Abstract
Background
The Collaborative Stage (CS) Data Collection System enables multiple cancer registration programs to document anatomic and molecular pathology features that contribute to the Tumor (T), Node (N), Metastasis (M) (TNM) system of the American Joint Committee on Cancer (AJCC). This chapter highlights changes in CS for colon and rectal carcinomas as TNM moved from the AJCC 6th to the 7th edition.
Methods
Data from 18 Surveillance, Epidemiology, and End Results (SEER) population-based registries were analyzed for the years 2004-2010, which included 191 361colon and 73 341 rectal carcinomas.
Results
Overall, the incidence of colon and rectal cancer declined, with the greatest decrease in stage 0. The AJCC's 7th edition introduction of changes in the subcategorization of T4, N1, and N2 caused shifting within stage groups in 25 577 colon and 10 150 rectal cancers diagnosed in 2010. Several site-specific factors (SSFs) introduced in the 7th edition had interesting findings: 1) approximately 10% of colon and rectal cancers had tumor deposits - about 30-40% occurred without lymph node metastases which resulted in 2.5% of colon and 3.3% of rectal cases becoming N1c (stage III A/B) in AJCC 7th edition ; 2) 10% of colon and 12% of rectal cases had circumferential radial margins <1 mm; 3) about 46% of colorectal cases did not have a CEA testing or documented CEA information; and 4) about 10% of colorectal cases had perineural invasion.
Conclusion
Adoption of AJCC 7th edition by the SEER Program provides an assessment tool for staging and SSFs on clinical outcomes. This evidence can be used for education and improved treatment for colorectal carcinomas.
Keywords: Colon, rectum, collaborative stage, AJCC, prognostic site-specific factors
INTRODUCTION
Colorectal cancer is the second leading cause of cancer death in the United States among both men and women. In 2013, an estimated 142 820 Americans were diagnosed with colorectal cancer, and 50 830 died of this disease.1 Between 1975 and 2010, the rate of new colorectal cancer diagnoses in Surveillance, Epidemiology, and End Results (SEER) registries decreased by 31% while colorectal death rates in the United States decreased by 45%.2 This decline in incidence and mortality has been observed across most racial and ethnic groups, including whites, blacks, Asians and Pacific Islanders, and Hispanics and is attributed to a combination of factors, including healthier diets,3 increased screening,4 and better treatments.5 Adenomatous polyps, which are precancerous growths in the large intestine, are the precursor of most colorectal cancers. Periodic screening with colonoscopy is recommended for people of average risk at 50 years of age and older and at an earlier age for those with high risk, such as a family history of colon cancer. In addition, some professional societies recommend that African Americans begin screening at age 45.6
In recent decades, innovative technologies in medicine have transformed cancer patient care. Bench discoveries are being applied to bedside practice, and cancer patients are being treated with “personalized” medicine and targeted therapies. Information on staging and tumor characteristics that traditionally has been collected by cancer registries is no longer sufficient for the changing landscape of patient care. In response to the need for greater precision in identifying subgroups with various prognoses, the American Joint Commission on Cancer (AJCC), in collaboration with the International Union for Cancer Control (UICC) and based on evidence generated from population-based cohorts of patients, introduced the 7th edition of the AJCC cancer staging manual7, effective with cases diagnosed in 2010. This edition includes additional data variables intended to support more detailed staging, allow for a more accurate prognosis, and help refine treatment options. Several cancer organizations, including the American College of Surgeons (ACoS), National Cancer Institute (NCI), and Centers for Disease Control and Prevention (CDC), adopted the AJCC 7th edition and its recommended data items into the Collaborative Stage (CS) Data Collection System Version 2 (CSv2) and use them as guidelines in data collection for their respective cancer surveillance programs: the National Cancer Data Base, the SEER Program, and the National Program of Cancer Registries. These additional data items on staging, prognosis, treatment, and clinical significance are referred to as site-specific factors (SSFs) in the CS Data Collection System.
Objectives
The objectives of this chapter are to: 1) examine the trends in CS-derived AJCC 6th edition8 stage distributions for colon and rectal cancer in the combined areas of 18 SEER registries from 2004 to 2010; 2) describe the changes in colorectal cancer staging in the AJCC 7th edition and examine how these changes impact stage at diagnosis by comparing stage distributions of 2010 cases between the AJCC 6th and 7th editions; and 3) assess the completeness and quality of SSFs recorded in the CS Data Collection System for 2010 colorectal cancer cases.
DATA DESCRIPTION
CS Schemas for Colon and Rectum and Case Exclusion Criteria
In the CS system, schemas for ‘Colon’ and ‘Rectum’ are defined slightly differently from the traditional SEER site codes. The CS schemas use both the topography and morphology of the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3)9 and follow the AJCC Cancer Staging guidelines regarding the specific histologies eligible for staging within a given cancer site. The ICD-O-3 topographies for the colon are C18.0, and C18.2-C18.9, and for the rectum are C19.9 and C20.9. The eligible morphologies for staging within these topographies include: M8000-M8152, M8154-M8231, M8243-M8245, M8247, M8248, M8250-M8934, M8940-M9136, M9141-M9582, and M9700-M9701.
The schemas for colon and rectum in the AJCC 6th edition were subdivided into the following schemas in AJCC 7th edition (CSv2): Colon, Rectum, Appendix, as well as gastrointestinal stromal tumors (GIST) and neuroendocrine tumors (NET) for the large intestine. For this analysis, GIST and NET occurring in the colon or rectum were excluded, as were cancers of the appendix. Histological types, for which AJCC stage does not apply within the colon or rectum in either the AJCC 6th or 7th editions7,8 were also excluded. In addition, cases diagnosed at autopsy or by death certificate only (DCO) were excluded as these cases often contained very limited information and represented only 1% of colon cancer cases and about 0.4% of rectal cancer cases in 2010.
Analysis Cohorts
In examining CS-derived AJCC 6th edition trends in stage distribution (objective #1), all eligible colorectal carcinomas (in situ and invasive) diagnosed between 2004 and 2010 were selected (Table 1). For comparing changes in stage distribution between the 6th and 7th editions of AJCC (objective #2), we limited our analysis to colorectal carcinomas diagnosed in 2010. Finally, when assessing the completeness and quality of CS SSFs (objective #3), we further excluded in situ cases and analyzed only invasive colorectal cancer cases diagnosed in 2010. In addition, we limited our analyses to appropriate subsets of the cohort when evaluating the completeness and quality of SSFs in 2010; e.g., patients who did not undergo surgical resection were excluded when assessing tumor deposits, circumferential resection margin (CRM), and perineural invasion. A subset trend analysis was conducted for the one colorectal SSF (CEA) available from 2004 through 2010.
TABLE 1.
Colon and Rectal Cancers: Exclusion Criteria for Analysis Cohort, SEER 18, 2004-2010
| Exclusion Criteria | Stage Trends | AJCC 6th & 7th Comparison | Site-Specific Factors (SSFs) |
|---|---|---|---|
| Objective 1 | Objective 2 | Objective 3 | |
| In situ cases | no | no | yes |
| Autopsy or death certificate cases | yes | yes | yes |
| Histologies for which AJCC 6th or 7th stage is not defined* | yes | yes | yes |
| Code 988 (not applicable), blank for each SSF | no | no | yes |
| Year of diagnosis | 2004-2010 | 2010 | 2010 |
| Final sample size: Colon | 191 361 | 25 577 | 24 437 |
| Final sample size: Rectum | 73 341 | 10 150 | 9678 |
American Joint Committee on Cancer (AJCC) 6th or 7th editions derived stage = NA identifies histologies for which stage is undefined for both colon and rectum.
Source: National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program: SEER 18 geographic areas: States of Connecticut, New Mexico, Utah, California (4 areas: San-Francisco, San Jose-Monterey, Los Angeles, Greater California), Hawaii, Iowa, New Jersey, Louisiana, Kentucky, Georgia (3 areas: Atlanta, Rural Georgia, and remainder of the state), Alaska Native Registry, and metropolitan areas of Detroit, Michigan and Seattle (Western Washington), Washington.
Key Changes between AJCC 6th and 7th Edition Staging
A major change in the AJCC 7th edition was the subdivision of T4 tumors into T4a (tumor penetrates the surface of the visceral peritoneum) and T4b (tumor directly invades or is histologically adherent to other organs or structures). This decision was based in part on an analysis of existing SEER data from 2004-2009 collected using the CS system available during that time period. Despite the fact that these tumors were grouped together in the AJCC 6th edition as T4, the CS system provided the ability to distinguish the two groups using internal CS codes. Results of this analysis indicated that patients whose cancers penetrated the visceral peritoneum had a better relative survival rate than those whose cancers invaded an adjacent organ or structure, even if it was completely resected.10
A second change from the 6th edition was adoption of N1c tumor deposit(s) without positive regional lymph node metastasis, upgrading a stage I or II tumor in the AJCC 6th edition to a stage III tumor under the AJCC 7th edition. This change was made to recognize the potential importance of satellite tumor deposits, allow clinicians to treat patients with these deposits with adjuvant therapy, and foster prospective data collection on frequency and incidence of tumor deposits.
A third change was the subdivision of the N1 category into categories N1a and N1b (1 versus 2-3 positive lymph nodes, respectively) and the N2 category into N2a and N2b (4-6 positive nodes versus 7 or more positive nodes, respectively). These decisions were again based on an analysis of SEER data which demonstrated that each subgroup comprised approximately 50% of either the N1 or N2 group, with significant differences in relative survival.10
A fourth change was the division of the M classifier into M1a for a single site of metastasis and M1b for more than one site of metastasis. This was done to collect data prospectively on whether patients with metastases to one organ site fare better than patients with multiple organ involvement. In addition, the AJCC 7th edition eliminated the category of “MX” (unknown distant metastasis) and instructed abstractors to assume these cases are M0 (no distant metastasis). This change in coding rules resulted in cases of unknown stage in the AJCC 6th edition now mapping to a specific stage in the AJCC 7th edition.
The effect of these changes on the AJCC stage grouping is diagrammed in Table 2.
TABLE 2.
Colon and Rectal Cancers: Comparison of Anatomic Stage and Prognostic Groups Based on AJCC 6th and 7th Editions
| AJCC 6th Edition | AJCC 7th Edition | ||||||
|---|---|---|---|---|---|---|---|
| Stage | T | N | M | Stage | T | N | M |
| 0 | Tis | N0 | M0 | 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 | I | T1 | N0 | M0 |
| T2 | N0 | M0 | T2 | N0 | M0 | ||
| IIA | T3 | N0 | M0 | IIA | T3 | N0 | M0 |
| IIB | T4 | N0 | M0 | IIB | T4a | N0 | M0 |
| IIC | T4b | N0 | M0 | ||||
| IIIA | T1–T2 | N1 | M0 | IIIA | T1–T2 | N1/N1c | M0 |
| T1 | N2a | M0 | |||||
| IIIB | T3–T4 | N1 | M0 | IIIB | T3–T4a | N1/N1c | M0 |
| T2–T3 | N2a | M0 | |||||
| T1–T2 | N2b | M0 | |||||
| IIIC | Any T | N2 | M0 | IIIC | T4a | N2a | M0 |
| T3–T4a | N2b | M0 | |||||
| T4b | N1-N2 | M0 | |||||
| IV | Any T | Any N | M1 | IVA | Any T | Any N | M1a |
| IVB | Any T | Any N | M1b | ||||
Note: “MX” cases in the AJCC 6th edition are considered “M0” in AJCC 7th edition and staged accordingly.
AJCC: American Joint Committee on Cancer
Source: AJCC 6th: Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th Ed. Chicago: Springer-Verlag; 2002; AJCC 7th: Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th Ed. Chicago: Springer-Verlag; 2010.
Site-Specific Factors (SSFs)
In 2004 when CS Version 1 (CSv1) was first implemented, only carcinoembryonic antigen (CEA) laboratory interpretation (SSF1) was collected for colon and rectal cancers. Clinical assessment of regional lymph nodes (SSF2) was added for cases diagnosed in 2007. With CSv2 implemented for cases diagnosed in 2010, all SEER registries were required to collect an additional 5 SSFs related to prognosis and treatment. These 7 SSFs are the same for colon and rectal cancers in the CSv2 schemas and are listed in Tables 3a and 3b, respectively. A detailed description of each of these SSFs is presented below.
TABLE 3a.
Colon Cancer: Site-Specific Factors (SSFs) Description and Codes, SEER 18, 2010 Cases
| Known | Unknown | Total | |||||
|---|---|---|---|---|---|---|---|
| Codes | N | % | Codes | N | % | N | |
| SSF 1: Carcinoembryonic Antigen (CEA) | 010, 020, 030 | 13 322 | 54.5 | 997, 998, 999 | 11 115 | 45.5 | 24 437 |
| SSF 2: Clinical Assessment of Regional Lymph Nodes | 000-120 | 18 884 | 77.3 | 200, 400, 999 | 5553 | 22.7 | 24 437 |
| 2a. Select Reg LN eval = 0, 1, 5, 9 | 000-120 | 3371 | 61.0 | 200, 400, 999 | 2157 | 39.0 | 5528 |
| 2b. Select Reg LN eval = 0, 1, 5, 9, and CS LN = 100-300, 800 | 010-120 | 129 | 22.6 | 000, 200, 400, 999 | 443 | 77.4 | 572 |
| SSF 3: Carcinoembryonic Antigen (CEA) Lab Value | 000-980 | 13 189 | 54.0 | 997, 998, 999 | 11 248 | 46.0 | 24 437 |
| SSF 4: Tumor Deposits | 000-081 | 17 614 | 72.1 | 990, 998, 999 | 6823 | 27.9 | 24 437 |
| 4a. Selected surgery codes 30-80 | 000-081 | 16 487 | 83.7 | 990, 998, 999 | 3213 | 16.3 | 19 700 |
| SSF 6: Circumferential Resection Margin (CRM) | 000-981, 990-996 | 14 812 | 60.6 | 998, 999 | 9625 | 39.4 | 24 437 |
| 6a.Selected surgery codes 30-80 | 000-981, 990-996 | 14 499 | 73.6 | 998, 999 | 5201 | 26.4 | 19 700 |
| SSF 8: Perineural Invasion | 000-010 | 17 817 | 72.9 | 998, 999 | 6620 | 27.1 | 24 437 |
| 8a.Selected surgery codes 30-80 | 000-010 | 16 685 | 84.7 | 998, 999 | 3015 | 15.3 | 19 700 |
| SSF 9: KRAS | 010-020 | 1998 | 8.2 | 997, 998, 999 | 22 439 | 91.8 | 24 437 |
| 9a. KRAS stage IV only | 010-020 | 1043 | 21.3 | 997, 998, 999 | 3858 | 78.7 | 4901 |
Source: National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program: SEER 18 geographic areas: States of Connecticut, New Mexico, Utah, California (4 areas: San-Francisco, San Jose-Monterey, Los Angeles, Greater California), Hawaii, Iowa, New Jersey, Louisiana, Kentucky, Georgia (3 areas: Atlanta, Rural Georgia, and remainder of the state), Alaska Native Registry, and metropolitan areas of Detroit, Michigan and Seattle (Western Washington), Washington.
LN: Lymph nodes; Reg: Regional; eval: evaluation; CS: Collaborative Stage
Note: there were no cases for not applicable (codes 988 or blank).
TABLE 3b.
Rectal Cancer: Site-Specific Factors (SSFs) Description, Codes, and Applicability, SEER 18, 2010 Cases
| Known | Unknown | Total | |||||
|---|---|---|---|---|---|---|---|
| Codes | N | % | Codes | N | % | N | |
| SSF 1: Carcinoembryonic Antigen (CEA) | 010, 020, 030 | 5214 | 53.9 | 997, 998, 999 | 4464 | 46.1 | 9678 |
| SSF 2: Clinical Assessment of Regional Lymph Nodes | 000-120 | 7626 | 78.8 | 200, 400, 999 | 2052 | 21.2 | 9678 |
| 2a. Select Reg LN eval = 0, 1, 5, 9 | 000-120 | 3549 | 72.9 | 200, 400, 999 | 1317 | 28.1 | 4866 |
| 2b. Select Reg LN eval = 0, 1, 5, 9, and CS LN = 100-300, 800 | 010-120 | 654 | 58.2 | 000, 200, 400, 999 | 469 | 41.8 | 1123 |
| SSF 3: Carcinoembryonic Antigen (CEA) Lab Value | 000-980 | 5194 | 53.7 | 997, 998, 999 | 4484 | 46.3 | 9678 |
| SSF 4: Tumor Deposits | 000-081 | 6281 | 64.9 | 990, 998, 999 | 3397 | 35.1 | 9678 |
| 4a. Selected surgery codes 30-80 | 000-081 | 5081 | 82.8 | 990, 998, 999 | 1053 | 17.2 | 6134 |
| SSF 6: Circumferential Resection Margin (CRM) | 000-981, 990-996 | 4972 | 51.4 | 998, 999 | 4706 | 48.6 | 9678 |
| 6a. Selected surgery codes 30-80 | 000-981, 990-996 | 4525 | 73.8 | 998, 999 | 1609 | 26.2 | 6134 |
| SSF 8: Perineural Invasion | 000-010 | 6263 | 64.7 | 998, 999 | 3415 | 35.3 | 9678 |
| 8a.Selected surgery codes 30-80 | 000-010 | 5043 | 82.2 | 998, 999 | 1091 | 17.8 | 6134 |
| SSF 9:KRAS | 010-020 | 716 | 7.4 | 997, 998, 999 | 8962 | 92.6 | 9678 |
| 9a. KRAS stage IV only | 010-020 | 362 | 21.3 | 997, 998, 999 | 1340 | 78.7 | 1702 |
Source: National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program: SEER 18 geographic areas: States of Connecticut, New Mexico, Utah, California (4 areas: San-Francisco, San Jose-Monterey, Los Angeles, Greater California), Hawaii, Iowa, New Jersey, Louisiana, Kentucky, Georgia (3 areas: Atlanta, Rural Georgia, and remainder of the state), Alaska Native Registry, and metropolitan areas of Detroit, Michigan and Seattle (Western Washington), Washington.
LN: Lymph nodes; Reg: Regional; eval: evaluation; CS: Collaborative Stage
Note: there were no cases for not applicable (codes 988 or blank).
CEA Interpretation (SSF1) and CEA Laboratory Value (SSF3)
CEA is a protein that is normally produced during prenatal development. After birth, CEA blood levels typically are very low or undetectable. Elevated CEA levels are associated with many carcinomas and other health conditions. Although colorectal cancer is a common cause of elevated CEA levels, CEA levels also increase from conditions such as biliary obstruction and inflammatory digestive disorders, and increased levels can be detected in “healthy” heavy smokers.11 Elevated CEA levels also are associated with liver or lung metastases in colorectal cancer patients. Therefore, CEA is recommended for use in assessing patients prior to surgery12 and for monitoring recurrence after surgery, but CEA is not used as a screening test for colorectal cancer.13 Both CEA laboratory values and CEA interpretations are to be recorded from the same test prior to treatment and for the test with the highest value if multiple tests are performed.
The CEA laboratory value is a 3-digit field with an implied decimal place between the second and third digits. Values should be coded as nanograms per milliliter (ng/ml), with values of 98.0 or greater recorded as “980.” The normal reference range may vary between laboratories; but the generally accepted normal range for plasma CEA level is <2.5 ng/ml in nonsmokers and <5 ng/ml in smokers. Because patients with metastatic colorectal carcinoma often have CEA blood levels in excess of 100 ng/ml,14 data quality control is needed to assure that CEA is expressed in ng/ml.
Clinical Assessment of Regional Lymph Nodes (SSF2)
Although AJCC 7th edition staging has very detailed pathologic evaluation of regional lymph node status when surgical resection is performed, information on lymph node status is not available when there is no resection or insufficient tissue to meet the criteria for pathologic staging, e.g., fine-needle aspiration (CS Lymph Node Evaluation = 0,1,9). In addition, in the presence of neoadjuvant therapy prior to surgery there are specific rules to be followed regarding when to utilize pathologic information on nodal status (CS Lymph Node Evaluation =5). Therefore, SSF2 was introduced in CSv2 to determine clinical lymph node status as part of the work-up by collecting such information based on imaging and physical examination in similar categories as the pathologic assessment (eg, clinically N0, N1, or N2). This assessment is separate from the pathologic assessment of the number of regional nodes examined and positive.
Number of Tumor Deposits (SSF4)
The CSv2 manual15 defines tumor deposits as “separate nodules or deposits of malignant cells in the perirectal or pericolic fat without evidence of residual lymph node tissue.” Other terminologies used in previous editions of the AJCC Cancer Staging Manual include “malignant tumor foci,” “malignant peritumoral deposits,” and “satellite nodules.” These deposits, if present, are found in the primary lymphatic drainage area of the tumor in resected specimens. The source document for tumor deposits is the pathology report, and the assessment and recording of tumor deposits are required in the College of American Pathologists’ (CAP) Cancer Case Summary protocol for colorectal cancer. A patient is considered without tumor deposits when the pathology report so indicates or when the report from a surgical resection does not mention tumor deposits.
Because the presence of tumor deposits is considered an adverse prognostic factor for cancers of the colon and rectum, the new designation of N1c is used in AJCC system when tumor deposits are found without lymph node involvement. The field “CS Lymph Nodes” captures information on cases with tumor deposits without regional nodal metastases (N1c), and SSF4 records the number of tumor deposits present.
Circumferential Resection Margin (CRM) (SSF6)
CRM, according to the CS manual,15 is the “measurement of the distance between the deepest invasion of the tumor and the closest soft tissue margin of the resected specimens.” Specifically, it is “the width of the surgical margin at the deepest part of the tumor in an area of the large intestine or rectum without serosa” (ie, the rectum and segments of the colon unencased or incompletely encased by the peritoneum). In segments of the colon completely encased by the peritoneum, the CRM also is referred to as the mesenteric resection margin. For rectal cancer, the CRM is the most important predictor of local-regional recurrence.16 The assessment and recording of the CRM is a requirement in the CAP protocol for colorectal cancer.
Perineural Invasion (SSF 8)
Perineural invasion is defined in the CS manual as “the infiltration of nerves in the area of the lesion by tumor cells or spread of tumor along the nerve pathway.”15 Several studies have found an association between poor prognosis and perineural invasion17. Biopsy specimens in general include mostly or only mucosa in which nerves are normally absent. Therefore perineural invasion is a finding that can only be evaluated in resection specimens and it is a required item in the CAP protocol for colorectal cancer. A positive finding is expected to be recorded in the pathology report; negative results are assumed if perineural invasion is not mentioned.
KRAS (SSF 9)
The presence of mutations in patients with advanced colorectal cancer makes them less likely to respond to the anti-epidermal growth factor receptor (anti-EGFR) drugs such as cetuximab or panitumumab. In 2008, the National Comprehensive Cancer Network (NCCN) updated its guidelines to include the recommendation that stage IV (metastatic) colorectal cancer patients be tested for KRAS gene mutation prior to treatment.18 Only patients with tumors characterized by the wild-type KRAS gene should be treated with EGFR inhibitors because of the toxicity and cost. The information on KRAS type is to be recorded for newly diagnosed cases and not for recurrent colorectal cases. KRAS results are part of the CAP protocol but reporting is optional; partly because this result is not always available when the pathology report is issued.
RESULTS
Trends in AJCC 6th Edition Stage Distributions for Colon and Rectal Cancers, 2004-2010
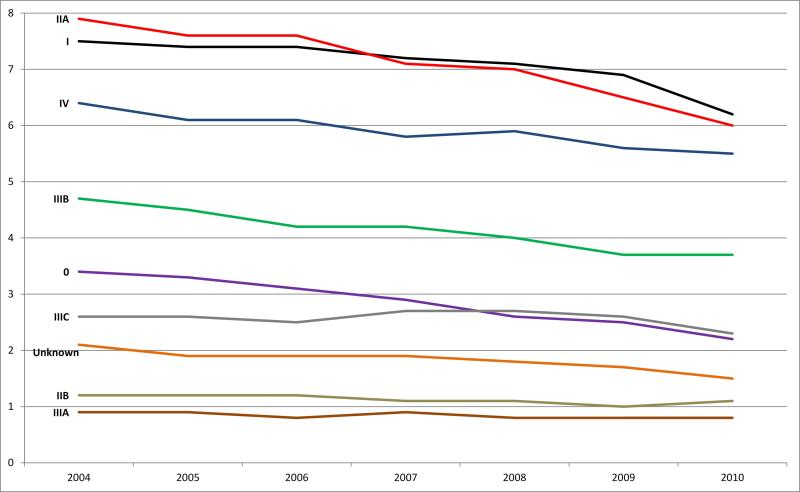
Using data from 18 SEER population-based registries, a total of 191 361 cases of colon cancer and 73 341 cases of rectal cancer were diagnosed during 2004-2010 and used in evaluating the time trends. Figure 1a displays annual colon cancer incidence rates per 100 000 persons by AJCC 6th edition stage over the 7-year period. Overall, the age-adjusted incidence rate for colon cancer declined approximately 21% between 2004 (36.8 per 100 000) and 2010 (29.1 per 100 000), with the largest annual decreases occurring in 2009 (5%) and 2010 (7%). The age-adjusted rate for stage 0 showed the greatest decline during the study period, at 35%. In addition, stages I, II, III, and IV declined 17%, 22%, 17%, and 14%, respectively between 2004 and 2010.
Figure 1a.
Colon cancer: Age-adjusted incidence rates by stage (Collaborative Stage derived AJCC 6th edition) and year of diagnosis, SEER 18 areas, 2004-2010. Note: Rates are per 100,000 and are age-adjusted to the 2000 US standard population (19 age groups). Data source for all figures: National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program: SEER 18 geographic areas: States of Connecticut, New Mexico, Utah, California (4 areas: San-Francisco, San Jose-Monterey, Los Angeles, Greater California), Hawaii, Iowa, New Jersey, Louisiana, Kentucky, Georgia (3 areas: Atlanta, Rural Georgia, and remainder of the state), Alaska Native Registry, and metropolitan areas of Detroit, Michigan and Seattle (Western Washington), Washington.
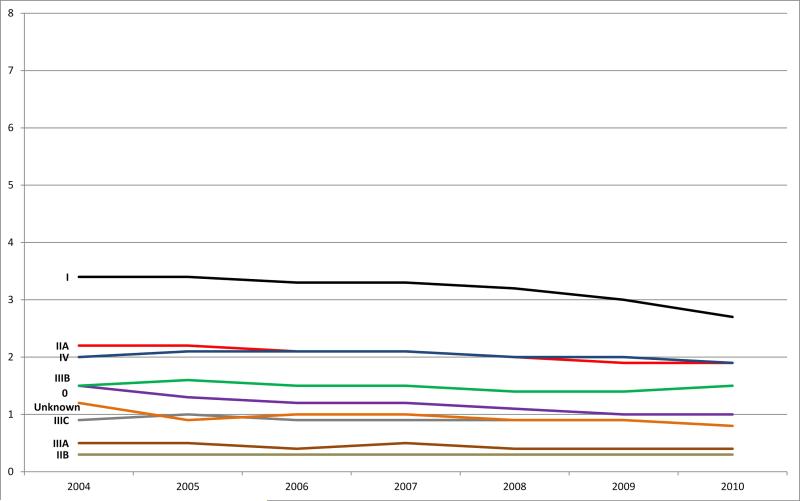
The age-adjusted overall rectal cancer incidence rate declined 16% between 2004 (13.5 per 100 000) and 2010 (11.3 per 100 000), with the largest annual decline of 5% between 2009 and 2010 (Fig. 1b). Similar to colon cancer, stage 0 showed the greatest decline of 33% between 2004 and 2010. Also during this time period, stage I cases declined 21%, stage II cases declined 12%, stage III cases declined 7%, and stage IV cases declined 5%.
Figure 1b.
Rectal cancer: Age-adjusted incidence rates by stage (Collaborative Stage derived AJCC 6th edition) and year of diagnosis, SEER 18 areas, 2004-2010. Note: Rates are per 100,000 and are age-adjusted to the 2000 US standard population (19 age groups). Data source for all figures: National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program: SEER 18 geographic areas: States of Connecticut, New Mexico, Utah, California (4 areas: San-Francisco, San Jose-Monterey, Los Angeles, Greater California), Hawaii, Iowa, New Jersey, Louisiana, Kentucky, Georgia (3 areas: Atlanta, Rural Georgia, and remainder of the state), Alaska Native Registry, and metropolitan areas of Detroit, Michigan and Seattle (Western Washington), Washington.
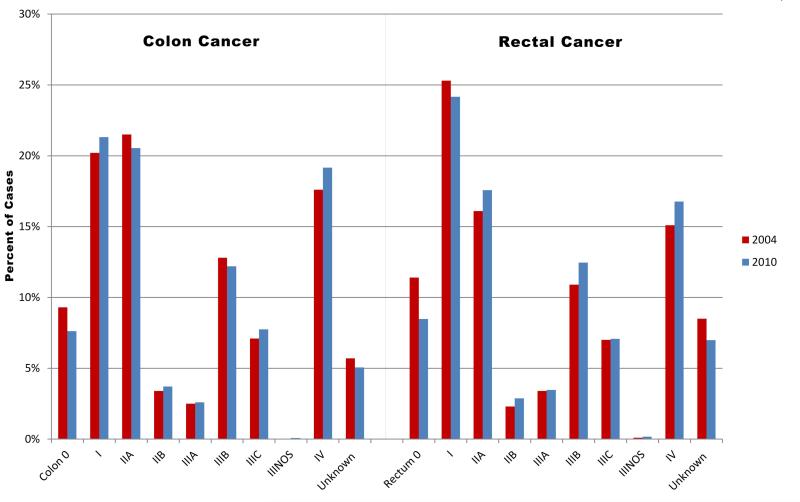
The proportions of cases by stage for colon and rectal cancer cases were relatively stable in 2004 and 2010, as illustrated in Figure 2, except that the decreasing proportion of stage 0 rectal cancer cases was offset by the increasing percentages of stage II, III, and IV diagnoses.
Figure 2.
Colon and rectal cancer: Collaborative Stage derived AJCC 6th edition stage distributions for 2004 and 2010, SEER 18 areas. Data source for all figures: National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program: SEER 18 geographic areas: States of Connecticut, New Mexico, Utah, California (4 areas: San-Francisco, San Jose-Monterey, Los Angeles, Greater California), Hawaii, Iowa, New Jersey, Louisiana, Kentucky, Georgia (3 areas: Atlanta, Rural Georgia, and remainder of the state), Alaska Native Registry, and metropolitan areas of Detroit, Michigan and Seattle (Western Washington), Washington.
Comparison of AJCC Staging in the 6th and 7th Editions
A summarized comparison of staging in the AJCC 6th and 7th editions for 25 577 colon and 10 150 rectal cancer cases diagnosed in 2010 is presented in Table 4. In this comparison, the exact same cases were staged using the two different standards. There were no differences between the 2 staging classifications in relation to stage 0 colon or rectal cancers. The AJCC 7th edition guidelines yielded 22 additional stage I colon cancer cases, 62 fewer stage II cases, and 179 additional stage III cases, as a result of the creation of N1c and the change from MX (unknown metastasis) to M0 (no metastasis) in the 7th edition. Likewise, the 7th edition guidelines yielded 6 additional stage I rectal cancer cases, 36 fewer stage II cases, and 72 additional stage III cases. Many cases formerly classified as “unknown” were reclassified into specific stage categories under the 7th edition's guidelines. Although cases were shifted among substages within stages II, III, and IV as the result of changes introduced in the AJCC 7th edition, there was little movement of cases between overall stages. The only notable exception was that 112 colon cancer cases and 65 rectal cancer cases that would have been considered stage I/II under the AJCC 6th edition became stage III under the 7th edition due to the new N1c category, which represents tumor deposit(s) without a positive regional lymph node metastasis (Table 5). These cases represented 2.5% of stage IIIA/B colon and 3.3% of stage IIIA/B rectal cancer cases in 2010.
TABLE 4.
Colon and Rectal Cancers: Stage and Subgroup Comparisons between the AJCC 6th and 7th Editions, SEER 18, 2010
| AJCC 6th Edition | AJCC 7th Edition | Difference (7th Ed. Total – 6th Ed Total) | |||
|---|---|---|---|---|---|
| Stage | Stage Group Total | Substage Counts | Stage Group Total | Substage Counts | |
|
COLON CANCER (N = 25 577)
| |||||
| 0 | 1949 | 1949 | 0 | ||
| I | 5450 | 5472 | +22 | ||
| II | 6202 | 6140 | –62 | ||
| IINOS | 5 | ||||
| IIA | 5254 | 5191 | |||
| IIB | 948 | 500 | |||
| IIC | -- | 444 | |||
| III | 5780 | 5959 | +179 | ||
| IIINOS | 20 | 3 | |||
| IIIA | 662 | 702 | |||
| IIIB | 3118 | 3868 | |||
| IIIC | 1980 | 1386 | |||
| IV | 4901 | 4901 | 0 | ||
| IVNOS | -- | 187 | |||
| IVA | -- | 2545 | |||
| IVB | -- | 2169 | |||
| Unknown | 1295 | 1156 | –139 | ||
|
RECTAL CANCER (N = 10 150) | |||||
| 0 | 860 | 860 | 0 | ||
| I | 2453 | 2459 | +6 | ||
| II | 2076 | 2040 | –36 | ||
| IINOS | -- | 18 | |||
| IIA | 1784 | 1749 | |||
| IIB | 292 | 68 | |||
| IIC | -- | 205 | |||
| III | 2351 | 2423 | +72 | ||
| IIINOS | 17 | 52 | |||
| IIIA | 352 | 386 | |||
| IIIB | 1264 | 1574 | |||
| IIIC | 718 | 411 | |||
| IV | 1702 | 1702 | 0 | ||
| IVNOS | -- | 138 | |||
| IVA | -- | 929 | |||
| IVB | -- | 635 | |||
| Unknown | 708 | 666 | –42 | ||
Source: National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program: SEER 18 geographic areas: States of Connecticut, New Mexico, Utah, California (4 areas: San-Francisco, San Jose-Monterey, Los Angeles, Greater California), Hawaii, Iowa, New Jersey, Louisiana, Kentucky, Georgia (3 areas: Atlanta, Rural Georgia, and remainder of the state), Alaska Native Registry, and metropolitan areas of Detroit, Michigan and Seattle (Western Washington), Washington.
NOS: Not Otherwise Specified; AJCC: American Joint Committee on Cancer
TABLE 5.
Colon and Rectal Cancers: Summarized Comparison of AJCC 6th and 7th Edition Case Counts by Stage, SEER 18, 2010
| COLON | AJCC 7th- 2010 Cases | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AJCC 6th-2010 Cases | 0 | I | II NOS | IIA | IIB | IIC | III NOS | IIIA | IIIB | IIIC | IV NOS | IVA | IVB | Unknown | Total |
| 0 | 1949 | 1949 | |||||||||||||
| I | 5439 | 11 | 5450 | ||||||||||||
| IIA | 5165 | 89 | 5254 | ||||||||||||
| IIB | 5 | 497 | 434 | 12 | 948 | ||||||||||
| III NOS | 20 | 20 | |||||||||||||
| IIIA | 662 | 662 | |||||||||||||
| IIIB | 2884 | 234 | 3118 | ||||||||||||
| IIIC | 3 | 24 | 827 | 1122 | 4 | 1980 | |||||||||
| IV | 187 | 2545 | 2169 | 4901 | |||||||||||
| Unknown | 33 | 26 | 3 | 10 | 5 | 56 | 30 | 1132 | 1295 | ||||||
| Total | 1949 | 5472 | 5 | 5191 | 500 | 444 | 3 | 702 | 3868 | 1386 | 187 | 2545 | 2169 | 1156 | 25 577 |
| RECTUM | AJCC 7th- 2010 Cases | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AJCC 6th-2010 Cases | 0 | I | IINOS | IIA | IIB | IIC | IIINOS | IIIA | IIIB | IIIC | IVNOS | IVA | IVB | Unknown | Total |
| 0 | 860 | 860 | |||||||||||||
| I | 2441 | 12 | 2453 | ||||||||||||
| IIA | 1737 | 47 | 1784 | ||||||||||||
| IIB | 18 | 66 | 202 | 6 | 292 | ||||||||||
| IIINOS | 17 | 17 | |||||||||||||
| IIIA | 352 | 352 | |||||||||||||
| IIIB | 1,167 | 97 | 1264 | ||||||||||||
| IIIC | 51 | 19 | 329 | 312 | 7 | 718 | |||||||||
| IV | 138 | 929 | 635 | 1702 | |||||||||||
| Unknown | 18 | 12 | 2 | 3 | 1 | 3 | 25 | 2 | 642 | 708 | |||||
| Total | 860 | 2459 | 18 | 1749 | 68 | 205 | 52 | 386 | 1574 | 411 | 138 | 929 | 635 | 666 | 10 150 |
Source: National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program: SEER 18 geographic areas: States of Connecticut, New Mexico, Utah, California (4 areas: San-Francisco, San Jose-Monterey, Los Angeles, Greater California), Hawaii, Iowa, New Jersey, Louisiana, Kentucky, Georgia (3 areas: Atlanta, Rural Georgia, and remainder of the state), Alaska Native Registry, and metropolitan areas of Detroit, Michigan and Seattle (Western Washington), Washington.
AJCC: American Joint Committee on Cancer; NOS: Not Otherwise Specified
A more detailed illustration of the migration between substages is presented in Table 5. Briefly, there was a shift from the “unknown” stage under the AJCC 6th edition to specific stages in the 7th edition. For colon cancer, approximately 13% of “unknown” stage cases were reassigned to a specific stage. The subdivision of T4 tumors into T4a and T4b and the creation of stage IIC caused roughly half of the cases that would have been staged as IIB according to the AJCC 6th edition to move to IIC according to the 7th edition. The changes in the definition of IIIC and changes related to the subdivision of N1 and N2 into N1a and N1b and N2a and N2b caused a substantial portion of cases to shift from stage IIIC to stage IIIB. The division of M1 resulted in 52% of cases being classified as stage IVA, 44% as stage IVB, and 4% as stage IV Not Otherwise Specified (NOS).
Similarly, approximately 9% of “unknown” stage cases of rectal cancer were assigned a specific stage. The subdivision of T4 tumors into T4a and T4b and the creation of stage IIC caused approximately three-fourths of the cases that would have been staged as IIB according to the AJCC 6th edition to be staged as IIC under the 7th edition. The changes in the definition of IIIC and changes related to the subdivision of N1 and N2 into N1a, N1b, N2a, and N2b caused a substantial number of cases to shift from stage IIIC to stage IIIB. The division of M1 resulted in more than half (55%) of rectal cases becoming classified as stage IVA, 37% as stage IVB, and 8% as stage IV NOS. There was, however, no clear up- or down-stage shift for either cancer site.
Site-Specific Factors (SSFs)
CEA Interpretation (SSF1) and CEA Laboratory Value (SSF3)
Of the 24 437 invasive colon cancer patients diagnosed in 2010 and included in the analyses of SSFs (Table 3a), only 54.5% had a known CEA interpretation. Among those with known values, 47.3% were interpreted as elevated CEA, 52.2% as within normal range, and 0.6% as borderline or undetermined. Of the 9678 invasive rectal cancer patients, 53.9% had a known CEA interpretation (Table 3b); of these, 48.8% were interpreted as elevated CEA, 50.5% as within normal range, and 0.8% as borderline. No preponderance of elevated CEA values was noted in any subsite of the large intestine.
There was a slight but steadily increasing trend of known CEA interpretation recorded over the 7-year period: from 48.6% in 2004 to 54.5% in 2010 for colon cancer patients, and from 49.2% in 2004 to 53.9% in 2010 for rectal cancer patients. Among cases with CEA interpretation, however, the proportion of cases interpreted as elevated remained quite constant over time at about 46-47% for colon cancer and 48% for rectal cancer (data not shown).
Because different CEA reference ranges are used depending on smoking status and the fact that registries do not have smoking information on all cases, we were not able to examine the consistency between CEA laboratory value and CEA interpretation.
Clinical Assessment of Regional Lymph Nodes (SSF2)
Information on the clinical assessment of lymph nodes (SSF2) was available for 61% of the colon cancer cases and 72.9% of the rectal cancer cases. When lymph nodes were positive clinically, only 22.6% of colon cancer patients had information on the number of nodes positive which is needed to calculate N; the corresponding figure for rectal cancer patients was much higher, at 58.2%.
Number of Tumor Deposits (SSF4)
Tumor deposits were evaluated and number of deposits recorded in 83.7% of the colon cancer patients (N = 19 700) and 82.8% of the rectal cancer patients (N = 6134) who underwent surgical resection. The majority had no tumor deposits; approximately 10% of patients (9.5% of colon and 10.2% of rectal) were identified with tumor deposits in the perirectal or pericolic fat. Of the colon cancer patients with tumor deposits, about 64% had an unknown number, 18% had only 1, and about 5% had more than 5 tumor deposits. Similarly, for rectal cancer patients with tumor deposits, approximately 53% had an unknown number, about 24% had only 1, and about 5% had more than 5 tumor deposits.
Thirty percent and 41.1%, respectively, of the colon or rectal cancer patients who had tumor deposits did not have positive lymph node involvement; nearly 75% of these patients had only 1 or 2 tumor deposits (data not shown).
CRM (SSF6)
Among patients who had surgical resection, CRM was evaluated in 73.6% of the colon cancer cases and in 73.8% of the rectal cancer cases. More than half of these cases (62.5% and 59.7%, respectively, for colon and rectum) had a clear margin (code = 991) or no residual tumor identified on the specimens (code = 990); less than 40% had a CRM measurement recorded (Table 6). Interestingly, 9.9% of colon and 11.7% of rectal cases had margins less than 1 mm.
TABLE 6.
Colon and Rectal Cancers: Frequency Distribution of Circumferential Resection Margins (CRMs) in Patients Who Underwent Surgical Resection+, SEER-18, 2010
| Codes | Description | Colon | Rectal | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Total | 19 700 | 100.0 | 6134 | 100.0 | |
| 000-009 | Margin is involved with tumor; CRM positive, less than 1 mm | 1953 | 9.9 | 716 | 11.7 |
| 010-019; 992 | Between 1 mm to <2 mm | 483 | 2.5 | 244 | 4.0 |
| 020-029; 993 | Between 2 mm to <3 mm | 312 | 1.6 | 137 | 2.2 |
| 030-039,994 | Between 3 mm to <4 mm | 311 | 1.6 | 105 | 1.7 |
| 040-049,995 | Between 4 mm to <5 mm | 243 | 1.2 | 57 | 0.9 |
| 050-981,996 | ≥5 mm | 2138 | 10.9 | 563 | 9.2 |
| 990,991 | No residual tumor identified on specimen; Margins clear; CRM negative, NOS | 9059 | 46.0 | 2703 | 44.1 |
| 998-999 | Unknown or no information, CRM not mentioned | 5201 | 26.4 | 1609 | 26.2 |
Source: National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program: SEER 18 geographic areas: States of Connecticut, New Mexico, Utah, California (4 areas: San-Francisco, San Jose-Monterey, Los Angeles, Greater California), Hawaii, Iowa, New Jersey, Louisiana, Kentucky, Georgia (3 areas: Atlanta, Rural Georgia, and remainder of the state), Alaska Native Registry, and metropolitan areas of Detroit, Michigan and Seattle (Western Washington), Washington.
+Patients underwent surgical resection, surgery codes=30-80 (Reference: Adamo MB, Johnson CH, Ruhl JL, Dickie, LA, (eds.). 2011SEER Program Coding and Staging Manual. National Cancer Institute, NIH Publication number 11-5581, Bethesda, MD)
NOS=Not otherwise specified
Perineural Invasion (SSF8)
Of all colon cancer patients who had surgical resection, 84.7% were evaluated for perineural invasion (a CAP Level II recommendation) and only one-tenth (10.2%) of those evaluated had neural invasion. Similarly, 82.2% of resected rectal cancer cases were assessed, and perineural invasion was present in only 10.8% of those evaluated.
KRAS Testing (SSF9)
Of cases diagnosed in 2010, information on the KRAS gene was found in only 8.2% of the colon cancer cases and in 7.4% of rectal cancer cases. When restricted to patients with stage IV disease (those with distant metastases) for whom KRAS gene testing is recommended by NCCN guidelines, KRAS gene status was available for 21.3% of both colon and rectal cancer patients. Among stage IV patients whose KRAS testing results were available, 43.1% of those with colon tumors and 39.2% of those with rectal tumors had a mutated KRAS gene.
DISCUSSION
The purpose of the CS Data Collection System is to stage cancer patients accurately using the current AJCC anatomic pathology nomenclature and to collect SSFs that will improve prognostic accuracy or inform treatment decisions for patients. CS represents a compromise among a number of stakeholders whose intent is to provide clinically useful information for physicians, epidemiologists, outcome researchers, and even patients. It is a compromise because not all clinically significant factors can be included in the registry workflow, which involves both considerable effort and cost. The intent of the CS system is to include prognostically informative molecular markers of sufficient quality as they become available in the future. This analysis of SEER data enables us to examine the impact of CSv2 on the CS-derived AJCC stage distribution as well as evaluate the availability and quality of the clinically significant SSFs.
The colorectal cancer incidence rates by stage show relatively steady trends over the 7 year period. The age-adjusted incidence rates for colon and rectal cancer declined from 2004 to 2010, largely attributable to decreasing in situ cancers. One likely factor contributing to this decrease is the detection and removal of precancerous polyps via colonoscopy, with fewer lesions typically found on subsequent colonoscopies. Another possible explanation could be the transition of terminology used by pathologists to describe lesions that are confined to the epithelium or invade the lamina propria alone. The World Health Organization Classification of Tumors19 suggests that “high-grade intraepithelial neoplasia” is a more appropriate term for such lesions than “adenocarcinoma in-situ” and helps to avoid overtreatment. Although adenocarcinoma in situ is reportable, high-grade dysplasia is not. This change in terminology may contribute to the decline in stage 0. Unfortunately, declines in advanced stage cancers have been modest. If inconspicuous and aggressive flat lesions are missed during colonoscopy and present as advanced stage cancer years later, they could contribute to a lag in the decreasing incidence of later stage cancers.20-22 An alternative explanation may be that there has been insufficient time elapsed for colonoscopy to affect incidence rates for advanced stage cancers because colonoscopy only became a standard modality within the past decade. If so, a decrease in early stage colon cancer due to preventive polypectomy23,24 should eventually decrease the rate of late stage colorectal cancer as well.
The collection of SSF2, clinical assessment of lymph nodes, was intended as part of the work-up to choose a treatment plan. It has been problematic, however, because the biopsy of regional or sentinel nodes performed as part of the work-up was not included in the latest CS instruction. This SSF will be revised in the near future.
During development of the AJCC TNM 7th edition, there was significant interest in the inclusion of tumor deposits because the presence of tumor deposits may be as significant a negative prognostic factor as is metastases in regional lymph nodes.25 A Netherland study suggested that lymph node negative colorectal cancers with isolated tumor deposits should be classified and treated as Stage III.26
The presence of tumor deposits, however, is not commonly documented in North American pathology reports. A concerted effort was generated by the AJCC and CAP to educate pathologists about the importance of reporting these deposits, and fields for collection were included both on the TNM staging sheet and in the CAP protocol for colorectal cancer surgical specimen reporting. This effort has been successful, with pathology reports for most cases annotating the presence or absence of tumor deposits in both colon and rectal carcinoma (Tables 3a and 3b). Survival of these patients can be followed prospectively to assess how these tumor deposits affect clinical outcome. The N1c category was created because oncologists were in a quandary about how to treat patients who had tumor deposits but lacked positive nodal metastases, with the literature leaning toward use of adjuvant therapy. Although the evidence supporting such a recommendation is limited and a small study raised the appropriateness of N1c among rectal cancer patients after pre-operative chemoradiation ,27 cancer registries will continue to collect tumor deposit information for assessing its prognostic significance and confirming the utility of treatment in a larger population. About 3% of colon and 4% of rectal stage III carcinomas had 1 or more tumor deposits without regional nodal metastasis.
Our study also found widespread adoption by pathologists of the practice of assessing and recording other significant prognostic factors such as CRM (SSF6) and perineural invasion (SSF8) which are part of the CAP protocols. While the 26% of colorectal cancer cases without documented CRM is lower than the finding of a Norwegian study (about 37% of rectal cancer patient who underwent total mesorectal excision did not have CRM measured),28 the documentation of CRM should be improved since CRM remains an important factor in rectal cancer for prediction of prognosis and clinical management. As the SEER registries follow these patients with various prognoses, their clinical outcomes can be used to guide adjuvant therapy options.
The data presented for SSF1 and SSF3 demonstrate that roughly one-half of the newly diagnosed patients had CEA test results. Because smoking and other factors that can cause an elevation in CEA are not collected by registries, justifying the effort needed to record both CEA laboratory values and their interpretation still is controversial. Given CEA is part of the American Society of Clinical Oncology (ASCO) guidelines12 and a CAP Level I recommendation as well as indices used for prognostication in patients with liver metastases,14 the collection of CEA values and their interpretation are important. Data sources and method of collection should be revised to ensure that CEA test results are captured as part of the pre-therapy assessment.
Despite NCCN's recommendation that all patients with metastatic colorectal cancer be tested for KRAS gene mutations18 and ASCO's recommendation that all patients who are candidates for anti-EGFR therapy be tested for such mutations,29 our analysis showed that only 21.3% of stage IV colorectal cancer patients had documented KRAS gene testing results. This low rate contrasts with findings of three cross-sectional surveys of physicians conducted in 14 countries in Europe, Latin America and Asia which reported an uptake of KRAS testing from 3% in 2008 to 47% in 2009 and to 61% in 2010 for metastatic colorectal cancer patients.30 A primary reason for the difference in KRAS testing rates is that the physician surveys included patients with initially diagnosed Stage IV disease and patients with recurrences where our study included only the former. Additionally, given that KRAS mutation analysis is a referral test in most institutions and that this test is often ordered directly by the oncologists, the results of this analysis may be available at the physician's office but may not be recorded in the hospital chart, which is the primary source of information for registry data. Other explanations include cost and insurance coverage for the test,31 as well as the lack of a clear protocol in place at a specific health care facility for the initiation and ordering of KRAS testing.
Recently released CAP checklist for biomarker testing suggested that more than exon 2 of KRAS be collected and patients with wild-type KRAS may benefit from testing the BRAF mutation, another prognostic marker. Hence, established clinical applications aside, it is important to continue collecting the KRAS status on as many patients as possible so that this subgroup can be better studied for clinical outcome.
This report has numerous strengths. First, the data source is a high-quality cancer surveillance program that covers about 28% of the US population. Second, the data suggest the feasibility of collecting more refined staging that allows better categorization of subgroups of patients with different clinical outcomes, such as patients with tumor deposits but no lymph node involvement. Third, most of the data on SSFs for colorectal cancer are quite complete and of good quality, especially when the source of information is from pathology reports or CAP protocols. Fourth, the CSv2 collects numerous clinically significant factors and prognostic factors that provide an opportunity to evaluate therapy options and assess survival outcomes.
This study also has limitations. First, the 2010 data represent the first year when CSv2 was introduced and collected by SEER registries; extensive editing and quality control have not been implemented. Second, considerable information is missing on some SSFs such as KRAS testing, and better venues for capturing such information need to be explored. Finally, although the SEER Program covers over one-fourth of the US population, its patient cohort may not be nationally representative.
In summary, these data from an initial examination of colorectal carcinoma staged under the 7th edition of AJCC and its SSFs suggest that the overall stages are similar to those defined by the 6th edition. Trends suggest a decrease in stage 0 lesions that may reflect the impact of colonoscopy and that the relative prevalence of stages I-IV is similar to past reports. The addition of subcategories for T4, N1, and N2 did not shift cases between stage groups but shifted them within stage group subcategories, so that clinical outcome data may be slightly more precise. Finally, several important findings are noted from this first analysis of population-based data on the new SSFs for colorectal carcinoma: tumor deposits are present in approximately 10% of colon or rectal carcinoma; 10-12% of colorectal cancer patients had CRM less than 1 mm; about 46% of colorectal cases did not have a CEA testing or documented CEA information; and 10% of colorectal cancer patients had perineural invasion. As SEER registries follow these cohorts over the next few years, we hope to determine the impact of these clinically significant factors on relative survival. The collection of these SSFs, however, should take into consideration the current registry workload, cost, and resources.
Supplementary Material
Precis.
This chapter highlights the changes in CS for colon and rectal carcinoma as TNM moved from the AJCC 6th to the 7th edition. Significant findings include the presence of tumor deposits that resulted in upstaging 2.5% of colon and 3.3% or rectal cases from stages I/ II to stage III and that ~15% of resections had circumferential radial margins that were closer than accepted standard of practice.
Acknowledgments
This work was supported in part under NIH/NCI contract number HHSN261201000030C with Louisiana State University Health Sciences Center (VWC, MCH, BAR); NIH/NCI contract number HHSN261201000032C with University of Iowa (MEC); NIH/NCI contract number HHSN261201200422P with RiesSearch, LLC (LAR). No financial disclosures for JK, SA, JMJ
Footnotes
Disclaimer: The findings and conclusions herein are the authors’ and do not necessarily represent the official positions of their affiliations or those of the National Cancer Institute, National Institutes of Health, or the US Department of Health and Human Services.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. [August 5, 2013];SEER Cancer Statistics Review, 1975-2010. Available at: http://seer.cancer.gov/csr/1975_2010/.
- 3.Miller PE, Lazarus P, Lesko SM, et al. Meat-related compounds and colorectal cancer risk by anatomical subsite. Nutr Cancer. 2013;65:202–226. doi: 10.1080/01635581.2013.756534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phipps AI, Scoggins J, Rossing MA, Li CI, Newcomb PA. Temporal trends in incidence and mortality rates for colorectal cancer by tumor location: 1975-2007. Am J Public Health. 2012;102:1791–1797. doi: 10.2105/AJPH.2011.300393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 6.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th Ed. Springer-Verlag; Chicago: 2010. [Google Scholar]
- 8.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th Ed. Springer-Verlag; Chicago: 2002. [Google Scholar]
- 9.Fritz A, Jack A, Parkin DM, et al. International Classification of Diseases for Oncology. 3rd ed. World Health Organization; Geneva: 2000. [Google Scholar]
- 10.Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart A. Revised tumor and node categorization for rectal cancer based on Surveillance, Epidemiology, and End Results and rectal pooled analysis outcomes. J Clin Oncol. 2010;28:256–263. doi: 10.1200/JCO.2009.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas P, Toth CA, Saini KS, Jessup JM, Steele G., Jr The structure, metabolism and function of the carcinoembryonic antigen gene family. Biochim Biophys Acta. 1990;1032:177–189. doi: 10.1016/0304-419x(90)90003-j. [DOI] [PubMed] [Google Scholar]
- 12.Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 13.Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology. J Clin Oncol. 1996;14:2843–2877. doi: 10.1200/JCO.1996.14.10.2843. [DOI] [PubMed] [Google Scholar]
- 14.Cady B, Stone MD, McDermott WV, Jr., et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg. 1992;127:561–568. doi: 10.1001/archsurg.1992.01420050085011. discussion 68-69. [DOI] [PubMed] [Google Scholar]
- 15.Collaborative Stage Work Group of the American Joint Committee on Cancer . Collaborative Stage Data Collection System User Documentation and Coding Instructions, version 02.04.40. Published by American Joint Committee on Cancer; Chicago, IL: [August 5, 2013]. Available from: http://web2.facs.org/cstage0204/schemalist.html. [Google Scholar]
- 16.Gosens MJ, Klaassen RA, Tan-Go I, et al. Circumferential margin involvement is the crucial prognostic factor after multimodality treatment in patients with locally advanced rectal carcinoma. Clin Cancer Res. 2007;13:6617–6623. doi: 10.1158/1078-0432.CCR-07-1197. [DOI] [PubMed] [Google Scholar]
- 17.Guillem JG, Chessin DB, Cohen AM, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829–836. doi: 10.1097/01.sla.0000161980.46459.96. discussion 36-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Colon Cancer & Rectal cancer. 2014 doi: 10.6004/jnccn.2009.0056. Version 3.2014. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. IARC Press; Lyon, France: 2000. [Google Scholar]
- 20.American Society for Gastrointestinal Endoscopy (ASGE) [August 5, 2013];Frequently Asked Questions: Flat Polyps. Available from: http://www.asge.org/press/press.aspx?id=8144.
- 21.Parra-Blanco A, Gimeno-Garcia AZ, Nicolas-Perez D, et al. Risk for high-grade dysplasia or invasive carcinoma in colorectal flat adenomas in a Spanish population. Gastroenterol Hepatol. 2006;29:602–609. doi: 10.1016/s0210-5705(06)71700-9. [DOI] [PubMed] [Google Scholar]
- 22.Lau PC, Sung JJ. Flat adenoma in colon: two decades of debate. J Dig Dis. 2010;11:201–207. doi: 10.1111/j.1751-2980.2010.00439.x. [DOI] [PubMed] [Google Scholar]
- 23.Pox CP, Altenhofen L, Brenner H, et al. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology. 2012;142:1460–1467. e2. doi: 10.1053/j.gastro.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Cheng L, Eng C, Nieman LZ, Kapadia AS, Du XL. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol. 2011;34:573–580. doi: 10.1097/COC.0b013e3181fe41ed. [DOI] [PubMed] [Google Scholar]
- 25.Nagtegaal ID, Tot T, Jayne DG, et al. Lymph nodes, tumor deposits, and TNM: are we getting better? J Clin Oncol. 2011;29:2487–2492. doi: 10.1200/JCO.2011.34.6429. [DOI] [PubMed] [Google Scholar]
- 26.Belt EJTh, van Stijn MFM, Bril H. Lymph node negative colorectal cancers with isolated tumor deposits should be classified and treated as Stage III. Ann Surg Oncol. 2010;17:3203–3211. doi: 10.1245/s10434-010-1152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song JS, Chang HJ, Kim DY, et al. Is the N1c category of the new American Joint Committee on Cancer staging system applicable to patients with rectal cancer who receive preoperative chemoradiotherapy? Cancer. 2011;117:3917–24. doi: 10.1002/cncr.25968. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein TE, Endreseth BH, Romundstad P. Wibe A on behalf of the Norwegian Colorectal Cancer Group. Circumferential resection margin as a prognostic factor in rectal cancer. Brit J Surg. 2009;96:1348–1357. doi: 10.1002/bjs.6739. [DOI] [PubMed] [Google Scholar]
- 29.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 30.Ciardiello F, Tejpar S, Normanno N, et al. Uptake of KRAS mutation testing in patients with metastatic colorectal cancer in Europe, Latin America and Asia. Target Oncol. 2011;6(3):133–45. doi: 10.1007/s11523-011-0181-x. [DOI] [PubMed] [Google Scholar]
- 31.Morton RF, Hammond EH. ASCO Provisional Clinical Opinion: KRAS, cetuximab, and panitumumab—clinical implications in colorectal cancer. J Oncol Pract. 2009;5:71–72. doi: 10.1200/JOP.0924603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.