analyzed by fluorography at several exposure times. To quantify the levels of 14C label incorporation in mannogen, LPG and phospholipid fractions, counts/min, were obtained by duplicate for each sample assayed before its chromatographic analysis and normalized to protein content. In some experiments, twin plates were stained using the carbohydrate-specific orcinol-H2SO4 stain and heating to 100 °C for 10 min (7).
RESULTS
Generation and Characterization of the L. mexicana Δgk, Δppdk, and Δpepck Null Mutants
To define the role of the L. mexicana GK, PPDK, and PEPCK in gluconeogenesis, we generated the specific null mutants (referred as Δgk, Δppdk, and Δpepck, respectively) by targeted gene replacement (see “Experimental Procedures”). The loss of the respective GK, PPDK, and PEPCK ORF in the Δgk, Δppdk, and Δpepck null mutants, respectively, was confirmed by Southern blots of genomic DNA (Fig. 1A). Moreover, the loss of PPDK and PEPCK protein expression in Δppdk and Δpepck was confirmed by Western blot (Fig. 1B), using specific antiserum generated against the respective proteins. Unfortunately, specific antibodies against the recombinant GK protein could not be generated after two attempts. To determine whether the lack of expression of these enzymes affects promastigote growth, wild type, Δgk, Δppdk, and Δpepck parasites were incubated in RPMI 1640 medium containing 10 mm glucose (glucose-replete condition) or without glucose (glucose-limited condition). No difference in growth was observed between wild type and the null mutant parasites in any of the assayed conditions (Fig. 1C).
Role of GK, PEPCK, and PPDK in the Gluconeogenesis Pathway of Promastigotes
In protozoan parasites such as Leishmania, glucose-6-phosphatase is absent (7, 25) and the de novo synthesis of d-glucose cannot be used to evaluate gluconeogenesis. The absence of this enzyme is not unexpected because unicellular parasites do not need to generate and export free glucose to the extracellular environment. Thus, to determine the participation of GK, PEPCK, and PPDK in gluconeogenesis, we studied the de novo synthesis of the Leishmania storage carbohydrate, mannogen. The Δgk, Δpepck, and Δppdk null mutant promastigotes were incubated with radiolabeled glycerol, aspartate, and alanine in glucose-starving conditions, and the incorporation of 14C label into mannogen was evaluated as described previously (7). Wild type parasites were used as positive control for all substrates assayed. As expected, the incorporation of 14C label into mannogen was strongly reduced in Δgk (86.5 ± 1.5% reduction) and Δpepck (92.2 ± 1.3% reduction) promastigotes incubated with glycerol and aspartate, respectively, compared with that of wild type parasites incubated in similar conditions (Fig. 2A). The Δgk and Δpepck promastigotes had normal cell shape and motility when observed under the microscope and were able to incorporate other precursors (alanine and aspartate in the case of Δgk, and glycerol and alanine in the case of Δpepck) into mannogen (Fig. 2A). Thus, the inability of Δgk and Δpepck promastigotes to synthesize mannogen from glycerol and aspartate, respectively, was not due to cell death or lack of metabolic activity. These results demonstrated that in promastigotes most of the glycerol and aspartate enter into the gluconeogenesis pathway through GK and PEPCK, respectively, as happens in mammalian cells (8).
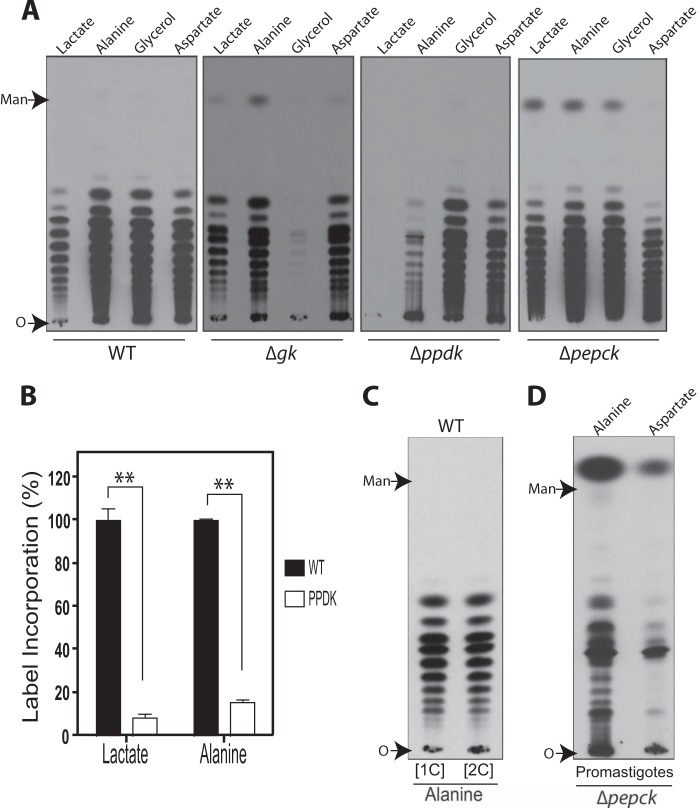
FIGURE 2.
Incorporation of glucogenic substrates into mannogen by Δgk, Δpepck, and Δppdk promastigotes. A, HPTLC plates showing 14C-labeled mannogen fractions extracted from wild type (WT), Δgk, Δpepck, and Δppdk promastigotes, respectively. The same number of late log/early stationary phase promastigotes from each cell line was metabolically labeled with the glucogenic substrate shown on the top, in the absence of glucose by 12 h. Mannogen fractions were analyzed on HPTLC plates followed by fluorography. Mannogen samples were exposed by 96 h. B, wild type promastigotes were starved from glucose by 12 h, followed by metabolically labeling for 90 min with [1-14C]alanine ([1C]) and [2-14C]alanine ([2C]). The mannogen fractions were separated on HPTLC plates followed by fluorography. Mannogen samples were exposed by 24 days. A and B, O marks the origin. C, quantification of the relative mannogen levels in WT, Δpepck, and Δpepck[PEPCK] promastigotes starved (right) or not (left) from glucose during 24 h. Wild type samples were set to a relative amount value of 100%, and other samples were normalized to those wild type samples. Data represent the mean ± S.D. measurements from three independent experiments. Significant differences are shown as *, p ≤ 0.05.
In contrast, Δppdk promastigotes were able to incorporate 14C label into mannogen when they were incubated with alanine, glycerol, and aspartate (Fig. 2A). The label incorporation in wild type and Δppdk promastigotes incubated with alanine was 98.6 ± 2.1 and 78.9 ± 8.2%, respectively. Thus, the participation of PPDK in gluconeogenesis as well as the entry of alanine into this pathway in this life cycle stage remain unclear. Saunders and co-workers (14) described that in promastigotes growing in abundant glucose most of the internalized alanine was converted into pyruvate and entered the TCA cycle, via acetyl-CoA. To evaluate whether this substrate follows a similar entry route under glucose starvation, wild type promastigotes were metabolically labeled with [1-14C]alanine and [2-14C]alanine. If most of the alanine is decarboxylated after its conversion into pyruvate, cells labeled with [1-14C]alanine would have a significant decrease in label incorporation into mannogen, because the labeled carbon 1 of alanine is lost as CO2 in its conversion to acetyl-CoA. Indeed, we found that 66.9 ± 11.2% (p ≤ 0.05) of the label (Fig. 2B) was lost in the mannogen fraction when wild type parasites were labeled with [1-14C]alanine compared with those labeled with [2-14C]alanine (Fig. 2B). As expected, the label incorporation into the phospholipid fraction was significantly reduced in cells labeled with [1-14C]alanine compared with those labeled with [2-14C]alanine ([1-14C]alanine/[2-14C]alanine ratio was 0.13 ± 0.01). Our results showed that the decarboxylation of pyruvate obtained after transamination of alanine plays an important role in the alanine entry into gluconeogenesis in promastigotes. The use of two-carbon molecules in the de novo synthesis of carbohydrates has been previously described in Leishmania promastigotes (6, 7); however, their entry route into the pathway in these parasites is unknown.
PPDK Is Expressed in Promastigotes When Glucose Is Depleted from the Medium
The low participation of PPDK, if any, in gluconeogenesis observed in promastigotes could be explained by lack of protein expression under glucose starvation. To evaluate this possibility, the expression of PPDK and PEPCK as well as the key enzymes for gluconeogenesis (FBPase) and glycolysis (pyruvate kinase, PYK) were compared in wild type promastigotes by Western blot (Fig. 3B). Surprisingly, PPDK expression increased 3–4-fold by day 6 of growth when wild type parasites grew in glucose-limited condition (glucose was depleted from the medium by day 2 of growth in this condition). Thus, the lack of participation of this enzyme in gluconeogenesis, observed in Fig. 2A,was not due to a down-regulation of this protein in the glucose-starved promastigotes. In contrast, PEPCK was constitutively expressed in promastigotes (Fig. 3, B and C), supporting the major role of this enzyme in this life cycle stage, as suggested previously (14).
FIGURE 3.
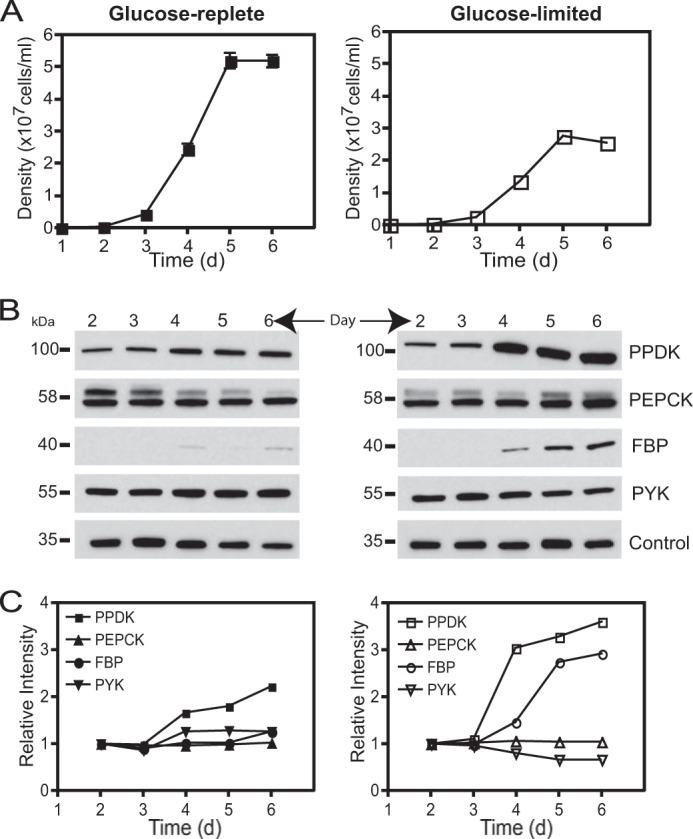
Protein expression levels of PPDK and PEPCK during the growth of the L. mexicana wild type promastigotes in glucose-replete and glucose-limited conditions. A, wild type parasites were diluted at 2 × 105 cells/ml in RPMI 1640 media supplemented with 10% iFBS, and cultures were counted every day. In the glucose-repleted condition, the medium contains ∼10.5 mm glucose, and in glucose-limited condition, medium only contains ∼0.5 mm glucose at the beginning of the growth curve. Values were plotted as the mean ± S.D. for the three replicate measurements. B, Western blot of cell lysate (10 μg) prepared from days 2–6 of growth in both conditions. Immunoblots were probed with each antiserum labeled at right. Control represents the signal from a cross-reacting band (30 kDa) and was employed for normalization. C, quantification of the Western blot signal from each enzyme (PPDK, PEPCK, FBPase, and PYK) normalized to the intensity of the control in the same lane. Glucose-replete condition is shown in the left panel and glucose-limited condition in the right panel. Samples from day 2 were set to a relative intensity value of 1.0, and other samples (from days 3 to 6) were compared with those on day 2.
In the case of FBPase and PYK, we found a correlation between the protein expression observed in this study and their enzymatic activities previously reported in promastigotes growing in abundant glucose (26, 27); FBPase was poorly expressed, whereas the PYK was expressed at higher levels (Fig. 3B). Interestingly, FBPase expression increased as sugar was depleted from the medium, a condition in which gluconeogenesis is induced in promastigotes. In contrast, FBPase was expressed at higher levels than PYK in wild type axenic amastigotes (data not shown), which correlated with the enzymatic activities described in the intracellular stage (26).
Δpepck Promastigotes Were Impaired in the Ability to Synthesize Mannogen
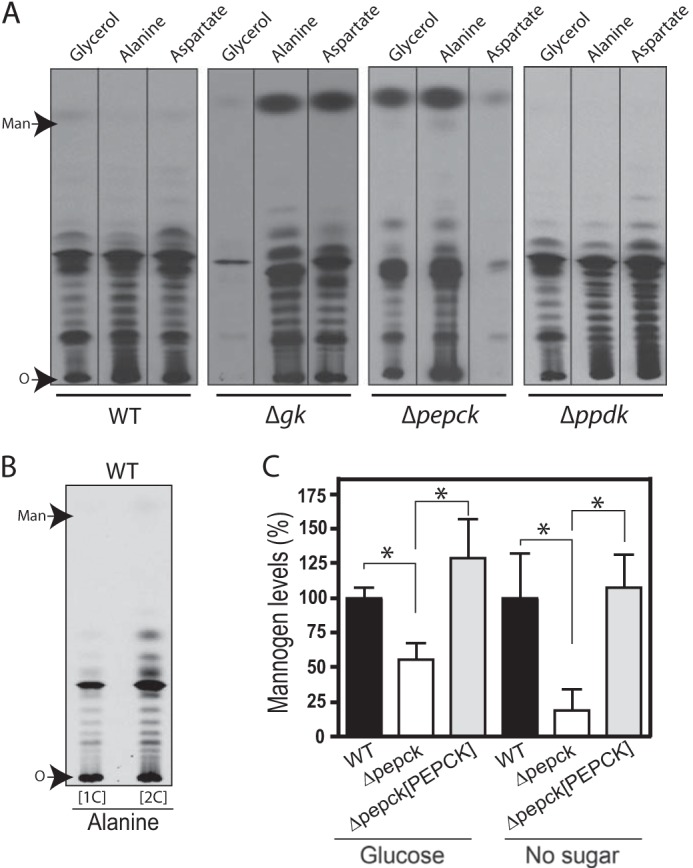
Twin HPTLC plates from the metabolic experiments described above were selectively stained for carbohydrates, using orcinol·H2SO4 stain. Interestingly, we observed a strong reduction in mannogen levels in the Δpepck samples, compared with that of wild type or the other null mutant parasites (data not shown), suggesting that Δpepck promastigotes were impaired in the ability to synthesize this carbohydrate under glucose starvation. To evaluate this possibility, wild type and Δpepck promastigotes were incubated in the presence or absence of 10 mm glucose for 24 h, and then mannogen fractions were extracted, and carbohydrates were quantified as described previously (7). In glucose-starving conditions, mannogen levels in Δpepck null mutants were reduced by 81.3% compared with wild type levels (Fig. 2C). This reduction was restored in the add-back Δpepck[PEPCK] promastigotes (Fig. 2C). Surprisingly, a moderate but significant reduction of mannogen levels (44.5%) was also observed in Δpepck null mutants when glucose was present in the culture medium (Fig. 2C). This phenotype was not due to the PEPCK role in gluconeogenesis described in this study, because Δpepck promastigotes incubated in the presence of sugar were only able to incorporate label into mannogen from mannose but not from alanine or aspartate (Fig. 4A). Moreover, the expression of FBPase was not modified in this null mutant compared with wild type (Fig. 3B) and Δpepck[PEPCK] parasites in such condition (Fig. 4B). In trypanosomatids, PEPCK is bidirectional and also participates in the succinate fermentation pathway, which is the principal route of maintaining the glycosomal redox/ATP balance during the glucose catabolism (14, 17). Perhaps as a consequence of the absence of this pathway, the glucose consumption would be affected, which could indirectly restrict the carbohydrate biosynthesis. The observed phenotype could be an indirect consequence of the lack of this pathway, although other possibilities cannot be ruled out. Thus, the results shown in Fig. 2C suggested that PEPCK was also important to maintain the rate of mannogen biosynthesis in L. mexicana promastigotes.
FIGURE 4.
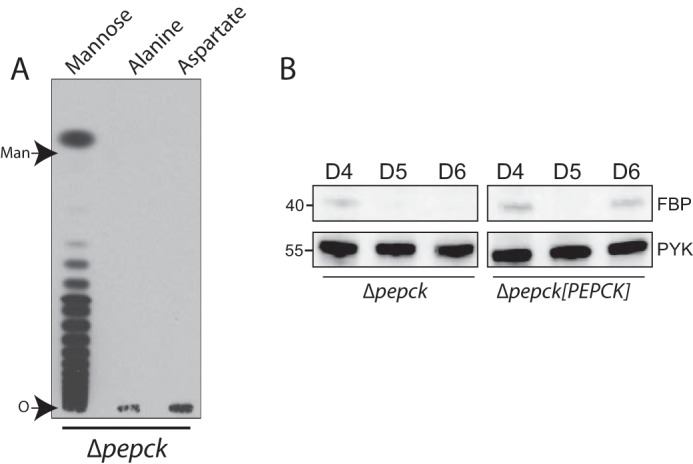
A, incorporation of radiolabeled substrates into mannogen by Δpepck promastigotes incubated in the presence of glucose. Late log/early stationary phase Δpepck promastigotes were metabolically labeled with [14C]mannose, l-[14C]alanine, or l-[14C]aspartate (shown on the top) in the presence of 5 mm sugar by 12 h, and mannogen fractions were extracted as described under “Experimental Procedures.” The mannogen fractions were separated on HPTLC plates followed by fluorography. Mannogen samples were exposed by 96 h. O marks the origin. B, Western blot from total lysates (10 μg) of Δpepck and Δpepck[PEPCK] promastigotes at late logarithmic and stationary phase of growth (days 4–6) in glucose-replete condition. Immunoblots were probed with the specific antisera against FBPase (top) and PYK as loading control (bottom), as described under “Experimental Procedures.” D4–D6 indicate days 4–6 of parasite growth.
Molecular Ablation of PEPCK Affects the Rate of Glucose Consumption in Promastigotes
External glucose provides a major source of carbon and energy to Leishmania promastigotes (28). We evaluated whether the lack of expression of PEPCK, the first enzyme of the glycosomal succinate fermentation pathway, would modify the rate of d-glucose uptake in the promastigotes at early/middle logarithmic and/or late logarithmic/early stationary phase of growth (see “Experimental Procedures” section), due to variations in the use of this substrate during the promastigote growth. For example, the intense replication rate observed in logarithmic phase promastigotes is associated with a higher energy consumption (29), whereas an increase in the biosynthesis of abundant glycoconjugates (30, 31) and mannogen (10) is observed in promastigotes reaching stationary phase of growth. Our results revealed that the rate of glucose uptake in the Δpepck null mutants was moderate but significantly reduced by 41.7% in early/middle logarithmic phase of growth (Table 1). This phenotype was restored to wild type levels by complementation of the Δpepck null mutant with an episomal copy of PEPCK ORF. In Trypanosoma brucei, a similar reduction in glucose uptake was obtained by null mutant parasites lacking of fumarate reductase (17), another enzyme of the succinate fermentation pathway. Thus, our results are in agreement with a major role of this enzyme in the catabolism of glucose described previously (14); however, they also demonstrated that PEPCK is not essential for maintaining the glycosomal homeostasis in promastigotes.
TABLE 1.
Rate of glucose uptake by L. mexicana Δpepck and Δppdk promastigotes
All values were normalized to picomoles/mg of protein/min. The rates of glucose uptake are reported as mean ± S.D. The “n” represents the number of biological samples assayed for each condition. Promastigotes were grown in RPMI 1640 medium supplemented with 10 mm glucose. The rate of 100 μm glucose uptake by the L. mexicana Δppdk and Δpepck null mutants and the respective added-back cell lines grown at early/middle logarithmic phase or late logarithmic/stationary phase of growth were compared with those of the parenteral wild type cell line.
| Cell line | Early/middle log | Late log/stationary |
|---|---|---|
| pmol/mg protein/min | pmol/mg protein/min | |
| WT | 156.0 ± 13.5 (n = 4) | 158.5 ± 9.9 (n = 3) |
| Δpepck | 90.9 ± 20.2 (n = 4)a | 143.1 ± 36.4 (n = 3) |
| Δpepck[PEPCK] | 139.5 ± 7.2 (n = 3) | 153.8 ± 13.1 (n = 2) |
| Δppdk | 130.2 ± 4.7 (n = 4) b | 157.8 ± 20.3 (n = 3) |
| Δppdk[PPDK] | 166.2 ± 49.1 (n = 3) | 169.5 ± 7.5 (n = 2) |
a p ≤ 0.01.
b p ≤0.05.
In the case of Δppdk null mutants, the uptake of glucose was only slightly reduced (16.5%) in early/middle logarithmic phase of growth (Table 1), similar to that previously described in T. brucei Δppdk procyclic form parasites (32). These results are in agreement with the predicted metabolic flux of the glucose catabolism proposed in trypanosomatid insect stages (14, 17). No significant difference in glucose uptake was observed when Δpepck and Δppdk cells were evaluated at late log/stationary phase of growth (Table 1).
Lactate as Glucogenic Precursor in Leishmania Parasites
Lactate is another classic glucogenic precursor used by mammalian cells to synthesize sugar (8), which enters the pathway by conversion into pyruvate. It could be a useful precursor in Leishmania; however, there is no evidence of its use as a carbon source in these parasites. We used a mixture of d- and l-lactate to determine whether Leishmania parasites were able to utilize lactate as a glucogenic precursor. Our results showed no incorporation of 14C label into mannogen by wild type promastigotes incubated with dl-lactate (Fig. 5A). This lack of label incorporation was also observed in the LPG fraction (data not shown), an abundant glycoconjugate present in promastigotes (30). Moreover, these parasites appeared normal in morphology and were motile. In contrast, wild type axenic amastigotes were able to utilize dl-[14C]lactate for mannogen biosynthesis whether they were incubated in the presence or absence of glucose, similar to our previous observation using other glucogenic precursors (7).
FIGURE 5.
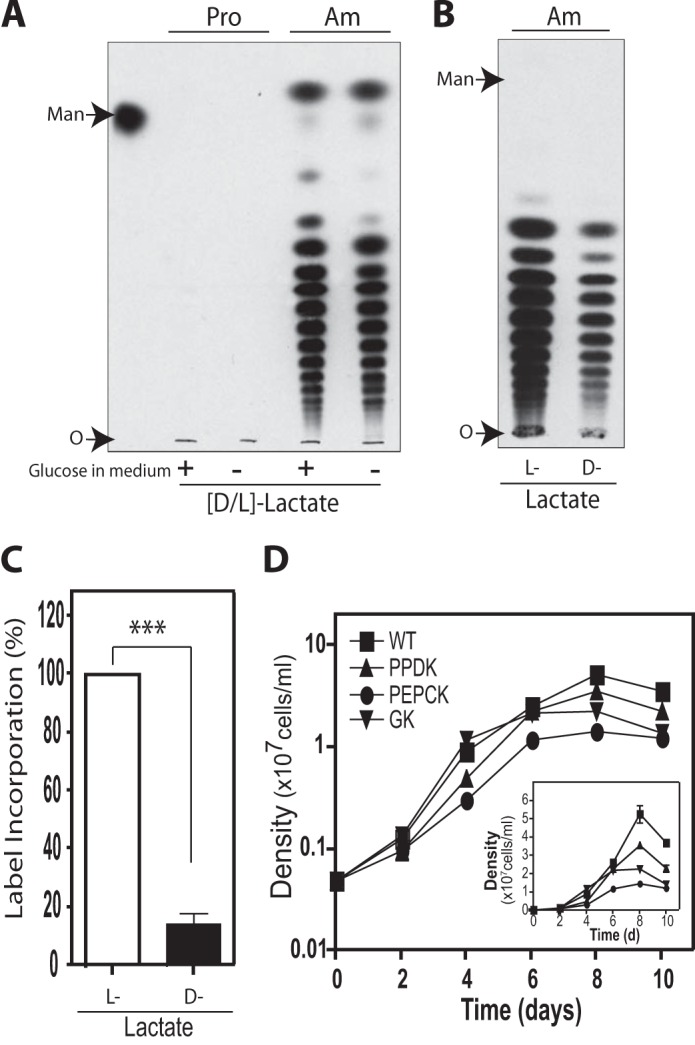
Incorporation of lactate into mannogen by L. mexicana wild type parasites. A, incorporation of dl-lactate into mannogen by promastigotes (Pro) and amastigotes (Am) in the presence (+) or absence (−) of 10 mm glucose in the culture medium. Parasites were metabolically labeled with ld-[14C]lactate by 12 h and mannogen fractions were analyzed on HPTLC plates followed by fluorography. Mannogen samples were exposed by 72 h. In amastigotes, the label incorporation ratio in the presence/absence of glucose was 0.90 ± 0.02. B, amastigotes were metabolically labeled with l-[14C]lactate or d-[14C]lactate in medium without sugar, and mannogen fractions were analyzed by HPTLC. Mannogen samples were exposed by 96 h. A and B, O marks the origin. C, percentage of 14C label of incorporation of l-lactate and d-lactate into the mannogen fraction. Data represent the mean ± S.D. for two independent experiments. Significant differences are shown as follows: ***, p ≤ 0.001. D, growth of axenic amastigotes at 32.5 °C. Stationary phase promastigotes were inoculated at higher temperature and low pH to be transformed into culture-form amastigotes. The transformed parasites were inoculated at an initial density of 5 × 105 cells/ml in DME-L medium supplemented with 20% iFBS and 10 mm glucose. Values were plotted as the mean ± S.D. for the three replicate measurements of one representative experiment. The y axis was graphed in log10 scale (large squares) and in linear scale (small squares, inset), for better visualization of data at lower and higher densities, respectively.
l-Lactate is abundant in mammalian tissues, being the major end product of glucose metabolism in activated macrophages and muscle (33, 34). It is also a precursor for gluconeogenesis in the liver and kidney, and a respiratory fuel for cardiac and skeletal muscle (35). d-Lactate is formed in mammals at nanomolar concentrations via the methylglyoxal pathway (34). To determine which lactate isomer was used by Leishmania amastigotes as precursor for gluconeogenesis, wild type parasites were labeled with l-[14C]lactate and d-[14C]lactate separately. Our results showed that both l- and d-lactate were incorporated into mannogen by the Leishmania amastigotes (Fig. 5B). Surprisingly, the incorporation of l-lactate was 5-fold higher than those observed with d-lactate (Fig. 5C). This is the first evidence of utilization of lactate by Leishmania amastigotes as well as its role as a precursor for gluconeogenesis. This result opens the possibility that l-lactate could be used as a carbon source by the intracellular stage of trypanosomatid parasites inside the mammalian hosts.
Role of GK, PPDK, and PEPCK in the Gluconeogenesis Pathway of Amastigotes
To determine the role of GK, PPDK, and PEPCK enzymes in the intracellular stage of Leishmania, axenic amastigotes from wild type, Δgk, Δppdk, and Δpepck cell lines were obtained by transformation of the respective stationary phase promastigotes. All the null mutants had the ability to grow in culture as axenic amastigotes (Fig. 5D). Moreover, the expression of PPDK and PEPCK in wild type amastigotes as well as their absences in the corresponding null mutants was confirmed by Western blot (data not shown). As observed in promastigotes, a strong reduction (98.8 ± 0.02%) in the incorporation of glycerol into mannogen was observed when Δgk amastigotes were assayed for their ability to incorporate such precursors (Fig. 6A), confirming the role of GK in the entry of glycerol into gluconeogenesis in Leishmania parasites. However, the incorporation of 14C label into mannogen was significantly reduced in Δppdk amastigotes incubated with dl-lactate (91.4 ± 2.2%) and alanine (84.6 ± 4.5%) (Fig. 6, A and B), demonstrating that in this life cycle stage both substrates enter into the pathway mainly by action of PPDK. Thus, in amastigotes most of the alanine enters into the pathway by pyruvate conversion into PEP, opposite that observed in the promastigote stage. In agreement with these results, no significant differences in the incorporation of [1-14C]- or [2-14C]alanine into mannogen were found in wild type amastigotes ([1-14C]alanine/[2-14C]alanine ratio was 0.97 ± 0.21 (n = 2); Fig. 6C).
FIGURE 6.
Incorporation of glucogenic precursors into mannogen by Δgk, Δppdk, and Δpepck amastigotes. A, wild type (WT), Δgk, Δppdk, and Δpepck amastigotes were metabolically labeled with the glucogenic substrate (dl-[14C]lactate, l-[U-14C]alanine, [14C]glycerol, or l-[14C]aspartate) shown on the top by 12 h, and each mannogen fraction was analyzed by HPTLC followed by fluorography. Mannogen samples were exposed by 24 h. B, percentage of 14C label incorporation of dl-lactate and l-alanine into mannogen by wild type (WT) and Δppdk amastigotes. Data represent the mean ± S.D. for two independent experiments. Significant differences are shown as follows: **, p ≤ 0.01. C, incorporation of [1-14C]alanine ([1C]) and [2-14C]alanine ([2C]) into mannogen by wild type amastigotes. D, Δpepck amastigotes were transformed into promastigotes and metabolically labeled with l-[U-14C]alanine and l-[14C]aspartate shown on the top of the HPTLC plate. Parasites were starved from glucose for 12 h, followed by metabolically labeling for 90 min with each substrate. The mannogen fractions were separated on HPTLC plates followed by fluorography. Mannogen samples were exposed by 27 days. A, C, and D, O marks the origin.
Taking into account the result obtained with Δppdk amastigotes, we were expecting that Δpepck amastigotes could not incorporate aspartate but could incorporate alanine and glycerol into mannogen, as was the case for this null mutant in the promastigote stage. Surprisingly, the metabolic labeling experiments revealed that the Δpepck amastigotes were able to incorporate lactate, alanine, glycerol, and aspartate into mannogen (Fig. 6A). The label incorporation in wild type and Δpepck amastigotes incubated with aspartate was 100.8 ± 1.3 and 89.9 ± 2.7%, respectively. These observations are not the result of a random event because they were reproducible using different biological samples from Δpepck parasites transformed into amastigotes totally independent of the one shown in Fig. 6A. To evaluate whether revertant parasites could be generated during the transformation of Δpepck promastigotes into amastigotes, the Δpepck amastigotes were incubated in RPMI 1640 medium, pH 7.4, at 26 °C to transform them back into promastigotes. Labeling experiments using the transformed-back Δpepck cultures showed that this cell line as promastigotes did not incorporate aspartate but alanine (Fig. 6D), i.e. they maintained the phenotype observed previously in the sand fly stage (Fig. 2A). This result argues against the possible generation of revertants as explanation for the observed aspartate incorporation into mannogen in Δpepck amastigotes.
Taking together these results, we can conclude that GK plays an important role in the entry of glycerol, and PPDK in the entry of alanine and lactate, into the gluconeogenesis pathway in amastigotes; however, the entry of aspartate into the pathway is undefined.
DISCUSSION
Gluconeogenesis is an essential pathway for the maintenance of many organisms when sufficient external sugar sources are not available (8, 36–38). This pathway is actively carried out in Leishmania amastigotes (7), and it has been suggested to be also responsible for supplying sugar for the biosynthesis of glycoproteins and glycoinositol phospholipids in the T. cruzi amastigotes (39). To contribute to the understanding of this pathway in these parasites, we studied the role of GK, PEPCK, and PPDK in the incorporation of glycerol, aspartate, and alanine into the storage carbohydrate mannogen.
In this study, we showed for the first time that the L. mexicana GK, PEPCK, and PPDK play a role in gluconeogenesis. GK participates in the entry of glycerol into this pathway in both life cycle stages, whereas the participation of PEPCK and PPDK differs between promastigotes and amastigotes. In promastigotes, most of the aspartate used for gluconeogenesis enters into the pathway through PEPCK, whereas in amastigotes the absence of this enzyme can be bypassed without preventing the use of aspartate for sugar biosynthesis. Furthermore, PPDK has a main role in the entry of alanine and lactate into this pathway in amastigotes; however, its participation in gluconeogenesis, if needed, can be circumvented in promastigotes. It has been shown in other organisms (40, 41) that during gene expression perturbations, like the genetic ablation of PEPCK and PPDK reported here, compensatory routes could take place to prevent the accumulation of metabolites inside the cells. It could explain, in part, the minimal participation of PPDK and PEPCK in gluconeogenesis observed in promastigotes and amastigotes, respectively. Both life cycle stages are quite different at morphologic and metabolic levels (3, 42–44). Interestingly, our results also revealed differences in the degree of flexibility to perturbations of the central metabolic network between promastigotes and amastigotes.
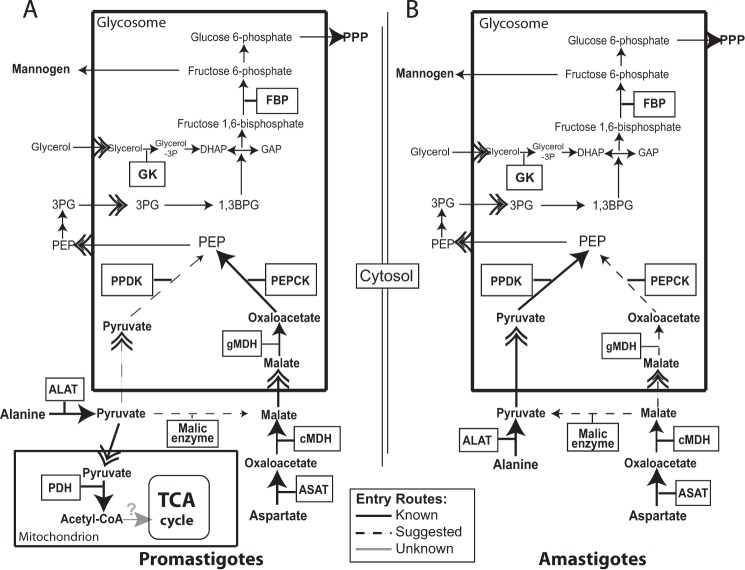
All GK, PPDK, and PEPCK are localized in the glycosomes, meaning that glycerol, pyruvate, and oxaloacetate have to be transported and/or generated into these organelles to be used for carbohydrate biosynthesis by these enzymes. It is possible that the difference in the compensatory mechanisms between promastigotes and amastigotes observed in this study may be influenced by differences in the trafficking of substrates between the glycosomes and the cytosol between both life cycle stages. The membrane of glycosomes, like other membranes, is impermeable to solutes and may contain transport proteins specific for certain metabolites (45). However, little is known about the transport systems operating in the glycosomal membrane and their expression during the parasite's life cycle. Our results suggested that the rate of pyruvate traffic between the cytosol and the glycosomal lumen is higher in amastigotes than in promastigotes.
The low participation of PPDK in the utilization of alanine for mannogen biosynthesis in promastigotes can be explained in part by the high rate of pyruvate decarboxylation observed in this life cycle stage (this study and see Ref. 14). In amastigotes, PPDK and PEPCK may play redundant roles in the entry of aspartate (Fig. 7), although other possibilities cannot be excluded. Aspartate could enter through PEPCK (as happens in promastigotes) and by the combined action of malic enzyme (ME) and PPDK (Fig. 7). The ME catalyzes the reversible oxidative decarboxylation of malate into pyruvate with the concomitant conversion of NADP+ to NADPH (8), which is required for the detoxification of reactive oxygen and nitrogen species (46) and for reductive biosyntheses (47) in trypanosomatids. This alternative gluconeogenesis route, combining ME and PPDK activities for the formation of PEP from TCA cycle intermediates, has been previously demonstrated in plants (48). A gluconeogenesis entry route involving the participation of ME (49) in combination with PPDK could be favorable in amastigotes, which live in environments where glucose is thought to be very low or absent, and in which the pentose phosphate pathway cannot serve as a major source of essential reducing power. Moreover, the ∼35% of alanine converted into pyruvate to be directly used as glucogenic precursor by promastigotes could be entering the pathway through PPDK and/or by the combined action of ME and PEPCK (Fig. 7). However, to clarify these issues will require an additional substantial body of work that is beyond the scope of this study.
FIGURE 7.
Schematic representation of the putative gluconeogenesis pathway in Leishmania promastigotes (A) and amastigotes (B) suggested in this study. Enzymes are shown in boxes. Not all enzymes and reactions in the pathway are shown for simplicity. Double chevron arrows indicate traffic through membranes. Dashed lines indicate suggested reactions or situations. Gray arrows indicate unknown reactions or situations. B, mitochondrial compartment is not shown for simplicity. Abbreviations used are as follows: ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; 1,3BPG, 1,3-bisphosphoglycerate; DHAP, dihydroxyacetone phosphate; FBPase, fructose-1,3-bisphosphatase; GAP, glyceraldehyde 3-phosphate; 3PG, 3-phosphoglycerate; cMDH, cytosolic malate dehydrogenase; gMDH, glycosomal malate dehydrogenase; PDH, pyruvate dehydrogenase; PPP, pentose phosphate pathway; TCA cycle, tricarboxylic acid cycle.
We found that Leishmania amastigotes but not promastigotes were able to use l-lactate as a precursor for gluconeogenesis. We could not detect the 14C label in the mannogen nor in the LPG fractions from promastigotes. Interestingly, label incorporation from lactate was also undetectable in the phospholipid fractions. The reason for the differences observed between both life cycle stages is unknown; however, there are several explanations involving, for example, possible stage-specific differences in the transport of substrates across the plasma membrane and/or the glycosome membranes as well as in the expression of specific enzyme activities between promastigotes and amastigotes. To date, it is unknown how this substrate is transported and converted into pyruvate in these parasites. In the Leishmania intracellular stage, lactate may enter into the parasites either by a mediated transport system (50) or it is possible that protonated lactate diffuses slowly across the plasma membrane of amastigotes, due to the acidic environment of the phagolysosomes. This situation has been described before in mammalian cells incubated in acidic conditions (34). Interestingly, T. cruzi amastigotes live inside cardiac cells, which take up l-lactate to be oxidized as a respiratory fuel (34), suggesting that this substrate would be also available in the environment in which the T. cruzi amastigotes develop. In this particular case, the participation of l-lactate transport proteins would be required to allow the utilization of this substrate because the T. cruzi amastigotes develop in a neutral pH environment. The conversion of lactate into pyruvate that would allow its use by L. mexicana PPDK in gluconeogenesis is a mystery. Lactate dehydrogenase activities have not been described in Leishmania or Trypanosoma parasites, and only two putative uncharacterized d-lactate dehydrogenase genes have been annotated in the L. mexicana genome database. However, there are several examples in the literature of trypanosomal proteins with well known functions and/or activities but quite divergent in origin from their mammalian counterparts (50–52). Functional complementation assays plus a more detailed study of the Leishmania genome database could help to clarify this issue.
Glycosomal succinate fermentation is considered the major pathway for regenerating glycosomal pools of ATP/NAD+ during the glucose catabolism in trypanosomatids (14, 17, 53). Although this pathway may increase the metabolic flexibility of the glycosomes (14), our results demonstrate that it is not essential for maintaining the glycosomal homeostasis is Leishmania promastigotes, as is the case in T. brucei procyclic stages (17, 53). In the absence of PEPCK, the presence of PPDK and GK could be important for maintaining the glycosomal ADP/ATP balance under such conditions. Indeed, PPDK seems not to play a major role in gluconeogenesis in promastigotes; however, its expression increased in this life cycle stage when parasites ran out of sugar, a condition in which gluconeogenesis was induced. These results suggest that PPDK may play an important role in maintaining the glycosomal energy balance under glucose starvation.
In conclusion, the nutritional differences among the habitats where these parasites thrive could lead to remarkable variations in their energy and carbon metabolism. It has been previously described that PEPCK, PPDK, and GK may contribute to the energy balance inside the glycosomes. In this study, we demonstrate that they also play a role in the synthesis de novo of carbohydrates in Leishmania parasites, showing stage-specific differences in their participation in the gluconeogenesis pathway (Fig. 7). The novel findings reported here substantially advance our knowledge about gluconeogenesis in Leishmania and make an important contribution to our understanding of the carbon metabolism and the central metabolic network in the clinically relevant amastigote stage. The information described in this study could help in the development of specific dynamic metabolic models that could be useful in finding drug targets more effective against intracellular trypanosomatid parasites. Nevertheless, more information about experimental sources in amastigotes, among other things, is still needed.
Supplementary Material
Acknowledgments
We appreciate the support of Scott M. Landfear for providing the L. mexicana wild type parasites and Claudia Lopez for technical advice. We acknowledge the members of the Landfear laboratory for critical comments on the paper before submission.
This work was supported by American Heart Association Grant 10BGIA2610035.

This article contains supplemental Fig. 1 and Table S1.
- GK
- glycerol kinase
- PEPCK
- phosphoenolpyruvate carboxykinase
- PPDK
- pyruvate phosphate dikinase
- PEP
- phosphoenolpyruvate
- FBPase
- fructose 1,6-bisphosphatase
- PYK
- pyruvate kinase
- LPG
- lipophosphoglycan
- ME
- malic enzyme
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- iFBS
- heat-inactivated FBS.
REFERENCES
- 1. Desjeux P. (2004) Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 27, 305–318 [DOI] [PubMed] [Google Scholar]
- 2. Murray H. W., Berman J. D., Davies C. R., Saravia N. G. (2005) Advances in leishmaniasis. Lancet 366, 1561–1577 [DOI] [PubMed] [Google Scholar]
- 3. Burchmore R. J., Hart D. T. (1995) Glucose transport in amastigotes and promastigotes of Leishmania mexicana mexicana. Mol. Biochem. Parasitol. 74, 77–86 [DOI] [PubMed] [Google Scholar]
- 4. Rainey P. M., MacKenzie N. E. (1991) A carbon-13 nuclear magnetic resonance analysis of the products of glucose metabolism in Leishmania pifanoi amastigotes and promastigotes. Mol. Biochem. Parasitol. 45, 307–315 [DOI] [PubMed] [Google Scholar]
- 5. Burchmore R. J., Landfear S. M. (1998) Differential regulation of multiple glucose transporter genes in Leishmania mexicana. J. Biol. Chem. 273, 29118–29126 [DOI] [PubMed] [Google Scholar]
- 6. Simon M. W., Martin E., Mukkada A. J. (1978) Evidence for a functional glyoxylate cycle in the leishmaniae. J. Bacteriol. 135, 895–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodríguez-Contreras D., Landfear S. M. (2006) Metabolic changes in glucose transporter-deficient Leishmania mexicana and parasite virulence. J. Biol. Chem. 281, 20068–20076 [DOI] [PubMed] [Google Scholar]
- 8. Nelson D. L., Cox N. N. (2005) Principles of Biochemistry, 4th Ed., pp. 551–559, W. H. Freeman & Co., New York [Google Scholar]
- 9. Naderer T., Ellis M. A., Sernee M. F., De Souza D. P., Curtis J., Handman E., McConville M. J. (2006) Virulence of Leishmania major in macrophages and mice requires the gluconeogenic enzyme fructose-1,6-bisphosphatase. Proc. Natl. Acad. Sci. U.S.A. 103, 5502–5507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ralton J. E., Naderer T., Piraino H. L., Bashtannyk T. A., Callaghan J. M., McConville M. J. (2003) Evidence that intracellular β1–2 mannan is a virulence factor in Leishmania parasites. J. Biol. Chem. 278, 40757–40763 [DOI] [PubMed] [Google Scholar]
- 11. Bringaud F., Baltz D., Baltz T. (1998) Functional and molecular characterization of a glycosomal PPi-dependent enzyme in trypanosomatids: pyruvate, phosphate dikinase. Proc. Natl. Acad. Sci. U.S.A. 95, 7963–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cymeryng C., Cazzulo J. J., Cannata J. J. (1995) Phosphoenolpyruvate carboxykinase from Trypanosoma cruzi. Purification and physicochemical and kinetic properties. Mol. Biochem. Parasitol. 73, 91–101 [DOI] [PubMed] [Google Scholar]
- 13. Steinborn K., Szallies A., Mecke D., Duszenko M. (2000) Cloning, heterologous expression and kinetic analysis of glycerol kinase (TbGLK1) from Trypanosoma brucei. Biol. Chem. 381, 1071–1077 [DOI] [PubMed] [Google Scholar]
- 14. Saunders E. C., Ng W. W., Chambers J. M., Ng M., Naderer T., Krömer J. O., Likic V. A., McConville M. J. (2011) Isotopomer profiling of Leishmania mexicana promastigotes reveals important roles for succinate fermentation and aspartate uptake in tricarboxylic acid cycle (TCA) anaplerosis. J. Biol. Chem. 286, 27706–27717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michels P. A., Hannaert V., Bringaud F. (2000) Metabolic aspects of glycosomes in trypanosomatidae–new data and views. Parasitol. Today 16, 482–489 [DOI] [PubMed] [Google Scholar]
- 16. Pieretti S., Haanstra J. R., Mazet M., Perozzo R., Bergamini C., Prati F., Fato R., Lenaz G., Capranico G., Brun R., Bakker B. M., Michels P. A., Scapozza L., Bolognesi M. L., Cavalli A. (2013) Naphthoquinone derivatives exert their antitrypanosomal activity via a multi-target mechanism. PLoS Negl. Trop. Dis. 7, e2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Besteiro S., Biran M., Biteau N., Coustou V., Baltz T., Canioni P., Bringaud F. (2002) Succinate secreted by Trypanosoma brucei is produced by a novel and unique glycosomal enzyme, NADH-dependent fumarate reductase. J. Biol. Chem. 277, 38001–38012 [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez-Contreras D., Feng X., Keeney K. M., Bouwer H. G., Landfear S. M. (2007) Phenotypic characterization of a glucose transporter null mutant in Leishmania mexicana. Mol. Biochem. Parasitol. 153, 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iovannisci D. M., Ullman B. (1983) High efficiency plating method for Leishmania promastigotes in semidefined or completely-defined medium. J. Parasitol. 69, 633–636 [PubMed] [Google Scholar]
- 20. Robinson K. A., Beverley S. M. (2003) Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol. Biochem. Parasitol. 128, 217–228 [DOI] [PubMed] [Google Scholar]
- 21. Feng X., Rodriguez-Contreras D., Polley T., Lye L. F., Scott D., Burchmore R. J., Beverley S. M., Landfear S. M. (2013) 'Transient' genetic suppression facilitates generation of hexose transporter null mutants in Leishmania mexicana. Mol. Microbiol. 87, 412–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seyfang A., Landfear S. M. (2000) Four conserved cytoplasmic sequence motifs are important for transport function of the Leishmania inositol/H+ symporter. J. Biol. Chem. 275, 5687–5693 [DOI] [PubMed] [Google Scholar]
- 23. Christie W. W. (2003) Lipid Analysis: Isolation, Separation, Identification and Structural Analysis of Lipids, 3rd Ed., pp. 343–372, The Oil Press, Bridgwater, UK [Google Scholar]
- 24. Tran K. D., Rodriguez-Contreras D., Vieira D. P., Yates P. A., David L., Beatty W., Elferich J., Landfear S. M. (2013) KHARON1 mediates flagellar targeting of a glucose transporter in Leishmania mexicana and is critical for viability of infectious intracellular amastigotes. J. Biol. Chem. 288, 22721–22733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blume M., Hliscs M., Rodriguez-Contreras D., Sanchez M., Landfear S., Lucius R., Matuschewski K., Gupta N. (2011) A constitutive pan-hexose permease for the Plasmodium life cycle and transgenic models for screening of antimalarial sugar analogs. FASEB J. 25, 1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mottram J. C., Coombs G. H. (1985) Leishmania mexicana: subcellular distribution of enzymes in amastigotes and promastigotes. Exp. Parasitol. 59, 265–274 [DOI] [PubMed] [Google Scholar]
- 27. Cazzulo J. J., Franke de Cazzulo B. M., Engel J. C., Cannata J. J. (1985) End products and enzyme levels of aerobic glucose fermentation in trypanosomatids. Mol. Biochem. Parasitol. 16, 329–343 [DOI] [PubMed] [Google Scholar]
- 28. Cazzulo J. J. (1992) Aerobic fermentation of glucose by trypanosomatids. FASEB J. 6, 3153–3161 [DOI] [PubMed] [Google Scholar]
- 29. Costa T. L., Ribeiro-Dias F., Oliveira M. A., Bezerra J. C., Vinaud M. C. (2011) Energetic metabolism of axenic promastigotes of Leishmania (Viannia) braziliensis. Exp. Parasitol. 128, 438–443 [DOI] [PubMed] [Google Scholar]
- 30. Yao C., Leidal K. G., Brittingham A., Tarr D. E., Donelson J. E., Wilson M. E. (2002) Biosynthesis of a major surface protease GP63 of Leishmania chagasi. Mol. Biochem. Parasitol. 121, 119–128 [DOI] [PubMed] [Google Scholar]
- 31. Ilgoutz S. C., McConville M. J. (2001) Function and assembly of the Leishmania surface coat. Int. J. Parasitol. 31, 899–908 [DOI] [PubMed] [Google Scholar]
- 32. Coustou V., Besteiro S., Biran M., Diolez P., Bouchaud V., Voisin P., Michels P. A., Canioni P., Baltz T., Bringaud F. (2003) ATP generation in the Trypanosoma brucei procyclic form: cytosolic substrate level is essential, but not oxidative phosphorylation. J. Biol. Chem. 278, 49625–49635 [DOI] [PubMed] [Google Scholar]
- 33. Newsholme P., Costa Rosa L. F., Newsholme E. A., Curi R. (1996) The importance of fuel metabolism to macrophage function. Cell Biochem. Funct. 14, 1–10 [DOI] [PubMed] [Google Scholar]
- 34. Poole R. C., Halestrap A. P. (1993) Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J. Physiol. 264, C761–C782 [DOI] [PubMed] [Google Scholar]
- 35. Ewaschuk J. B., Naylor J. M., Zello G. A. (2005) d-Lactate in human and ruminant metabolism. J. Nutr. 135, 1619–1625 [DOI] [PubMed] [Google Scholar]
- 36. Dougherty M. J., Boyd J. M., Downs D. M. (2006) Inhibition of fructose-1,6-bisphosphatase by aminoimidazole carboxamide ribotide prevents growth of Salmonella enterica purH mutants on glycerol. J. Biol. Chem. 281, 33892–33899 [DOI] [PubMed] [Google Scholar]
- 37. McKinney J. D., Höner zu Bentrup K., Muñoz-Elías E. J., Miczak A., Chen B., Chan W. T., Swenson D., Sacchettini J. C., Jacobs W. R., Jr., Russell D. G. (2000) Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406, 735–738 [DOI] [PubMed] [Google Scholar]
- 38. Lorenz M. C., Bender J. A., Fink G. R. (2004) Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 3, 1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Atwood J. A., 3rd, Weatherly D. B., Minning T. A., Bundy B., Cavola C., Opperdoes F. R., Orlando R., Tarleton R. L. (2005) The Trypanosoma cruzi proteome. Science 309, 473–476 [DOI] [PubMed] [Google Scholar]
- 40. Usui Y., Hirasawa T., Furusawa C., Shirai T., Yamamoto N., Mori H., Shimizu H. (2012). Investigating the effects of perturbations to pgi and eno gene expression on central carbon metabolism in Escherichia coli using 13C metabolic flux analysis. Microb. Cell Fact. 11, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zelezniak A., Sheridan S., Patil K. R. (2014). Contribution of network connectivity in determining the relationship between gene expression and metabolite concentration changes. PLoS Comput. Biol. 10, e1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coombs G. H., Craft J. A., Hart D. T. (1982) A comparative study of Leishmania mexicana amastigotes and promastigotes. Enzyme activities and subcellular locations. Mol. Biochem. Parasitol. 5, 199–211 [DOI] [PubMed] [Google Scholar]
- 43. McConville M. J., Naderer T. (2011) Metabolic pathways required for intracellular survival of Leishmania. Annu. Rev. Microbiol. 65, 543–561 [DOI] [PubMed] [Google Scholar]
- 44. Bates P. A. (1994) Complete developmental cycle of Leishmania mexicana in axenic culture. Parasitology 108, 1–9 [DOI] [PubMed] [Google Scholar]
- 45. Igoillo-Esteve M., Mazet M., Deumer G., Wallemacq P., Michels P. A. (2011) Glycosomal ABC transporters of Trypanosoma brucei: characterisation of their expression, topology and substrate specificity. Int. J. Parasitol. 41, 429–438 [DOI] [PubMed] [Google Scholar]
- 46. Kerkhoven E. J., Achcar F., Alibu V. P., Burchmore R. J., Gilbert I. H., Trybiło M., Driessen N. N., Gilbert D., Breitling R., Bakker B. M., Barrett M. P. (2013) Handing uncertainty in dynamic models: the pentose phosphate pathway in Trypanosoma brucei. PLoS Comput. Biol. 9, e1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee S. H., Stephens J. L., Englund P. T. (2007) A fatty acid synthesis mechanism specialized for parasitism. Nat. Rev. Microbiol. 5, 287–297 [DOI] [PubMed] [Google Scholar]
- 48. Osterås M., Driscoll B. T., Finan T. M. (1997) Increased pyruvate orthophosphate dikinase activity results in an alternative gluconeogenic pathway in Rhizobium (Sinorhizobium) meliloti. Microbiology 143, 1639–1648 [DOI] [PubMed] [Google Scholar]
- 49. Leroux A. E., Maugeri D. A., Opperdoes F. R., Cazzulo J. J., Nowicki C. (2011) Comparative studies on the biochemical properties of the malic enzymes from Trypanosoma cruzi and Trypanosoma brucei. FEMS Microbiol. Lett. 314, 25–33 [DOI] [PubMed] [Google Scholar]
- 50. Sanchez M. A. (2013) Molecular identification and characterization of an essential pyruvate transporter from Trypanosoma brucei. J. Biol. Chem. 288, 14428–14437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang T., Bar-Peled M. (2010) Identification of a novel UDP-sugar pyrophosphorylase with a broad substrate specificity in Trypanosoma cruzi. Biochem. J. 429, 533–543 [DOI] [PubMed] [Google Scholar]
- 52. Dean S., Marchetti R., Kirk K., Matthews K. R. (2009) A surface transporter family conveys the trypanosome differentiation signal. Nature 459, 213–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Coustou V., Biran M., Breton M., Guegan F., Rivière L., Plazolles N., Nolan D., Barrett M. P., Franconi J. M., Bringaud F. (2008) Glucose-induced remodeling of intermediary and energy metabolism in procyclic Trypanosoma brucei. J. Biol. Chem. 283, 16342–16354 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.