Abstract
As part of this special section on genetics and behavioral intervention, we discuss the papers by McGue et al. and by Davey Smith. In the second half of our paper, we consider the integration of genetics and intervention research more broadly.
The two papers describe ways to use genetic controls to infer causation from correlational (‘observational’) data without intervention. McGue et al. discuss the use of twins discordant for exposure, which is a variant of the co-twin control method. This method can show that the link between an exposure and outcome is not entirely mediated genetically. Davey Smith discusses a method called Mendelian randomization that uses DNA to draw causal inferences without the need for experimental intervention.
Despite the possibilities for using genetic controls to infer causation from correlational data in order to attenuate the need for intervention studies, we are most excited about the opportunities for integrating genetics and intervention research, especially as new DNA technologies make it possible to incorporate genetics in any intervention research.
Now that nearly everyone accepts the importance of both nature and nurture in behavioral development, interest is increasing in the interplay (interactions and correlations) between nature and nurture as well as identifying ways to study nurture controlling for nature and vice versa. What’s new in this special section is an attempt to move beyond investigating the genetic and environmental etiology of naturally occurring phenotypic variation to consider ways in which genetics can facilitate behavioral interventions. That is, instead of studying the genetic and environmental origins of what is, how can we use genetics in conjunction with interventions in order to help understand what could be?
The papers by McGue et al. and by Davey Smith attempt to use genetic controls to infer causation from correlational (‘observational’) data without experimental intervention. Putting the words correlation and causation in the same sentence might still evoke the habitual response that ‘correlation does not imply causation’, although the aphorism really means that correlation does not necessarily imply causation. The real issue is the relative strength of a design to draw causal inferences from correlational data. Although experiments with randomized assignment remain the gold standard for intervention studies (Rubin, 2008), genetic control studies of the types described by McGue et al. and by Davey Smith provide opportunities to triangulate on the causes of a correlation before jumping to expensive randomized controlled trials (Kendler & Campbell, 2009). The need for such genetic control studies is especially relevant where ethics or practicality prohibits interventions that can definitively demonstrate causality.
After discussing the papers by McGue and by Davey Smith, we consider the integration between genetics and intervention more broadly.
1.0 Twins Discordant for Exposure: Co-twin Control Studies
Members of identical twin pairs are perfect genetic controls because they do not differ genetically, that is, they have the same inherited differences in DNA sequence. Co-twin control studies control for genetics by investigating the relationship between exposure and outcomes within pairs of identical twins who differ in exposure. An important distinction is between the original use of the co-twin control method as part of an intervention experiment in contrast to most current studies that do not impose an intervention. Both intervention and non-intervention co-twin control studies have strengths and weaknesses; McGue et al. focus on non-intervention co-twin control studies.
1.1 Intervention Co-twin Control Studies
Eighty years ago, the first genetically sensitive behavioral intervention studies were conducted using what was called the method of co-twin control (Gesell & Thompson, 1929; Hilgard, 1933; Strayer, 1930). One member of an identical twin pair was trained for accelerated motor or language development, both highly heritable traits, in order to investigate how much an intervention can create differences within pairs of genetically identical individuals. The method investigates the effect of an intervention while holding genetics (i.e., DNA sequence variation) constant throughout the genome. In contrast, instead of controlling for genetic influence, the traditional between-subject experimental design randomly assigns genetically heterogeneous individuals to experimental and control groups.
Co-twin control is a powerful design for behavioral intervention research, especially for interventions that are too intensive and expensive for traditional between-subject randomized control trials. The relative efficiency of the two designs depends on the ratio of variance between individuals and variance within identical twin pairs (Christian & Kang, 1972). Because identical twins are highly similar for most traits, their within-pair variance is low and thus the relative efficiency of the co-twin control intervention design is high. In an intervention study of the effects of vitamin C on biochemical blood tests, the traditional experimental design required about five times as many subjects as the co-twin control intervention design in order to achieve the same power to detect an effect of the intervention (Carr, Martin, & Whitfield, 1981).
1.2 Non-intervention Co-twin Control Studies
Despite the power of the co-twin control intervention design, the design has rarely been used in the last 50 years. A good example from 30 years ago is an intervention study on the effect of vitamin C on cold symptoms (Miller et al., 1977). A literature search for co-twin control studies yields dozens of studies but these are not intervention studies – they use pre-existing differences in exposure within pairs of identical twins to investigate the extent to which differences in exposure relate to differences in outcome. This non-intervention type of co-twin control study is the focus of the paper by McGue et al. which reviews these studies and presents new data using this method to draw causal inferences from the correlation between moderate drinking and late-life cognitive function. Because these are not intervention studies, when they show links between exposures and outcomes within pairs of identical twins, they cannot definitively prove that the exposure itself caused the outcome. The reason is that the within-pair differences in exposure might be due to any pre-existing differences within the pairs such as personality, health or non-shared experiences. In addition, failure to find a significant link between exposure and outcome within pairs of identical twins needs to be interpreted carefully in relation to power.
However, if a phenotypic link between exposure and outcome can also be found within pairs of identical twins, we can safely conclude that the link is at least in part mediated environmentally even if we cannot conclude that the exposure itself caused the outcome. This is an important finding because most exposures show genetic influence and links between exposures and outcomes often show genetic mediation (Plomin, 1994). Given the relatively low cost of a non-intervention co-twin control study as compared to an intervention study, one might suggest that the former is a reasonable prerequisite for the latter. However, as McGue et al. show (their Table 1), studies of identical twins discordant for exposure generally confirm individual-level analyses showing links between exposures and outcomes, thus warranting the inference that the links between exposures and outcomes are at least in part mediated environmentally. We suggest that the reason for this finding is that phenotypic links between exposure and outcome, although often significantly mediated genetically, have never been shown to be entirely mediated genetically.
In summary, non-intervention co-twin control studies using identical twins can show that the link between exposure and outcome is not entirely mediated genetically. However, we suggest that this is almost a foregone conclusion because it depends only on the power of the study to detect non-shared environmental mediation of the link between exposure and outcome, which limits the value of this information for behavioral interventions. As McGue et al. demonstrate, more information can be gleaned by including exposure-discordant fraternal twins in addition to identical twins. We suggest that more useful information for planning behavioral interventions might come from expanding the approach to a full twin multivariate genetic analysis of the continuous range of individual differences in exposures and outcomes in the population rather than studying exposure-discordance as a categorical variable. This is usually easy to do because the exposure-discordant twins are nearly always selected from a reference twin sample that includes the full range of exposure. Rather than just studying twin differences, the full multivariate twin design uses variance between as well as within twin pairs to decompose the covariance between an environmental measure and an outcome measure into genetic and shared and non-shared environmental sources of covariance. This approach could provide more useful information for planning behavioral interventions because it can estimate the magnitude of genetic and shared and non-shared environmental links between exposures and outcomes in the population to which the intervention will be applied. Although quantitative genetic analyses of what is are not necessarily related to what could be, it could be useful in planning an intervention study to know what causes links at the individual differences level between relevant exposures and outcomes. For example, finding that existing exposure-outcome links are substantially mediated genetically might motivate the use of genetic controls and the exploration of genetic differences in response to the intervention.
We recommend the original design of the co-twin control method as an intervention design because, by completely controlling for genome-wide genetic influence, the co-twin control method is many times more efficient than a standard case-control randomized controlled trial. In addition, intervening within pairs of genetically identical individuals offers unique opportunities to identify genetic-free biomarkers of environmental change such as genome-wide gene expression (transcriptome or methylome) profiles of change that could provide more sensitive targets for behavioral interventions.
2.0 Candidate Genes and Mendelian Randomization
If the genes responsible for heritability could be identified, there would be no need for quantitative genetic approaches to genetic control in behavioral intervention studies because genetic effects could be assessed directly from each individual’s DNA rather than implied indirectly by genetic relatedness in special groups such as twins and adoptees. DNA sequence variation (polymorphism) is the ultimate genetic tool for behavioral interventions because it has unique causal status. Reverse causation is not possible because nothing changes the inherited DNA sequence – not other genes, gene expression, or the environment. For this reason, a correlation between the correct DNA polymorphism and an outcome implies causality, which is not true for any other category of biomarker such as gene expression and brain structure and function. The irony is that this sole causal factor (inherited DNA sequence variation) that can be inferred directly from simple correlations is not amenable to intervention. For complex mental disorders, genetic engineering is not a realistic option; behavioral and environmental engineering is necessary to moderate the causal association between genes and mental disorders.
For the thousands of severe but rare monogenic disorders, genetics is central to any intervention. For example, interventions that target Huntington’s disease would make no sense unless they targeted individuals with the allele for the disease because the Huntington’s allele is necessary for the disease to occur. Although most genetic associations appear to have very small effects, a few have large effects. For example, a polymorphism in the gene that codes for apolipoprotein E increases risk six fold for late-onset Alzheimer’s disease. Most intervention research on late-onset Alzheimer’s genotypes its participants for the apolipoprotein E gene. These studies include behavioral interventions such as walking (Eggermont, Swaab, Hol, & Scherder, 2009) and drug interventions such as antioxidant therapy (Ancelin, Christen, & Ritchie, 2007) and atorvastatin treatment (Sparks et al., 2006). In these studies, the genotype information has primarily been used to investigate GE interaction, that is, whether the intervention works better or worse for individuals at genetic risk. Other behavioral intervention research is likely to follow a similar course, as seen in the paper by Niklas in this special section, in which the effect of exercise on physical functioning in older adults is shown to depend on a polymorphism in the gene that codes for angiotensin-converting enzyme.
Davey Smith uses candidate gene information to draw causal inferences in order to lessen the need for intervention research. A novel feature of this approach is that it depends on a candidate gene strongly associated, not with a disorder, but with an exposure. If a candidate gene is strongly associated with exposure, then individuals with genotypes for high exposure are analogous to an intervention group and individuals with genotypes for low exposure are analogous to a control group. The point of the method’s name, Mendelian randomization, is that Mendel’s first law (segregation) posits that parents’ alleles at a locus are randomly allocated to their offspring. At first glance, it seems that random allocation of alleles to genotypes within families is a long way from random allocation of individuals to experimental and control conditions, but the following example might help to explain the logic of the approach.
As mentioned in Davey-Smith’s paper, in Asian individuals, a polymorphism in the gene that codes for aldehyde dehydrogenase 2 (ALDH2) is strongly associated with exposure to alcohol. 30 percent of Asian individuals have an ALDH2 genotype that yields a deficient form of ALDH2 which leads to unpleasant side effects of drinking alcohol because alcohol is not properly metabolized. These ALDH2-deficient individuals have lower exposure to alcohol in that their rates of drinking are lower. Irons et al. (2007) used the Mendelian randomization approach to test the gateway hypothesis which posits that alcohol leads to other drug use and abuse. The essence of the approach is that the two genotypic groups – ALDH2-normal and ALDH2-deficient – should be similar except for their ALDH2-driven alcohol exposure. The gateway hypothesis would predict that the ALDH2-deficient genotypic group which was much less exposed to alcohol would be less likely to use other drugs. The results strongly disconfirmed this gateway hypothesis because the ALDH2-deficient genotypic group was just as likely to use other drugs.
These results appear to warrant strong causal inference even though the assumption of the equivalence of the two genotypic groups can be questioned given the highly pleiotropic effects of most genes. Note that a randomized controlled trial, for example an intervention using disulfiram to produce similar decreases in alcohol exposure, would have its own problems such as selective participation and compliance. The Mendelian randomization method is especially relevant for assessing the effects of long-term exposures such as alcohol intake, diet and lifestyle because randomized controlled trials generally examine only short-term effects.
As acknowledged in the many papers on Mendelian randomization by Davey Smith, one practical limitation for the use of this method as a substitute for randomized controlled trials is that it depends on the availability of a candidate gene strongly associated with the intervention, as in the example of the ALDH2 association with exposure to alcohol in Asians. In his paper, Davey Smith mentions other candidate gene proxies for exposures such as blood cholesterol levels; several candidate genes relevant to nutritional interventions are also available (Qi, 2009). The problem for behavioral interventions is that it seems unlikely that any candidate genes will be strongly associated with relevant exposures, for example, exposures such as social engagement that lower the probability of developing dementia.
3.0 Integrating Genetics and Intervention
Both the discordant-twin approach and the Mendelian randomization approach provide some purchase on causal inference from correlational data using genetic controls. However, in addition to using genetic controls to infer causation from correlational data without intervention, much can be gained from integrating genetics research and intervention research. We suggest that intervention has more to offer genetic research than randomized controlled trials and that genetics has more to offer intervention research than GE interaction.
In many ways, genetic research and intervention research seem to have little in common. Genetic research addresses the genetic and environmental origins of individual differences as they exist in a particular population at a particular time (what is), whereas intervention research focuses on creating an average difference between experimental and control groups in order to understand what could be and to prove causality of the intervention. A sign of the gulf between the two levels of analysis is that individual differences are called the ‘error term’ in statistical analysis of experiments. More fundamentally, knowing what is has no necessary implications for what could be. A highly heritable disorder can be changed dramatically by a novel environmental intervention, as in the extreme example of severe obesity and gastric bands (Aditya & Wilding, 2009).
As a result of the focus on mean differences between experimental and control groups, the impact of genetics on intervention research has largely been limited to GE interaction, asking the extent to which the effect of an intervention depends on genotype, as indicated in the previous section. Although GE interaction opens the door on individual differences in response to intervention, genetic research can be integrated to a much greater extent if we focus on individual differences (heterogeneity) in response to an intervention. Individual differences in response to an intervention can be investigated within a randomized controlled trial, but the integration with genetics will be more rapid in intervention studies that do not randomly assign subjects to experimental and control groups and instead focus on within-subject comparisons between pretest and posttest. Without randomized assignment of subjects to experimental and control groups, such research cannot definitively demonstrate that the intervention is causal. However, in many cases it is sufficient to know that an intervention is followed by certain changes in behavior even if the mechanism of change is not clear.
For example, consider a school-based intervention called FRIENDS, which is a program based on structured cognitive behavior therapy designed to provide practical skills to help children identify and overcome anxious thoughts (Stallard, Simpson, Anderson, & Goddard, 2008). FRIENDS was administered to an unselected sample of school children over 10 one-hour sessions without a control group. A mean reduction in total anxiety was found at 3 months post-intervention and was maintained 12 months later. What is most novel in this ‘universal’ intervention is the ability to investigate individual differences in response to the intervention. One feature of an individual differences approach is that it goes beyond statistical significance to highlight effect size. In the FRIENDS study, the effect size was ‘medium’ (Cohen, 1988) in the sense that the mean reduction in total anxiety was about half a standard deviation in the sample which implies that the mean effect accounted for about 6% of the variance. As discussed below, an important goal for such individual differences intervention research is to identify individuals who benefit most from the intervention in order to target interventions on that group and to understand processes of change. Although the effect of the intervention accounts for about 6% of the variance on average in the sample, the effects are normally distributed around the mean. In other words, some individuals will benefit much more than others. A key goal of individual differences intervention research is to identify individuals who benefit most from the intervention in order to target interventions on that group and to understand processes of change. Individuals who benefit most from the intervention might not be individuals at greatest risk for the targeted outcome; this search needs to go well beyond the targeted behavior itself, as described below. Equally important is the identification of children who might suffer adverse effects; for example, the emotional health of some children might worsen by focusing on anxious thoughts.
Universal intervention in community samples leads to thinking about improving lives for the entire population and preventing problems, not just intervening to treat problems after they occur. Investigating the neglected positive end of the normal distribution might lead to interventions that promote healthy outcomes. Moreover, interventions that are aimed at making people healthier might be more effective at a public health level than those that aim to make people avoid harm. Finally, although the mean effect of an intervention is likely to be smaller for an unselected sample than for a sample selected for risk, from a public health perspective a small mean difference in the population could have a far greater overall effect on society than a large mean difference for a small group. Indeed, from an individual differences perspective it would not matter if an intervention had no overall mean effect.
If a universal intervention were embedded in a quantitative genetic design such as a twin design, it would be possible to investigate the genetic and environmental etiology of individual differences in response to the intervention. One area where much research of this type has been conducted is pharmacogenetics, the genetics of individual differences in response to drugs. For the past 30 years, animal studies (Crabbe, Belknap, & Buck, 1994), and to a much less extent human twin studies (Vesell, 1989), have indicated genetic influence on individual differences in response to drugs. A surge of molecular genetic research in recent years has identified genes that contribute to these individual differences (van der Straaten & van Schaik, 2010). Although the potential for personalized drug treatments is exciting (Wagner, 2009), an important cautionary note is that molecular genetic research in relation to psychotropic drugs has not yet been translated successfully into personalized drug treatment in psychiatry (Kirchheiner, Seeringer, & Viviani, 2010).
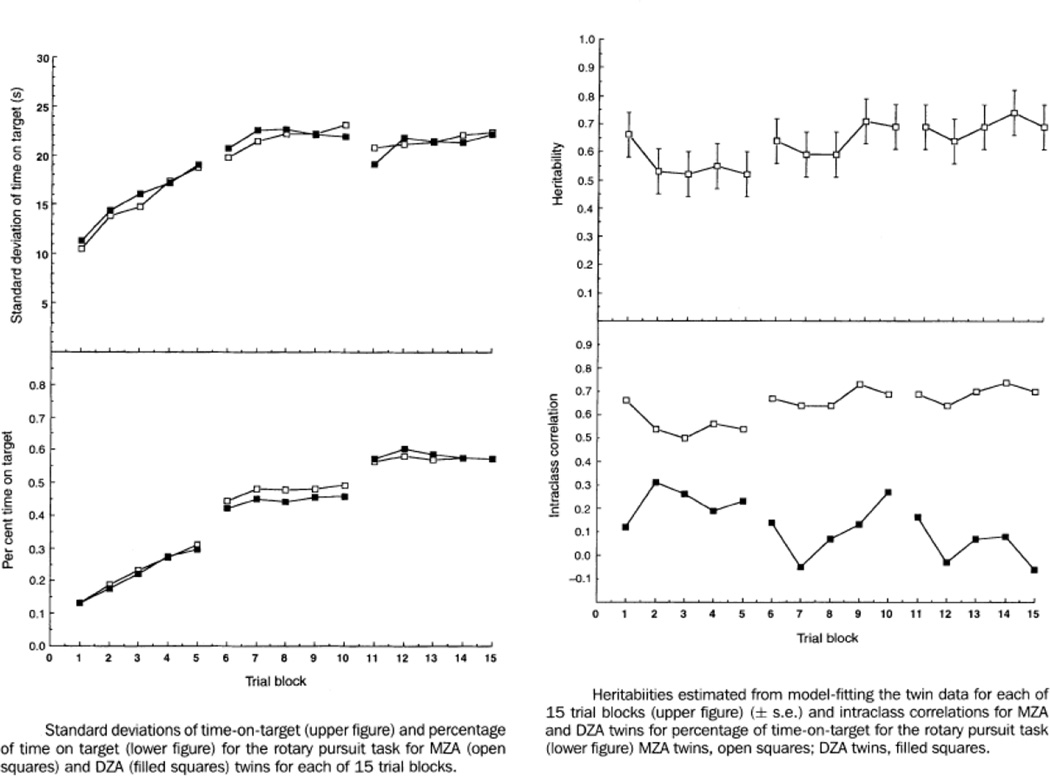
There are very few quantitative genetic studies that have incorporated behavioral interventions. A good example is a twin study that investigated feedback on performance in a motor learning task given over 15 blocks of trials across three days (Fox, Hershberger, & Bouchard, Jr., 1996). The subjects were 64 pairs of identical twins and 32 pairs of fraternal twins – remarkably, all the twins had been reared apart. As shown in Figure 1 (lower left), on average both MZ and DZ twins improved and there was a ‘reminiscence’ improvement following the rest between each day’s training. From an individual differences perspective, it is interesting that variance greatly increased during training, indicating that some subjects improved more than others (Figure 1, upper left). The twin correlations (lower right) and heritability estimates (upper right) indicate that genetic influence on individual differences in response to this intervention is substantial not only at the beginning of the training, but also during training and at the end of training, with a suggestion of an increase in heritability from about 55% to about 70%. The study also found that individual differences in change in performance as assessed by each individual’s slope within each block of five trials also showed high heritability, suggesting genetic influence on individual differences in response to the intervention. The authors end their paper with an important distinction between means and individual differences: “This conclusion [about the importance of genetics] does not diminish the importance of practice with feedback for the acquisition of skill. Even the least gifted of our twins attained levels of skill after practice that were superior to those achieved in initial trials by the most gifted” (p. 357).
Figure 1.
A behavioral intervention in a twin study of individual differences in performance on a motor skill task with feedback given over 15 blocks of trials administered across three days. Open squares indicate MZ twins, closed squares DZ twins. (See text for explanation.) (Adapted from Fox, Hershberger & Bouchard, 1996.) [Note to editor: Figure will be re-drawn.]
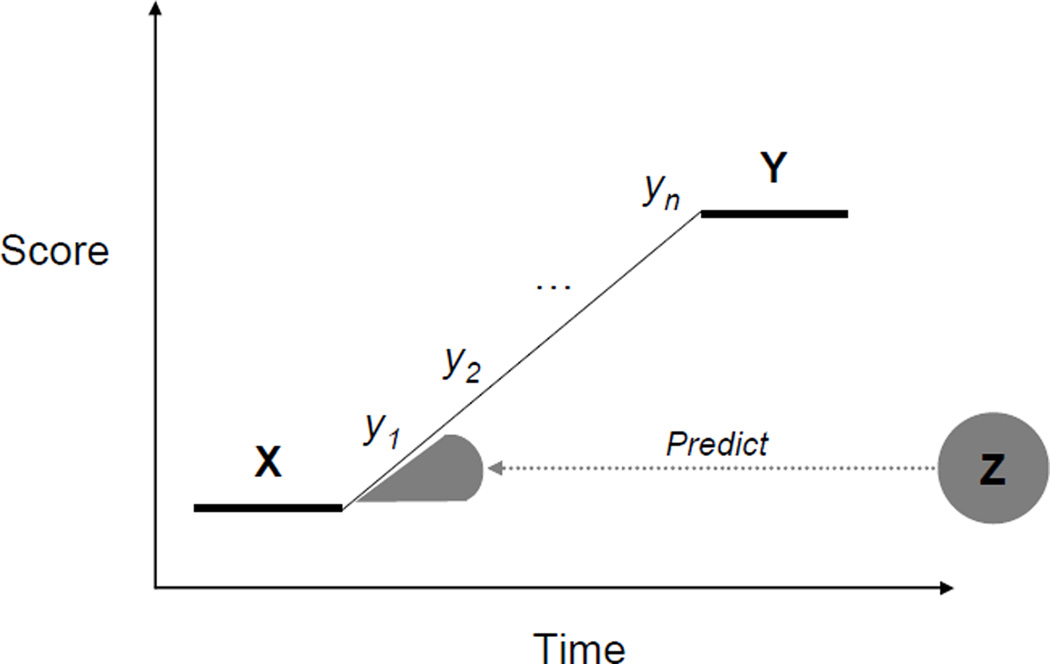
This twin study provides examples of individual differences questions that can be addressed with a ‘universal’ intervention. However, much more can be mined from an individual differences approach. Three examples are illustrated in Figure 2 which are relevant to any intervention study of individual differences but which have special significance for interventions embedded in quantitative genetic designs such as a twin study or for interventions that obtain DNA. First, we can investigate a new set of phenotypes that are the essence of individual differences in response to an intervention: post-intervention phenotypes (Y) independent of pre-intervention phenotypes (X), which we will call Y·X. Measures of Y during or after the intervention could be highly correlated with the pre-intervention measure, whereas Y·X focuses on change. In a twin study, genetic results for Y·X could be completely different from results for X because Y·X is uncorrelated with X. In the twin study results shown in Figure 1, performance during training could be highly correlated with performance at the beginning of training; to this extent, the heritabilities during training could involve the same genetic influences because Y includes X to some unknown extent.
Figure 2.
Individual differences in response to intervention. (See text for explanation.)
In an intervention study with DNA, genetic associations with Y·X could facilitate identification of individuals who would most benefit or least from the intervention. Importantly, genes associated with Y·X will be different from genes associated with X because Y·X is uncorrelated with X. For mental disorders as well as the exposures relevant to them, heritability appears to be caused by many genes of small effect (Plomin, Haworth, & Davis, 2009). Although the effect of each DNA marker is likely to be very small, their effects can be aggregated in polygenic risk scores, which are already beginning to be used to predict population-wide genetic risk for common disorders such as breast cancer, atherosclerosis, coronary heart disease and Type-2 diabetes (Plomin et al., 2009) and for quantitative traits such as height (Weedon et al., 2008) and weight (Li et al., 2010).
Second, multivariate analysis can be used to describe and explain the processes by which an intervention leads to behavioral change at the level of individual differences. In a twin study, multivariate genetic analysis can address the extent to which the same genetic and environmental factors affect X and Y. If Y is assessed throughout the intervention as in Figure 2, a ‘longitudinal’ version of multivariate genetic analysis can address the etiology of change during the intervention. In contrast, in the twin study described in Figure 1, although heritability remained high throughout training, different genes might have affected performance at the beginning and end of training. Similarly, in a DNA study, different genes could be associated with different phases of the change process.
The third example is key to an individual differences approach to intervention: identifying individuals who will benefit most from the intervention and those who might be harmed by it. In addition to identifying genes that could predict Y·X, we can look outside X and Y to other traits and experiences (Z). In a twin study, multivariate genetic analysis can be used to assess the genetic and environmental origins of the links between Y·X and Z. DNA studies can also examine the extent to which links between Y·X and Z are mediated by specific genes.
Many refinements are possible on the rudimentary individual differences intervention framework depicted in Figure 2, such as cross-over designs, or in the case of a twin study, co-twin control cross-over designs. A no-intervention comparison group, although doubling the size of the study, would be useful to make sure that the individual differences results in response to the intervention are indeed in response to the intervention rather than to repeated testing, the passage of time or maturation.
Historically, incorporating interventions in genetically sensitive designs represents a third stage in quantitative genetic research. In the first stage, quantitative genetic designs decomposed phenotypic variance into genetic and environmental components of variance without measuring genes or environments. The second stage, which began in the mid-1980s, included measures of the environment and treated them as dependent variables in quantitative genetic research. This research resulted in evidence for major influence of genetics on measures of the environment and on the relationship between environmental measures and outcome measures, that is, GE correlation. GE correlation implies that what we measure as environment is not purely environmental, which is part of the reason why it is so difficult to infer causation from correlations between exposure and outcomes, as discussed in the first part of this paper. From this historical perspective, what is exciting about incorporating interventions in genetically sensitive designs is that an environmental intervention is imposed and is thus free of GE correlation in the investigation of behavioral change.
Acknowledgement
This work was supported by grants from the U.K. Medical Research Council (G500079) and the U.S. National Institute of Child Health and Human Development (HDF444554, HD46167, HD49861). CMAH is supported by an MRC/ESRC Interdisciplinary Fellowship (G0802681).
References
- Aditya BS, Wilding JPH. Modern management of obesity. Clinical Medicine. 2009;9:617–621. doi: 10.7861/clinmedicine.9-6-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin ML, Christen Y, Ritchie K. Is antioxidant therapy a viable alternative for mild cognitive impairment? Examination of the evidence. Dementia and Geriatric Cognitive Disorders. 2007;24:1–19. doi: 10.1159/000102567. [DOI] [PubMed] [Google Scholar]
- Carr AB, Martin NG, Whitfield JB. Usefulness of the co-twin control design in investigations as exemplified in a study of effects of ascorbic-acid on laboratory test results. Clinical Chemistry. 1981;27:1469–1470. [PubMed] [Google Scholar]
- Christian JC, Kang KW. Efficiency of human monozygotic twins in studies of blood lipids. Metabolism: clinical and experimental. 1972;21:691–699. doi: 10.1016/0026-0495(72)90118-7. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Crabbe JC, Belknap JK, Buck KJ. Genetic animal-models of alcohol and drug abuse. Science. 1994;264:1715–1723. doi: 10.1126/science.8209252. [DOI] [PubMed] [Google Scholar]
- Eggermont LHP, Swaab DF, Hol EM, Scherder EJA. Walking the line: a randomised trial on the effects of a short term walking programme on cognition in dementia. Journal of Neurology Neurosurgery ad Psychiatry. 2009;80:802–804. doi: 10.1136/jnnp.2008.158444. [DOI] [PubMed] [Google Scholar]
- Fox PW, Hershberger SL, Bouchard TJ., Jr Genetic and environmental contributions to the acquisition of a motor skill. Nature. 1996;384:356–357. doi: 10.1038/384356a0. [DOI] [PubMed] [Google Scholar]
- Gesell A, Thompson H. Learning and growth in identical infant twins: An experimental study by the method of co-twin control. Genetic Psychology Monographs. 1929;6:1–124. [Google Scholar]
- Hilgard JR. The effect of early and delayed practice on memory and motor performances studied by the method of co-twin control. Genetic Psychology Monographs. 1933;14:493–567. [Google Scholar]
- Irons DE, McGue M, Iacono WG, Oetting WS. Mendelian randomization: A novel test of the gateway hypothesis and models of gene-environment interplay. Development and Psychopathology. 2007;19:1181–1195. doi: 10.1017/S0954579407000612. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Campbell J. Interventionist causal models in psychiatry: repositioning the mind-body problem. Psychological Medicine. 2009;39:881–887. doi: 10.1017/S0033291708004467. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Seeringer A, Viviani R. Pharmacogenetics in Psychiatry - A Useful Clinical Tool or Wishful Thinking for the Future? Current Pharmaceutical Design. 2010;16:136–144. doi: 10.2174/138161210790112728. [DOI] [PubMed] [Google Scholar]
- Li S, Zhao JH, Luan J, Luben RN, Rodwell SA, Khaw KT, et al. Cumulative effects and predictive value of common obesity-susceptibility variants identified by genome-wide association studies. American Journal of Clinical Nutrition. 2010;91:184–190. doi: 10.3945/ajcn.2009.28403. [DOI] [PubMed] [Google Scholar]
- Miller JZ, Nance WE, Norton JA, Wolen RL, Griffith RS, Rose RJ. Therapeutic effect of vitamin C: Co-twin control study. Journal of the American Medical Association. 1977;237:248–251. [PubMed] [Google Scholar]
- Plomin R. Genetics and experience: The interplay between nature and nurture. Thousand Oaks, California: Sage Publications Inc; 1994. [Google Scholar]
- Plomin R, Haworth CMA, Davis OSP. Common disorders are quantitative traits. Nature Reviews Genetics. 2009;10:872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- Qi L. Mendelian randomization in nutritional epidemiology. Nutrition Reviews. 2009;67:439–450. doi: 10.1111/j.1753-4887.2009.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. For objective causal inference, design trumps analysis. Annals of Applied Statistics. 2008;2:808–840. [Google Scholar]
- Sparks DL, Connor DJ, Sabbagh MN, Petersen RB, Lopez J, Browne P. Circulating cholesterol levels, apolipoprotein E genotype and dementia severity influence the benefit of atorvastatin treatment in Alzheimer's disease: results of the Alzheimer's Disease Cholesterol-Lowering Treatment (ADCLT) trial. Acta Neurologica Scandinavica (Supplement) 2006;114:3–7. doi: 10.1111/j.1600-0404.2006.00690.x. [DOI] [PubMed] [Google Scholar]
- Stallard P, Simpson N, Anderson S, Goddard M. The FRIENDS emotional health prevention programme: 12 month follow-up of a universal UK school based trial. European Child and Adolescent Psychiatry. 2008;17:283–289. doi: 10.1007/s00787-007-0665-5. [DOI] [PubMed] [Google Scholar]
- Strayer LC. Language and growth: The relative efficacy of early and deferred vocabulary training, studied by the method of co-twin control. Genetic Psychology Monographs. 1930;8:209–319. [Google Scholar]
- van der Straaten T, van Schaik RHN. Genetic Techniques for Pharmacogenetic Analyses. Current Pharmaceutical Design. 2010;16:231–237. doi: 10.2174/138161210790112755. [DOI] [PubMed] [Google Scholar]
- Vesell ES. Pharmacogenetic perspectives gained from twin and family studies. Pharmacology & therapeutics. 1989;41:535–552. doi: 10.1016/0163-7258(89)90130-7. [DOI] [PubMed] [Google Scholar]
- Wagner MJ. Pharmacogenetics and personal genomes. Personalized Medicine. 2009;6:643–652. doi: 10.2217/pme.09.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM, Mangino M, et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nature genetics. 2008;40:575–583. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]