SUMMARY
Autophagy is a process in which cellular contents are captured in specialized, membrane-bounded vesicles and delivered to lysosomes for final degradation. Most studies support an inherent connection between autophagy and survival, but increasing evidence also suggests an association between autophagy and cell death. The therapeutic potential of targeting the autophagy pathway in cancer seems clear, but specific strategies for achieving successful eradication of cancer cells are less obvious. Recent developments in the fields of autophagy and programmed cell death, nevertheless, have shed light on therapeutic strategies with significant potential. In this review, we provide an overview of the autophagy process, pathways that modulate autophagy, and promising autophagy-based therapeutic strategies for cancer.
INTRODUCTION
The term autophagy was first used by Christian de Duve on the basis of electron microscopic observations (1). Autophagy, literally meaning "self-eating", is an intracellular process in which a double membrane, or membrane cistern, isolates cellular constituents such as proteins and organelles, which are then degraded by lysosomes. Three reported sub-types of autophagy are macroautophagy, microautophagy (2), and chaperone-mediated autophagy (CMA) (3). Here, we focus specifically on macroautophagy, hereafter referred to as autophagy.
Under basal, nutrient-replete conditions, the main function of autophagy is to maintain cellular homeostasis by removing damaged proteins and organelles and by providing the cell with energy and new building blocks. During conditions of high metabolic demand or stress, including nutrient starvation, micro-environmental changes, and DNA damage, the function of autophagy is ostensibly unchanged, but autophagic flux is dramatically increased in an attempt to maintain cell viability. Over 34 autophagy-related genes (ATGs) have been identified so far (4), and proteomic analysis recently uncovered a complex network of proteins that interact with and modulate autophagy (5).
AUTOPHAGY IS A MULTISTEP PROCESS
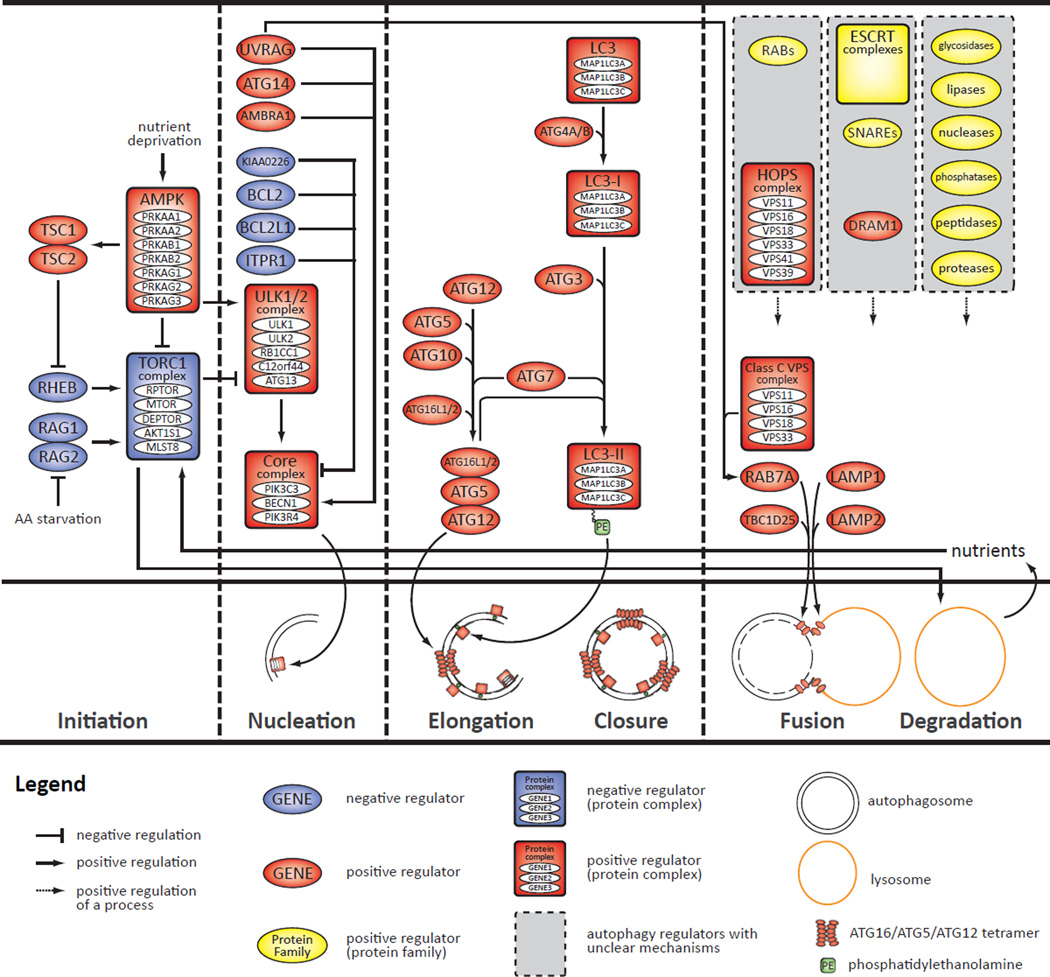
The autophagic process consists of four main steps: 1) initiation, 2) nucleation (mediated by Vps34/PIK3C3 and Beclin1/BECN1), 3) elongation and closure (mediated by two ubiquitin-like systems), and 4) fusion and degradation (Figure 1) (6).
Figure 1. Molecular features of the autophagy process.
The top panel shows specific entities (genes, proteins, complexes, and small molecules) associated with that process and, to the extent possible, their specific roles. The middle panel shows a schematic timeline of the macroautophagy process (four steps separated by vertical dashed lines). The bottom panel defines entities that appear in the top two panels.
Under basal, nutrient-replete conditions, mTOR complex 1 (TORC1) is active and negatively regulates initiation of autophagy, whereas cellular stresses such as hypoxia and nutrient starvation suppress mTOR function, thereby triggering autophagy initiation (7, 8). mTOR function can be decreased by three mechanisms: 1) TSC2 phosphorylation by AMPK deactivates Rheb GTPase and, consequently, inhibits TORC1 (9); 2) inhibition of Raptor/RPTOR, a component of TORC1, results in TORC1 recruitment to 14-3-3 proteins (10), and 3) amino acid starvation can decrease mTOR activity through Rag GTPases (11). Although TORC1 is a key component of autophagy induction, there are also a number of TORC1-independent mechanisms that can induce autophagy (12, 13).
The nucleation step of autophagy serves to prevent unbalanced activation of autophagy via two major pathways downstream of mTOR—the ULK1/2 complex and the Vps34/PIK3C3 “core complex” (Figure 1). The mammalian ULK1/2 kinase complex consists of ULK1 or ULK2, ATG13, ATG101/C12orf44, and FIP200/RB1CC1 (6). Under nutrient replete conditions, TORC1 negatively regulates ULK1 and ATG13 by phosphorylation, which prevents ULK1 interaction with AMPK (14, 15); hence, the nutrients generated by autophagy turn the pathway off through negative feedback. A series of recent publications propose an additional mechanism for ULK1 regulation in which AMPK activates autophagy by directly phosphorylating ULK1 (16). The other major regulator of nucleation is the core complex composed of PI3K class III (Vps34/PIK3C3), p150/Vps15/PIK3R4, and BECN1. That complex is required for generation of phosphoinositide signals on autophagosomal source membranes (ER, Golgi, plasma membrane, mitochondria, or de novo) (17) and for recruitment of other autophagy-related proteins, for which BECN1 functions as a scaffold. Binding of BECN1 to AMBRA1, ATG14, UVRAG, and a number of additional proteins (18), activates autophagosome nucleation, whereas binding to RUBICON/KIAA0226, BCL2, Bcl-Xl/BCL2L1, and IP3R/ITPR1 inhibits autophagosome nucleation (19).
Autophagosome elongation is mediated by the ubiquitin-like ATG5/ATG12 and LC3/MAP1LC3 conjugation systems (20, 21). During the initial phase, ATG12 covalently binds ATG5, which in turn promotes autophagosome elongation via recruitment of LC3 (Figure 1). LC3 is cleaved by ATG4 and conjugated to phosphatidylethanolamine (PE) by an ATG3/ATG7-dependent activation and transfer system. LC3 is then conjugated to the membrane and plays an important role in cargo recruitment and in determination of autophagosome size. Cargo selection depends on cargo adaptor proteins, such as NBR1, p62/SQSTM1, and NIX/BNIP3L. NBR1 and p62 contain ubiquitin binding domains and therefore can specifically recruit ubiquitinated cargo (22). p62 is degraded during the process of autophagy and accumulates when autophagy is impaired. NIX/BNIP3L, the other cargo adaptor protein, recruits mitochondria to the autophagosome (23). Autophagosome biogenesis ends with the closure of autophagosomal membranes, resulting in double-membrane vesicles containing selected, intracellular cargoes destined for degradation.
Autophagosomes then mature and fuse with endosome/lysosomes to form autolysosomes (Figure 1). OATL1/TBC1D25 regulates autophagosomal maturation through direct interaction with LC3 and is also involved in fusion of the outer membrane of autophagosomes with lysosomes (24). Fusion is additionally regulated by RAB7 (25) and LAMP1/2 (26). Ultimately, lysosomal hydrolases degrade the luminal and inner membrane constituents, including most autophagy proteins, and the resulting products are then released into the cytosol by lysosomal efflux permeases (e.g., Spinster/SPNS1 (27)) for reuse as building blocks or for generation of energy. Finally, LC3/MAP1LC3 lipidation is reversed by ATG4, and lysosome homeostasis is restored by autophagic lysosome reformation (ALR) through activation of mTOR (28). Hence, the nutrients generated by autophagy turn the pathway off through negative feedback.
SURVIVAL VS. CELL DEATH BY AUTOPHAGY
It is well accepted that autophagy can promote cell survival. By providing energy and building blocks and serving as a disposal system, autophagy helps cells to cope with a wide variety of intracellular and extracellular stresses. However, a growing number of studies suggest that autophagy can also turn into a cell death mechanism. As suggested by others (29), though, caution must be exercised when using the term “autophagic cell death,” since, to date, death by autophagy has not been unequivocally demonstrated. As the pro-survival role of autophagy has been widely accepted, we will briefly discuss the contentious topic of autophagic cell death.
Autophagy associated with cell death
Autophagic cell death is considered to be one of three major cellular mechanisms of programmed cell death, along with apoptosis and necroptosis. Apoptosis is the best characterized, with a variety of hallmarks that have been clearly defined (30). Necroptosis—programmed necrosis—was identified in the last decade (31) and is beginning to become more clearly understood (32). In contrast, autophagic cell death is not clearly defined. In fact, its existence is questionable and highly dependent on exclusion of other cell death mechanisms (33–35). That has proven difficult due to the high degree of interconnection between programmed cell death mechanisms. For example, ATG3, ATG5, ATG12, BCL2 family members, BECN1, CASP3, CASP8, DAPK, E2F1, FLIP/CFLAR, JNK/MAPK8, NFKB, p27/CDKN1B, p53/TP53, p62/SQSTM1, RB1, RIPK1,and Vps34/PIK3C3 have been found to play roles in both autophagy and apoptosis (36–40). In consideration of the overlap between autophagy and apoptosis, autophagic cell death was recently defined as cell death accompanied by 1) absence of apoptosis markers, 2) increased autophagic flux, and 3) increased viability when autophagy is genetically or pharmacologically inhibited (41). As that definition lacks distinction from necroptosis, “absence of necroptosis markers” should be added to the list. To provide evidence that autophagic cell death is distinct from other modes of cell death, it will be necessary to investigate the dependence of the various modes of cell death on several factors: 1) level of autophagy induction, 2) duration of autophagy induction, 3) nature of stimulus, 4) genetic background, and 5) microenvironmental influences. Such investigations may facilitate identification of biomarkers that clearly distinguish activation of each pathway, thereby enabling further refinement of the definition of autophagic cell death.
As there is still much to learn about the relationships between the various programmed cell death mechanisms, we conclude that it is not yet clear whether autophagic cell death exists and, hence, whether stimulating autophagy would have therapeutic utility. It should be clarified that many experiments have been mis-interpreted; use of autophagy inhibitors (either pharmacological or genetic) to demonstrate that autophagy is necessary to achieve cell death does not equate with demonstration of autophagic cell death. Under such conditions, the mode of cell death may technically be necroptosis. Developing a therapeutic strategy based on stimulation of autophagy will therefore require knowledge of the genetic context in which pharmacological stimulators of autophagy activate programmed cell death and which mode of cell death is being activated. Future studies will also need to address whether multiple cell death mechanisms are concurrently activated within a single cell and/or whether the pathways are mutually exclusive, either temporally or spatially.
AUTOPHAGY AS A TUMOR SUPPRESSOR AND TUMOR SUPPORTER
Autophagy has been proposed to mediate both tumor suppression and tumorigenesis, another seemingly contradictory pair of functions (34, 42, 43). We refer the reader to a recent review by Debnath (44) for an extensive description of the multiple functions of autophagy during tumor initiation and progression. With regard to tumor suppression, studies investigating ATG genes and autophagy-associated signaling pathways have identified ATG4C, ATG5, ATG7 (45), BECN1, and BECN1 interactors UVRAG (46) and Bif/SH3GLB1 as tumor suppressors (47–49). In addition, PTEN, DRAM, DAPK, TSC1, TSC2, and LKB1, which are tumor suppressors that positively regulate autophagy, have been found to be lost or inactivated in many cancers (50). Further support for the role of autophagy as a tumor suppressor has been provided by the observation that defective autophagy is associated with genomic damage that can promote tumor formation (45, 51). Finally, oncogenes such as AKT, BCL2, and PIK3CA, which negatively regulate autophagy, are frequently up-regulated and mutated in cancer (50). Possible mechanisms of tumor suppression by autophagy include suppression of inflammation (52, 53); mitigation of metabolic stress and genomic damage (54, 55); degradation of p62 (56), facilitating oncogene-induced senescence (57); inhibition of necrosis (52); and autophagic cell death, although the latter is controversial, as previously stated. Altogether, these reports suggest that autophagy prevents tumor initiation and is a key feature in the homeostasis of normal tissue.
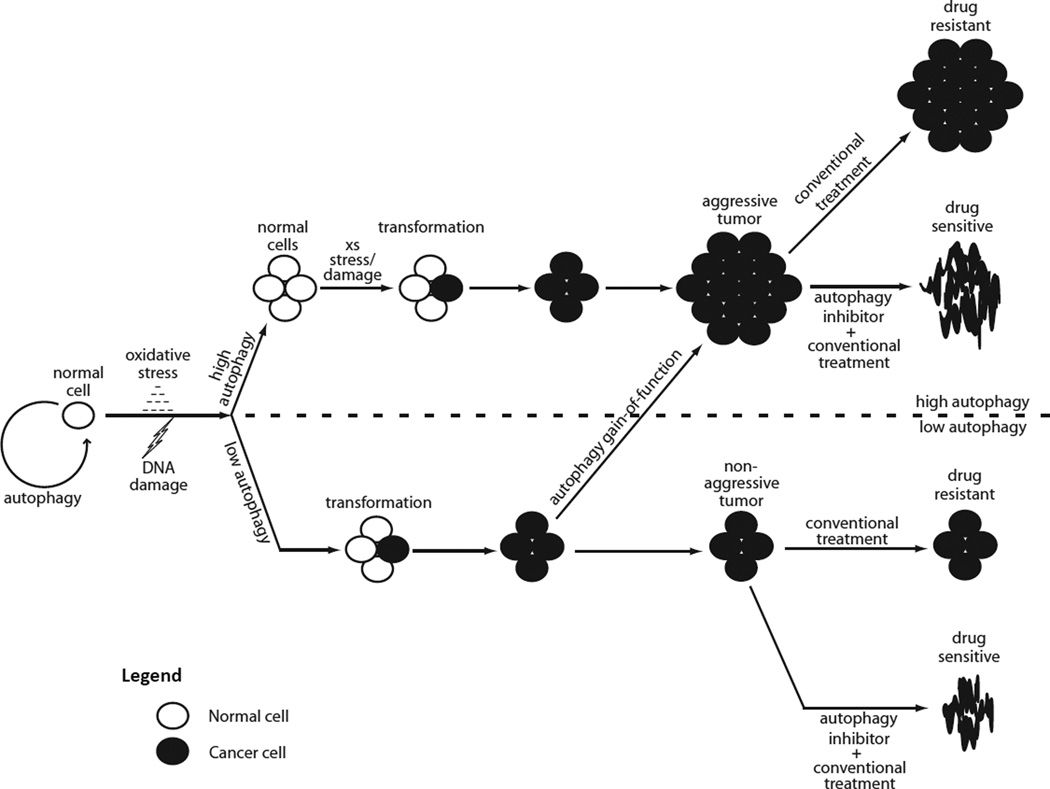
A role for autophagy in tumorigenesis, on the other hand, can be explained by the emerging concept of “autophagy addiction.” Specifically, autophagy may serve as a tumor suppressor in normal cells, but once a tumor is formed, autophagy may be required for progression to an aggressive tumor, as suggested by the observation that aggressive tumors (e.g., those exhibiting oncogenic RAS activation) exhibit a high degree of autophagy activation (58, 59). Since cancer cells are exposed to increased cellular and metabolic stress such as hypoxia (52) and anoikis (60), and since autophagy can prevent cell death by mitigating the effects of such stresses, it seems reasonable to hypothesize that an elevated autophagy rate could be selected during tumorigenesis in order to meet increasing metabolic demand and to tolerate the stress associated with that demand. Hence, the seemingly contradictory functions of autophagy as a tumor suppressor and tumor supporter may not be contradictory after all. Figure 2 illustrates those disparate functions.
Figure 2. Conceptual schematic of autophagy as a tumor suppressor, tumor supporter, and mediator of chemotherapy resistance.
In the absence of stress signals, normal cells require autophagy to maintain homeostasis. Stress signals such as DNA damage or reactive oxygen species induce autophagy, which recycles damaged proteins and organelles and prevents transformation. Autophagy therefore serves as a tumor suppressor in normal cells. If the accumulation of damage exceeds autophagic capacity (flux), transformation to a cancer cell can occur. Thereafter, autophagy supports tumorigenesis by supplying recycled building blocks and energy to tumor cells while also clearing damage. Cells that sustain high autophagy or acquire a gain-offunc tion mutation are likely to progress to an aggressive tumor. A majority of conventional anti-cancer treatments stimulate autophagy, which imparts drug resistance. Therefore, combination therapies that include an inhibitor are expected to enhance anticancer efficacy.
AUTOPHAGY MODULATION AS A STRATEGY FOR ANTICANCER THERAPY
Modulation of autophagy in cancer therapy has come sharply into focus over the past couple of years. Armed with our current knowledge of autophagy-regulating molecules, the critical question is, “Should the anticancer strategy be based on stimulation or inhibition of autophagy?” Unfortunately, due to the molecular complexities involved, there is no one-size-fits-all answer; it will likely depend on context. However, the molecular determinants of “context” are still being defined; although autophagy has been a subject of investigation since the mid-1960s, the complex networks that regulate the process have only begun to be elucidated. A recent report described the combined use of protein expression, immunoprecipitation, and mass spectrometry to identify an “Autophagy Interaction Network” composed of 409 proteins and 751 interactions (5). However, for the purposes of identifying candidate drug targets, for which it is desirable to have a measure of a gene’s contribution to the autophagy process, siRNA screening represents a powerful alternative strategy. In that regard, only three siRNA screens that employed autophagy-specific endpoints in human cell lines have been reported to date (12, 61, 62). Only one of those was a genome-wide screen (12). Further limiting the utility of the existing data, LC3-II expression has been the only endpoint used; downstream markers of autophagic flux (e.g., p62 turnover) have not been employed in siRNA screens. As LC3-II is formed early during autophagy, LC3-based autophagy screens may be limited to elucidation of early steps in the process and incomplete/abortive autophagy that results in autophagosome accumulation. Hence, the existing siRNA screen data provide a starting point for identifying already-characterized autophagy drug targets, but they are probably quite limited in scope and coverage of the phenomenon.
It is not yet clear whether stimulating autophagy will be a useful anticancer strategy. Such a strategy will first depend on the existence of an autophagy/cell death threshold. Furthermore, successful eradication of cancer cells by stimulation of autophagy will require that the autophagy/cell death threshold be surmountable. For aggressive tumors that are addicted to autophagy and have adapted to survive with high autophagy rates, the autophagy/cell death threshold is likely to have a high set point. A chemotherapy strategy for stimulating autophagy might, therefore, be better suited for tumors with a low autophagy/cell death threshold. However, the molecular determinants of the autophagy/cell death set point are not yet clear and are likely to involve a variety of factors. For example, the death-inducing signaling complex (DISC), which mediates necroptosis, must be intact for cell death to occur by necroptosis. That complex includes TRADD, FADD, FLIP/CFLAR, CASP8, RIPK1, and RIPK3 (32, 63).
Stimulation of autophagy has clearer potential in the context of cancer prevention, for which the efficacy of such a strategy would not depend on the existence of an autophagy/cell death threshold. Stimulation of autophagy in normal cells would be expected to repress the initiation of cancer through elimination of damaged proteins and abnormal mitochondria and by preventing accumulation of DNA damage. Despite the seemingly non-controversial potential of such a strategy, more work will be needed to assess that potential, especially since it is conceivable that stimulation of autophagy may be found to support tumorigenesis by enabling pre-malignant cells to acquire a pro-survival mechanism and to become addicted to autophagy (64). Rapamycin, a well-known autophagy stimulator (and MTOR inhibitor), would be a model compound to aid in such studies.
Compared with stimulating autophagy, there is much better understanding of the contexts in which inhibiting autophagy holds promise for eradication of cancer cells. Increased levels of autophagy are almost always observed following anti-cancer treatment and are thought to be associated with drug resistance. Not surprisingly, inhibition of autophagy has been found to augment drug efficacy (65). Toward the goal of achieving clinical efficacy, numerous trials are underway using chloroquine (CQ) or hydrochloroquine (HCQ) as an autophagy inhibitor (66) in combination with a variety of therapeutic agents. Of those trials, the one testing HCQ in combination with the rapamycin analog temsirolimus is worth particular attention. As single agents, TORC1 inhibitors like temsirolimus have shown limited activity in clinical trials (67), presumably due to induction of autophagy and other pro-survival pathways. Therefore, the combination of a TORC1 inhibitor (e.g., temsirolimus) with an autophagy inhibitor like HCQ might be expected to achieve greater cancer cell death (see Figure 2). Such a strategy may enable rapamycin (68), its analogs, and other autophagy-inducing anticancer agents to reach their full therapeutic potential. It should be acknowledged, however, that chronic suppression of autophagy may stimulate tumorigenesis due, for example, to a buildup of reactive oxygen species-induced damage and genomic instability (69). It will therefore be necessary to optimize the time frame during which autophagy inhibitors are used clinically to minimize potential long-term side effects.
CONCLUSION
Autophagy appears to function as both a pro-survival mechanism and a cell death mechanism. Whether stimulating or inhibiting autophagy will improve patient outcomes will depend on context. The balance between the two mechanisms is a delicate one. Significant challenges remain in elucidating the complicated relationships between autophagy and programmed cell death, but current understanding suggests that inhibition of autophagy may have significant potential as an anticancer strategy, perhaps in combination with inhibition of mTOR.
ACKNOWLEDGEMENTS
This work was supported in part by Komen Foundation grants KG 081694 and FAS0703849, Cancer Prevention and Research Institute of Texas, and the Ovarian Cancer Research Fund (GBM); the H.A. and Mary K. Chapman Foundation, The Michael & Susan Dell Foundation (honoring Lorraine Dell), the Ovarian Cancer Research Fund grant PPD/MDACC/01.08 03 (JNW); and U.S. National Cancer Institute (NCI) grant numbers CA143883 and CA083639 (JNW and PLL). Funding to SC as an Odyssey Fellow was supported by the Odyssey Program and the Theodore N. Law Endowment for Scientific Achievement at the University of Texas MD Anderson Cancer Center and NCI Breast SPORE Career Developmental Project award CA116199.
Footnotes
DISCLOSURES
The authors declare no competing interests.
REFERENCES
- 1.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 2.Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: Revisiting a 40-year-old conundrum. Autophagy. 2011;7(7):673–682. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- 3.Kaushik S, Bandyopadhyay U, Sridhar S, et al. Chaperone-mediated autophagy at a glance. J Cell Sci. 2011;124(Pt 4):495–499. doi: 10.1242/jcs.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klionsky DJ, Codogno P, Cuervo AM, et al. A comprehensive glossary of autophagy-related molecules and processes. Autophagy. 2010;6(4) doi: 10.4161/auto.6.4.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466(7302):68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehrpour M, Esclatine A, Beau I, Codogno P. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. Am J Physiol Cell Physiol. 2010;298(4):C776–C785. doi: 10.1152/ajpcell.00507.2009. [DOI] [PubMed] [Google Scholar]
- 7.Caron E, Ghosh S, Matsuoka Y, et al. A comprehensive map of the mTOR signaling network. Mol Syst Biol. 2010;6:453. doi: 10.1038/msb.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17(15):1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipinski MM, Hoffman G, Ng A, et al. A genome-wide siRNA screen reveals multiple mTORC1 independent signaling pathways regulating autophagy under normal nutritional conditions. Dev Cell. 2010;18(6):1041–1052. doi: 10.1016/j.devcel.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou X, Ikenoue T, Chen X, Li L, Inoki K, Guan KL. Rheb controls misfolded protein metabolism by inhibiting aggresome formation and autophagy. Proc Natl Acad Sci U S A. 2009;106(22):8923–8928. doi: 10.1073/pnas.0903621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosokawa N, Hara T, Kaizuka T, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20(7):1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roach PJ. Ampk -> Ulk1 ->Autophagy. Mol Cell Biol. 2011 doi: 10.1128/MCB.05565-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tooze SA. The role of membrane proteins in mammalian autophagy. Semin Cell Dev Biol. 2010;21(7):677–682. doi: 10.1016/j.semcdb.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell death and differentiation. 2011;18(4):571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20(6):355–362. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36(12):2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weidberg H, Shpilka T, Shvets E, Abada A, Shimron F, Elazar Z. LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev Cell. 2011;20(4):444–454. doi: 10.1016/j.devcel.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu M, Ichimura Y. Selective autophagy regulates various cellular functions. Genes Cells. 2010;15(9):923–933. doi: 10.1111/j.1365-2443.2010.01433.x. [DOI] [PubMed] [Google Scholar]
- 23.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh T, Kanno E, Uemura T, Waguri S, Fukuda M. OATL1, a novel autophagosome-resident Rab33B-GAP, regulates autophagosomal maturation. J Cell Biol. 2011;192(5):839–853. doi: 10.1083/jcb.201008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117(Pt 20):4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 26.Eskelinen EL. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med. 2006;27(5–6):495–502. doi: 10.1016/j.mam.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Rong Y, McPhee CK, Deng S. et al. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(19):7826–7831. doi: 10.1073/pnas.1013800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu L, McPhee CK, Zheng L, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465(7300):942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine B, Kroemer G. Autophagy in aging, disease and death: the true identity of a cell death impostor. Cell Death Differ. 2009;16(1):1–2. doi: 10.1038/cdd.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9(3):231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 31.Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 32.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 33.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9(12):1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy JM, Thorburn A. Targeting autophagy during cancer therapy to improve clinical outcomes. Pharmacol Ther. 2011;131(1):130–141. doi: 10.1016/j.pharmthera.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol. 2011 doi: 10.1016/j.tcb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang H, Martin V, Gomez-Manzano C, et al. The RB-E2F1 pathway regulates autophagy. Cancer Res. 2010;70(20):7882–7893. doi: 10.1158/0008-5472.CAN-10-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang J, Shao SH, Xu ZX, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9(2):218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 39.Ciavarra G, Ho AT, Cobrinik D, Zacksenhaus E. Critical role of the Rb family in myoblast survival and fusion. PLoS ONE. 2011;6(3):e17682. doi: 10.1371/journal.pone.0017682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciavarra G, Zacksenhaus E. Direct and indirect effects of the pRb tumor suppressor on autophagy. Autophagy. 2011;7(5) doi: 10.4161/auto.7.5.15056. [DOI] [PubMed] [Google Scholar]
- 41.Shen HM, Codogno P. Autophagic cell death: Loch Ness monster or endangered species? Autophagy. 2011;7(5) doi: 10.4161/auto.7.5.14226. [DOI] [PubMed] [Google Scholar]
- 42.Chen GC, Lee JY, Tang HW, Debnath J, Thomas SM, Settleman J. Genetic interactions between Drosophila melanogaster Atg1 and paxillin reveal a role for paxillin in autophagosome formation. Autophagy. 2008;4(1):37–45. doi: 10.4161/auto.5141. [DOI] [PubMed] [Google Scholar]
- 43.White E, Karp C, Strohecker AM, Guo Y, Mathew R. Role of autophagy in suppression of inflammation and cancer. Curr Opin Cell Biol. 2010;22(2):212–217. doi: 10.1016/j.ceb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debnath J. The multifaceted roles of autophagy in tumors-implications for breast cancer. J Mammary Gland Biol Neoplasia. 2011;16(3):173–187. doi: 10.1007/s10911-011-9223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takamura A, Komatsu M, Hara T, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25(8):795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, Jung JU. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nature cell biology. 2006;8(7):688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 47.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 48.Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112(12):1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100(25):15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009;16(1):87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- 51.Mathew R, White E. Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr Opin Genet Dev. 2011;21(1):113–119. doi: 10.1016/j.gde.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nature reviews Cancer. 2007;7(12):961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21(13):1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathew R, Kongara S, Beaudoin B, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21(11):1367–1381. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathew R, Karp CM, Beaudoin B, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137(6):1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young AR, Narita M, Ferreira M, et al. Autophagy mediates the mitotic senescence transition. Genes & development. 2009;23(7):798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo JY, Chen HY, Mathew R, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25(5):460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lock R, Roy S, Kenific CM, Su JS, Salas E, Ronen SM, Debnath J. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol Biol Cell. 2011;22(2):165–178. doi: 10.1091/mbc.E10-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Molecular biology of the cell. 2008;19(3):797–806. doi: 10.1091/mbc.E07-10-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan EY, Kir S, Tooze SA. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem. 2007;282(35):25464–25474. doi: 10.1074/jbc.M703663200. [DOI] [PubMed] [Google Scholar]
- 62.Szyniarowski P, Corcelle-Termeau E, Farkas T, Hoyer-Hansen M, Nylandsted J, Kallunki T, Jaattela M. A comprehensive siRNA screen for kinases that suppress macroautophagy in optimal growth conditions. Autophagy. 2011;7(8) doi: 10.4161/auto.7.8.15770. [DOI] [PubMed] [Google Scholar]
- 63.Peter ME. Programmed cell death: Apoptosis meets necrosis. Nature. 2011;471(7338):310–312. doi: 10.1038/471310a. [DOI] [PubMed] [Google Scholar]
- 64.Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Tanowitz HB, Sotgia F, Lisanti MP. Stromal-epithelial metabolic coupling in cancer: Integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol. 2011 doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Claerhout S, Verschooten L, Van Kelst S, De Vos R, Proby C, Agostinis P, Garmyn M. Concomitant inhibition of AKT and autophagy is required for efficient cisplatin-induced apoptosis of metastatic skin carcinoma. Int J Cancer. 2010;127(12):2790–2803. doi: 10.1002/ijc.25300. [DOI] [PubMed] [Google Scholar]
- 66.Amaravadi RK, Lippincott-Schwartz J, Yin XM, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17(4):654–666. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121(4):1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi Y, Frankel A, Radvanyi LG, Penn LZ, Miller RG, Mills GB. Rapamycin enhances apoptosis and increases sensitivity to cisplatin in vitro. Cancer research. 1995;55(9):1982–1988. [PubMed] [Google Scholar]
- 69.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nature reviews Molecular cell biology. 2010;11(3):220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]