Abstract
Facioscapulohumeral muscular dystrophy (FSHSD) is one of the most common adult muscular dystrophies and is divided into types 1 and 2 based on genetic mutation. Clinically both FSHD types 1 and 2 demonstrate often asymmetric and progressive muscle weakness affecting initially the face, shoulder, and arms, followed by the distal and then proximal lower extremities later in the disease course. Approximately 95% of patients, termed FSHD1, have a deletion of a key number of repetitive elements on chromosome 4q35. The remaining 5%, termed FSHD2, have no deletion on chromosome 4q35. Nevertheless, both FSHD types 1 and 2 share a common downstream mechanism making it possible that future disease-directed therapies will be effective for both FSHD types 1 and 2.
Keywords: Muscular dystophy, FSHD1, FDHD2, D4Z4 deletion, DUX4, SMCHD1 mutation
Introduction
Facioscapulohumeral muscular dystrophy (FSHD), one of the most prevalent adult muscular dystrophies (1:15,000 to 1:20,000), is characterized by asymmetric and often descending weakness affecting the face, shoulder, and arms, followed by weakness of the distal lower extremities and pelvic girdle.1, 2 FSHD is categorized as type 1 or type 2 based on the underlying genetic lesions. Approximately 95% of patients will have disease inherited in an autosomal dominant fashion associated with loss of part of a repeated sequence in the D4Z4 region on chromosome 4q35.3, 4 An additional 5% of patients will have disease with a variable inheritance pattern caused by a D4Z4 deletion-independent pathway.5 Recent advances suggest both FSHD types 1 and 2 exert their effect through a common pathophysiological pathway: de-repression of the retrogene DUX4 believed to cause disease in a toxic-gain-of-function manner.6 Studies have suggested FSHD1 and FSHD2 are clinically identical; although, the number of FSHD2 patients studied has been limited. Approximately 20% of FSHD patients greater than 50 years of age will require the use of a wheelchair.2, 7 FSHD1 patients with the largest contractions are more likely to have extra-muscular manifestations of FSHD, which include symptomatic retinal vascular disease and hearing loss.8, 9 The elucidation of a proposed common molecular mechanism behind both FSHD types 1 and 2 has opened the door to research in potential disease-directed therapies.
Clinical Findings
Both FSHD types 1 and 2 are clinically similar, characterized by:
Symptom onset typically in the first or second decade of life, but can present later in life
Often marked side to side asymmetry
Facial weakness seen as inability to squeeze the eyes shut or furrow the brow, a transvers smile, or flattening when puckering the lips
Shoulder weakness often with scapular winging and flattening of the clavicles
Arm weakness including the biceps and triceps often with forearm sparing
Asymmetric abdominal weakness which can be seen on exam as a positive Beevor’s sign
Usually distal lower extremity weakness before proximal, starting with a foot drop
FSHD can go on to affect most any skeletal muscle but typically spares extra-ocular muscles, cardiac muscles, and bulbar muscles. Patients can develop debilitating paraspinal muscle weakness, which can be an initial presentation.10 Although the most common presentation is with facial and shoulder weakness and a descending pattern of progression, many different initial presentations have been described, including bent-spine and less specific limb girdle patterns. The rate of progression has been evaluated in a large prospective natural history study, which demonstrated a loss of strength using combined quantitative strength testing and manual muscle testing of about 1-4% per year.11 Although not life-limiting FSHD can cause significant lifetime morbidity.2, 7, 12 The 6-year risk of wheelchair use overalll is about 24%. Risk of wheelchair use shows a bimodal distribution: FSHD1 patients with the largest D4Z4 deletions (1-3 remaining repeats) have the highest risk which peaks in the 2nd and 3rd decades, followed by a slow age-dependent increase in the risk. Respiratory involvement can be seen in about 10% of patients, most commonly in the most severely affected or wheelchair bound patients. Atrial arrhythmias can be seen in about 5% of FSHD patients but are rarely symptomatic.
Extra-muscular manifestations are also rarely symptomatic and include retinal vascular changes and hearing loss. Approximately half of FSHD patients show peripheral retinal vascular abnormalities on fluorescein angiography but symptomatic retinal vasculopathy (Coat’s syndrome) is only seen in approximately 1% of patients, typically patients with the largest D4Z4 deletions.9, 13, 14 High frequency hearing loss is reported in approximately half of FSHD patients; however symptomatic hearing loss resulting in the need for hearing aids is almost exclusively seen in patients with the largest D4Z4 deletions.8, 15
A more severe infantile form of FSHD has been described.16, 17 These patients typically:
Have the largest deletions (1-3 D4Z4 repeats remaining)
Have increased prevalence of symptomatic retinal vascular disease and hearing loss
Have more severe disease with earlier wheelchair use
Rarely mental retardation or seizures have been described
Diagnosis
Clinical criteria for the diagnosis of FSHD include the presence of characteristic findings and the absence of other explanations.18 FSHD is suggested by the presence of:
Facial weakness
Weakness of shoulder scapular stabilizers or foot dorsiflexors
The absence of:
Ptosis, weakness of extra-ocular muscles or bulbar weakness
Electromyography in a patient or affected family member showing myotonia or neurogenic changes
Electromyography shows changes characteristic of a chronic myopathy, small polyphasic motor units, but may be normal or only show changes in limited muscles, like the serratus anterior or pectorals. Muscle biopsy is not required for diagnosis but can show non-specific myopathic or dystrophic changes including variability in fiber size, rounded fibers, internal nuclei, necrotic or regenerating fibers, increased connective tissue and fatty deposition. Up to 1/3 of muscle biopsies can show a primarily mononuclear inflammatory infiltrate.19, 20
Muscle MRI is being used more frequently in the evaluation of patients with suspected muscular dystrophies, and although there are patterns of muscle involvement typical for FSHD, the role of MRI in diagnosis has yet to be determined.21-23 Patterns of muscle involvement on MRI in FSHD include:
Early involvement of trapezius, scapular girdle, and pectoral muscles
Early involvement of the gastrocnemius and tibialis anterior
A variable number of patients will demonstrate STIR positive signal in structurally normal appearing muscles, which may correspond to areas of inflammation
Ultimately the diagnosis of FSHD is confirmed by genetic testing. Approximately 95% of patients meeting clinical criteria, termed FSHD1, will turn out to have deletion of a key number of repetitive elements in the D4Z4 region of chromosome 4q35. Normal individuals have >10 repeats. Patients with FSHD1 have between 1-10 repeats.3, 4 An additional 5% of patients, termed FSHD2, will have disease without a deletion in the number of D4Z4 repeats. Nevertheless, these patients have reduced methylation in the D4Z4 region of 4q35 as is seen in the contracted 4q35 allele in FSHD1.5 Recently up to 2/3 of patients with FSHD2 were discovered to have mutations in the gene SMCHD1, believed to have a role in chromatin inactivation.24 Commercial genetic confirmation of FSHD2 is not yet available.
Pathophysiology
Recent studies suggest that activation of a normally repressed transcriptional regulator, DUX4, contained within the D4Z4 repeat elements on chromosome 4q35 causes disease in FSHD via a toxic-gain-of-function fashion.25
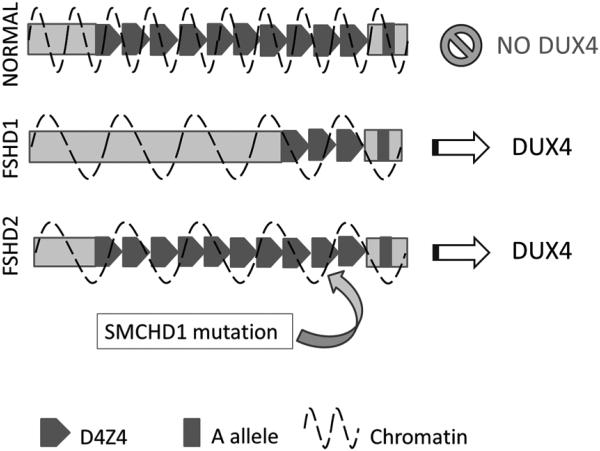
In FSHD1, a key number of repeated sequences, each 3.3 kb long, are lost in the D4Z4 region on chromosome 4q35. Loss of the repetitive elements leads to decreased methylation and opening up of the chromatin structure. Contained in each D4Z4 repeat is a putative retrogene, DUX4, not normally expressed in adult muscle. Loss of the repetitive elements opens the chromatin structure allowing DUX4 to be expressed from the most distal D4Z4 repeat. But that is not enough to cause disease in FSHD. In addition, patients must have a permissive genetic background: a certain polymorphism distal to the last repeat, known as the A variant, results in a polyadenylation sequence that is essential for the stability of nascent DUX4 transcripts, which would otherwise be degraded (Figure 1).6
Figure 1.
Pathological mechanism in FSHD. In normal individuals the chromatin is tightly wound keeping DUX4 in a repressed state. In FSHD1 deletion of a critical number of D4Z4 repeats opens up the chromatin structure allowing DUX4 to be expressed. However this only occurs when the D4Z4 deletion occurs on a permissive genetic background, the A allele, which contains a polyadenylation sequence which stabilizes the nascent DUX4 transcripts. In FSHD2 patients do not have deletions in the D4Z4 region, but do have decreased methylation, which in approximately 2/3 of patients is associated with mutations in the SMCHD1 gene. Decreased methylation also causes an opening of chromatin structure, and when this occurs on a permissive genetic background containing the A allele, DUX4 can be expressed. In both expression of DUX4 is believed to cause disease in a toxic gain-of-function fashion.
In FSHD2 patients do not have deletions in the D4Z4 region and yet there is loss of methylation in the D4Z4 region of chromosome 4q35. While the inheritance in FSHD1 is dominant (resulting from the occurrence of a contraction on one copy of 4q35 containing an A allele), the inheritance pattern in FSHD2 is more complex because it is a digenic disease. FSHD2 requires the inheritance of permissive A allele and separate mutation in a gene that regulates chromatic structure (eg: SMCHD1 on chromosome 18). This loss of methylation in conjunction with a permissive genetic background also leads to expression of DUX4.5 Recently approximately 2/3 of patients with FSHD2 were found to have mutations in SMCHD1, a gene on chromosome 18 believed to have a role in chromatin inactivation (Figure 1).24
Several lines of evidence suggest that low levels of DUX4 expression interfere with myogenic differentiation, lead to apoptotic cell death and make cells more susceptible to oxidative stress.24, 26-30
Necessary for FSHD:
Opening up of the chromatin structure in the D4Z4 region of chromosome 4q35 allowing the normally repressed DUX4 gene to be expressed
Stabilizing polymorphisms that prevent nascent DUX4 mRNA from being degraded
Therapeutic Strategies
There are currently no FDA-approved treatments for FSHD. A number of pharmacological strategies have been tested to determine if they slow down or halt disease progression in FSHD: trials of anabolic agents, a myostatin inhibitor, creatine supplementation, and corticosteroids were either negative or inconclusive.31-36 Future treatment strategies can be split into two categories: 1) therapies to increase muscle bulk or strength (anabolic agents, myostatin or follistatin inhibitors); 2) therapies to halt disease progression (molecular knock-down of DUX4, or downstream targets of DUX4).
A trial of exercise and albuterol, alone or in combination, showed improvement in isolated muscle strength with albuterol but was negative for individually trained muscles.37 Strength training consisted of a progressive overload program which included dynamic and isometric exercises focusing on elbow flexors and ankle dorsiflexors. Patients receiving strength training did not do worse than those who did not pursue strength training, and dynamic strength improved in elbow flexors. Aerobic therapy is likely beneficial in FSHD, not only improving cardiovascular health but possible increasing strength.38
There are a number of observational studies and case series documenting improvement in shoulder function, shoulder range of motion, or improvement in scapular pain following scapular fixation.39, 40 Scapulodesis (the fixation of the scapula with screws, wires or plates) with bone grafting is the preferred surgical procedure. Despite that there are no randomized trials to support the benefit seen in observational studies; nor are there clear recommendations on which patients are most likely to benefit from this procedure or the optimal timing of surgery. Intuitively patients considering surgery should have reasonable residual upper arm strength. The potential gain in range of motion from surgical fixation can be tested at the bedside by manual fixation of the scapula. Drawbacks to scapular fixation include postoperative immobilization, need for physiotherapy and potential complications (breaks in the wire with consequent loss of the functional gain, brachial plexus injuries, or possible loss of respiratory forced vital capacity).
Although no prospective studies have determined the optimal surveillance strategy for use of orthotics or extra muscular manifestations of FSHD, we recommend:
Baseline screening for retinal involvement with dilated ophthalmological exam in all patients; then yearly in patients with the largest D4Z4 deletions (1-3 residual repeats)
Baseline pulmonary function testing in patients with advanced disease, early pelvic girdle weakness, or significant kyphoscoliosis, then yearly follow up
Hearing test for all infantile onset FSHD
Yearly evaluation for need for orthotic devices for ambulation
Summary and Future Directions
Recent advances in our understanding of the molecular pathology of FSHD have identified potential molecular targets for future therapies. FSHD types 1 and 2 are clinically similar and share a final common pathway suggesting that similar treatment strategies may prove successful for both. Future therapeutic strategies will likely include targeting the expression of DUX4 or its downstream targets, and strategies geared towards increasing muscle bulk and strength.
Key Points.
Clinically, FSHD types 1 and 2 are similar: often asymmetric and descending weakness affecting the face, shoulder and arms, followed by the distal lower extremities and pelvic girdle
FSHD1 patients with the largest contractions are more likely to have symptomatic extra muscular involvement which includes: retinal vascular disease, hearing loss, and rarely, cognitive impairment or seizures
FSHD type 1 is caused by a deletion in the number of the macrosatellite repeat (D4Z4) elements on chromosome 4q35; this leads to decreased DNA methylation and opening of the chromatin structure.
FSHD type 2 is caused by mutations in genes elsewhere in the genome that lead to decreased methylation in the same D4Z4 region on chromosome 4q35
The opening of the chromatic structure seen in both FSHD types 1 and 2 results in derepression of the DUX4 gene, a transcriptional factor believed to cause disease through a toxic gain-of-function mechanism
The identification of a proposed disease mechanism opens the door to future disease-directed therapies.
Acknowledgments
Support:
J.S. MDA Clinical Research Training Grant
R.T. none.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Both R.T. and J.S. are consultants for Cytokinetics
References
- 1.Mostacciuolo ML, Pastorello E, Vazza G, et al. Facioscapulohumeral muscular dystrophy: epidemiological and molecular study in a north-east Italian population sample. Clin Genet. 2009;75(6):550–555. doi: 10.1111/j.1399-0004.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 2.Padberg GW, Frants RR, Brouwer OF, et al. Facioscapulohumeral muscular dystrophy in the Dutch population. Muscle Nerve. 1995;2:S81–84. [PubMed] [Google Scholar]
- 3.van Deutekom JC, Wijmenga C, van Tienhoven EA, et al. FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum Mol Genet. 1993;2(12):2037–2042. doi: 10.1093/hmg/2.12.2037. [DOI] [PubMed] [Google Scholar]
- 4.Wijmenga C, Hewitt JE, Sandkuijl LA, et al. Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat Genet. 1992;2(1):26–30. doi: 10.1038/ng0992-26. [DOI] [PubMed] [Google Scholar]
- 5.de Greef JC, Lemmers RJ, Camano P, et al. Clinical features of facioscapulohumeral muscular dystrophy 2. Neurology. 2010;75(17):1548–1554. doi: 10.1212/WNL.0b013e3181f96175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Maarel SM, Tawil R, Tapscott SJ. Facioscapulohumeral muscular dystrophy and DUX4: breaking the silence. Trends Mol Med. 2011;17(5):252–258. doi: 10.1016/j.molmed.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Statland JM, Tawil R. Risk of functional impairment in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2013 doi: 10.1002/mus.23949. [DOI] [PubMed] [Google Scholar]
- 8.Lutz KL, Holte L, Kliethermes SA, et al. Clinical and genetic features of hearing loss in facioscapulohumeral muscular dystrophy. Neurology. 2013;81(16):1374–1377. doi: 10.1212/WNL.0b013e3182a84140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Statland JM, Sacconi S, Farmakidis C, et al. Coats syndrome in facioscapulohumeral dystrophy type 1: frequency and D4Z4 contraction size. Neurology. 2013;80(13):1247–1250. doi: 10.1212/WNL.0b013e3182897116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan B, Eger K, Koesling S, et al. Camptocormia phenotype of FSHD: a clinical and MRI study on six patients. J Neurol. 2011;258(5):866–873. doi: 10.1007/s00415-010-5858-z. [DOI] [PubMed] [Google Scholar]
- 11.FSH-DY A prospective, quantitative study of the natural history of facioscapulohumeral muscular dystrophy (FSHD): implications for therapeutic trials. Neurology. 1997;48(1):38–46. doi: 10.1212/wnl.48.1.38. The FSH-DY Group. [DOI] [PubMed] [Google Scholar]
- 12.Laforet P, de Toma C, Eymard B, et al. Cardiac involvement in genetically confirmed facioscapulohumeral muscular dystrophy. Neurology. 1998;51(5):1454–1456. doi: 10.1212/wnl.51.5.1454. [DOI] [PubMed] [Google Scholar]
- 13.Fitzsimons RB, Gurwin EB, Bird AC. Retinal vascular abnormalities in facioscapulohumeral muscular dystrophy. A general association with genetic and therapeutic implications. Brain. 1987;110:631–648. doi: 10.1093/brain/110.3.631. Pt 3. [DOI] [PubMed] [Google Scholar]
- 14.Padberg GW, Brouwer OF, de Keizer RJ, et al. On the significance of retinal vascular disease and hearing loss in facioscapulohumeral muscular dystrophy. Muscle Nerve. 1995;2:S73–80. [PubMed] [Google Scholar]
- 15.Trevisan CP, Pastorello E, Tomelleri G, et al. Facioscapulohumeral muscular dystrophy: hearing loss and other atypical features of patients with large 4q35 deletions. Eur J Neurol. 2008;15(12):1353–1358. doi: 10.1111/j.1468-1331.2008.02314.x. [DOI] [PubMed] [Google Scholar]
- 16.Funakoshi M, Goto K, Arahata K. Epilepsy and mental retardation in a subset of early onset 4q35-facioscapulohumeral muscular dystrophy. Neurology. 1998;50(6):1791–1794. doi: 10.1212/wnl.50.6.1791. [DOI] [PubMed] [Google Scholar]
- 17.Lunt PW, Jardine PE, Koch MC, et al. Correlation between fragment size at D4F104S1 and age at onset or at wheelchair use, with a possible generational effect, accounts for much phenotypic variation in 4q35-facioscapulohumeral muscular dystrophy (FSHD) Hum Mol Genet. 1995;4(5):951–958. doi: 10.1093/hmg/4.5.951. [DOI] [PubMed] [Google Scholar]
- 18.Tawil R, McDermott MP, Mendell JR, et al. Facioscapulohumeral muscular dystrophy (FSHD): design of natural history study and results of baseline testing. Neurology. 1994;44(3):442–446. doi: 10.1212/wnl.44.3_part_1.442. FSH-DY Group. Pt 1. [DOI] [PubMed] [Google Scholar]
- 19.Arahata K, Ishihara T, Fukunaga H, et al. Inflammatory response in facioscapulohumeral muscular dystrophy (FSHD): immunocytochemical and genetic analyses. Muscle Nerve. 1995;2:S56–66. [PubMed] [Google Scholar]
- 20.Carpenter S KG . Pathology of Skeletal Muscle. Second Oxford University Press; New York: 2001. [Google Scholar]
- 21.Friedman SD, Poliachik SL, Carter GT, et al. The magnetic resonance imaging spectrum of facioscapulohumeral muscular dystrophy. Muscle Nerve. 2012;45(4):500–506. doi: 10.1002/mus.22342. [DOI] [PubMed] [Google Scholar]
- 22.Kan HE, Scheenen TW, Wohlgemuth M, et al. Quantitative MR imaging of individual muscle involvement in facioscapulohumeral muscular dystrophy. Neuromuscul Disord. 2009;19(5):357–362. doi: 10.1016/j.nmd.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Masciullo M, Iannaccone E, Bianchi ML, et al. Myotonic dystrophy type 1 and de novo FSHD mutation double trouble: a clinical and muscle MRI study. Neuromuscul Disord. 2013;23(5):427–431. doi: 10.1016/j.nmd.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Lemmers RJ, Tawil R, Petek LM, et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat Genet. 2012;44(12):1370–1374. doi: 10.1038/ng.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemmers RJ, van der Vliet PJ, Klooster R, et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science. 2010;329(5999):1650–1653. doi: 10.1126/science.1189044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosnakovski D, Daughters RS, Xu Z, et al. Biphasic myopathic phenotype of mouse DUX, an ORF within conserved FSHD-related repeats. PLoS One. 2009;4(9):e7003. doi: 10.1371/journal.pone.0007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowaljow V, Marcowycz A, Ansseau E, et al. The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein. Neuromuscul Disord. 2007;17(8):611–623. doi: 10.1016/j.nmd.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Snider L, Asawachaicharn A, Tyler AE, et al. RNA transcripts, miRNA-sized fragments and proteins produced from D4Z4 units: new candidates for the pathophysiology of facioscapulohumeral dystrophy. Hum Mol Genet. 2009;18(13):2414–2430. doi: 10.1093/hmg/ddp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanderplanck C, Ansseau E, Charron S, et al. The FSHD atrophic myotube phenotype is caused by DUX4 expression. PLoS One. 2011;6(10):e26820. doi: 10.1371/journal.pone.0026820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wuebbles RD, Long SW, Hanel ML, et al. Testing the effects of FSHD candidate gene expression in vertebrate muscle development. Int J Clin Exp Pathol. 2010;3(4):386–400. [PMC free article] [PubMed] [Google Scholar]
- 31.Kissel JT, McDermott MP, Mendell JR, et al. Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology. 2001;57(8):1434–1440. doi: 10.1212/wnl.57.8.1434. [DOI] [PubMed] [Google Scholar]
- 32.Payan CA, Hogrel JY, Hammouda EH, et al. Periodic salbutamol in facioscapulohumeral muscular dystrophy: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90(7):1094–1101. doi: 10.1016/j.apmr.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 33.Rose MR, Tawil R. Drug treatment for facioscapulohumeral muscular dystrophy. Cochrane Database Syst Rev. 2004;(2) doi: 10.1002/14651858.CD002276.pub2. CD002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tawil R, McDermott MP, Pandya S, et al. A pilot trial of prednisone in facioscapulohumeral muscular dystrophy. FSH-DY Group. Neurology. 1997;48(1):46–49. doi: 10.1212/wnl.48.1.46. [DOI] [PubMed] [Google Scholar]
- 35.Wagner KR, Fleckenstein JL, Amato AA, et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63(5):561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 36.Walter MC, Lochmuller H, Reilich P, et al. Creatine monohydrate in muscular dystrophies: A double-blind, placebo-controlled clinical study. Neurology. 2000;54(9):1848–1850. doi: 10.1212/wnl.54.9.1848. [DOI] [PubMed] [Google Scholar]
- 37.van der Kooi EL, Vogels OJ, van Asseldonk RJ, et al. Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology. 2004;63(4):702–708. doi: 10.1212/01.wnl.0000134660.30793.1f. [DOI] [PubMed] [Google Scholar]
- 38.Olsen DB, Orngreen MC, Vissing J. Aerobic training improves exercise performance in facioscapulohumeral muscular dystrophy. Neurology. 2005;64(6):1064–1066. doi: 10.1212/01.WNL.0000150584.45055.27. [DOI] [PubMed] [Google Scholar]
- 39.Orrell RW, Copeland S, Rose MR. Scapular fixation in muscular dystrophy. Cochrane Database Syst Rev. 2010;(1) doi: 10.1002/14651858.CD003278.pub2. CD003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Tongel A, Atoun E, Narvani A, et al. Medium to long-term outcome of thoracoscapular arthrodesis with screw fixation for facioscapulohumeral muscular dystrophy. J Bone Joint Surg Am. 2013;95(15):1404–1408. doi: 10.2106/JBJS.L.01097. [DOI] [PubMed] [Google Scholar]