Abstract
The increasing prevalence of type 1 and type 2 diabetes mellitus combined with advancement in early detection of cardiovascular disease (CVD) has placed CVD as a significant concern for preventative pediatric medicine. The public health burden of type 2 diabetes is predicted to parallel increasing obesity in children with a projected increase of early CVD in adulthood. In this article, we review practice guidelines for cardiovascular health in children and adolescents with diabetes and data on which they are based. We then focus on imaging modalities that are promising tools to expand our understanding of the cardiovascular risk imposed on youths with diabetes.
Introduction
The rising prevalence of type 1 and type 2 diabetes (T1D and T2D, respectively) mellitus combined with advancement in early detection of cardiovascular disease (CVD) places future CVD complications of diabetes at the forefront of preventative pediatric medicine. CVD in diabetes patients manifests as macrovascular disease, which includes cerebrovascular events, peripheral vascular disease, cardiac dysfunction, and myocardial ischemia, and such complications are major causes of morbidity and mortality in both T1D and T2D.1–4 According to two major international registries, EURODIAB and DIAMOND, most regions in the world are seeing a steady increase in T1D, with a worldwide T1D incidence increasing by 3% per year, yielding an estimate of 65,000 newly diagnosed cases per year.5 In the United States, the prevalence of T1D was estimated by the SEARCH for Diabetes in Youth study (a multicenter study comprising six centers with multiple races and ethnicities) to be 2.28/1,000 in youths younger than 20 years of age, with 154,369 youths with diabetes in 2001.6 Similarly, the burden of T2D is predicted to worsen and parallel increasing obesity in children7 with a projected increase of early CVD in adulthood.8
Although overt CVD rarely presents during childhood, evidence from autopsy studies suggests that subclinical disease is already present as early as adolescence. The landmark U.S. Army study in 1953 looking at autopsies of young men who died during the Korean War (average age, 22 years) reported a high frequency of advanced atherosclerosis in coronary arteries.9 In a substudy of the Bogalusa Heart Study, aortic fatty streaks had a strong relationship to postmortem levels of total cholesterol and low-density lipoprotein-cholesterol (LDL-c) in those without diabetes with a mean age of death at 18 years.10 Similarly, in a large study of 2,876 subjects between 15 and 34 years old, the Patholobiological Determinants of Atherosclerosis in Youth Study11 showed that atherosclerotic processes begin as early as the late teen years in autopsy specimens. Findings of atherosclerotic lesions in adolescent patients make a powerful argument that the coronary artery disease (CAD) process begins very early in life and that risk factors need to be determined in the subclinical disease state if any impact is going to be had in decreasing early CVD.
Long-term follow-up of research cohorts into adulthood has been used to make determinations of longitudinal CVD risk in children,8 and surrogate markers have been required to substitute for clinical end points.12 Inherent to this approach is the uncertainty of the relationship between surrogate markers and CVD events. That is, surrogate markers may be associated with a disease but not be part of the pathophysiology of the disease. Furthermore, although surrogate markers are often used to determine the effect of an intervention, they may not be sensitive to the intervention or are part of a pathway that is not affected by the intervention.13 The limitations of surrogates must be understood to manage and stratify risk for CVD. Surrogates may be biomarkers, structural markers, or functional markers. Biomarkers include measures of glycemia (hemoglobin A1c [HbA1c]), lipoprotein metabolism (lipid panel), and inflammation (high-sensitivity C-reactive protein). Structural markers may be echocardiographic and computed tomography (CT) assessment of coronary artery calcification (CAC). Examples of functional markers are noninvasive measurement of endothelial function with pulse-wave velocity, augmentation index, and brachial artery dilation. All surrogates inadequately predict cardiovascular events, but some such as LDL-c14,15 have been shown in large studies to strongly predict CAD.
In this article, we review practice guidelines for cardiovascular health in children and adolescents with diabetes and the data on which they are based. Whenever possible and for the ease of comprehension, we will discuss T1D patients first under each topic, followed by T2D patients. We then focus on imaging modalities that are very promising tools to expand our understanding of the impact of CVD on youths with diabetes.
Burden of CVD
T1D
Historically, the estimated cumulative mortality rate from CAD in T1D patients is 35% by 55 years of age compared with 4–8% for subjects without diabetes,16 making it the most frequent cause of mortality in people with T1D.17,18 In the third decade after the diagnosis of T1D, CVD accounts for 67% of deaths.19
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study found that intensive glycemic control in T1D patients decreased the risk of CVD events by 57%.20 This study provides compelling evidence to support targeting glycemic control in an effort to reduce macrovascular disease. Similarly, the Pittsburgh Epidemiology of Diabetes Complications Study21 found that each 1% decrease in HbA1c was associated with a decrease in CAD by 23% (defined as angina, myocardial infarction, ischemia, revascularization, or fatal CAD).
T2D
Obese children are at risk for developing metabolic syndrome, which includes insulin resistance and glucose intolerance, and are at a high risk for becoming obese adults with progression to T2D.22 The United Kingdom Prospective Diabetes Study followed a cohort of 5,102 T2D patients over 20 years and found that intensive glycemic control was associated with a 16% reduction in fatal and nonfatal myocardial infarction, although this was not quite statistically significant.23
Pathophysiology
T1D
In T1D youths with suboptimal HbA1c, lipid profiles are higher than in control subjects, whereas those with optimal HbA1c have similar or even less atherogenicity than youths without diabetes.24 Nonetheless, T1D patients as a whole are susceptible to oxidative damage and have high-density lipoprotein (HDL)-cholesterol (HDL-c) dysfunction.25 Elevated apolipoprotein B levels and small, dense LDL-c particles were observed in T1D patients compared with controls without diabetes regardless of their glycemic control,24 suggesting that even mild hyperglycemia can be associated with atherogenic lipoprotein changes.26 Although less commonly considered a component of T1D, insulin resistance is part of the pathophysiology of T1D27 and likely contributes to the increased CVD in T1D. Additionally, inflammation, fibrosis, mitochondrial dysfunction, and autonomic disorder have been hypothesized as potential causes of cardiac dysfunction in T1D.28,29 Cardiac dysfunction is defined as impaired systolic or diastolic function in the absence of clear ischemic heart disease.30
T2D
The promotion of atherogenesis in T2D is a complex process involving compensatory mechanisms for hyperglycemia and elevated circulating free fatty acids and inflammation.23 Increased abdominal adiposity stimulates the liver to increase production of triglyceride-rich lipoproteins and a decrease in HDL, all of which lead to the dyslipidemia seen in T2D patients. Adipocytes stimulate a cascade of inflammatory response beginning with tumor necrosis factor-α, which stimulates the liver to produce C-reactive protein, fibrinogen, and plasminogen activator inhibiter-1, with the last two promoting thrombosis.23
Furthermore, in both forms of diabetes, glucose reacts with protein molecules forming conjugates called advanced glycation end-products, which when stimulated further activate inflammatory responses from endothelial cells, smooth muscle cells, and macrophages, all of which are involved in atherogenesis.23 As a consequence of hyperglycemia-induced activation of protein kinase C, pro-inflammatory gene expression increases, which in turn increase CVD risk. Also involved are blood flow abnormalities, vascular permeability, angiogenesis, and capillary occlusion.31
Risk Factors
Among the list of risk factors of early onset of atherosclerosis in the pediatric population, the American Diabetes Association (ADA) panel included diabetes, along with elevated LDL-c, family history of premature CVD or peripheral vascular disease, smoking, hypertension, HDL-c <35 mg/dL, obesity (body mass index [BMI] ≥95%), and physical inactivity.32
Dyslipidemia
T1D children with poorly controlled glucose are prone to lipid abnormalities, as previously discussed.32 In T2D, similar to obese patients, lipid profiles are often characterized by an elevated triglyceride level, normal to elevated LDL-c levels, and decreased HDL-c levels. Studies in T2D adult populations have suggested the condition is also associated with lipid and lipoprotein abnormalities such as small, dense LDL-c particles,33,34 which are associated with increased CVD risk.24 The association between lipid abnormalities and diabetes places both T1D and T2D children at risk for development of early CVD. In fact, the longitudinal Bogalusa35 and Muscatine36 studies indicate that children with increased CVD risk factors, including dyslipidemia, are also at increased CVD risk as adults. It is important that the Bogalusa Heart study showed correlation of LDL-c, blood pressure (BP), BMI, and fasting insulin with aortic and coronary fatty streaks with autopsy results in teens.35
Other factors
Lipid abnormalities are just one of many factors that increase the risk of CVD in both T1D and T2D pediatric patients. Traditional cardiovascular risk factors in T1D patients include duration of diabetes, hypertension, smoking, inflammatory markers, and renal disease.37 Among pediatric T2D studies the mean age of diagnosis of T2D is 13.5 years—also the period of peak physiologic pubertal insulin resistance, which contributes to the onset of dysglycemia.38 Hypertension is also seen in T2D patients, ranging from 17% to 32% in published reports.39 Among children and adolescents with increased CVD risk factors, there is often a strong family history of early CVD.39 It is surprising that in the SEARCH for Diabetes in Youth study, among 3,466 T1D and T2D youths, tobacco use was reported in 17% of 15–19-year-old and 34% of ≥20-year-old T1D subjects and 16% of 15–19-year-old and 40% of ≥20-year-old T2D subjects.40 Tobacco use was associated with higher odds of elevated triglyceride levels and physical inactivity.40
Guidelines for Managing Hypertension, Dyslipidemia, and Other CVD Risk Factors in Children and Adolescents with Diabetes
Dyslipidemia
The ADA consensus statement outlined the standard approach to managing dyslipidemia in all types of children and adolescents with diabetes.32,41 Initial screening was recommended after achieving glycemic control and differs for T1D versus T2D.
T1D
In the T1D group, those without a family history of early CVD or unknown family history and who are ≥12 years or at the onset of puberty should have screening at diagnosis once achieving glycemic control. This should be repeated every 5 years if the initial screen is normal. If family history is of concern, T1D children ≥2 years of age should have a fasting lipid profile soon after diagnosis and after achieving glucose control. If lipid screening is abnormal, annual screening is recommended.
T2D
For T2D patients, a lipid profile should be obtained at diagnosis and every 2 years if it is normal.
The targets for lipid management for all children with diabetes as defined by the ADA32,41 and the American Heart Association (AHA)42 are LDL-c <100 mg/dL, HDL >35 mg/dL, and triglycerides <150 mg/dL. Both the ADA and AHA emphasize integration of appropriate diets and weight reduction and minimizing other risk factors like hypertension, smoking, obesity, and inactivity before initiating medical treatment. Medications can be considered based on the child's age, other CVD risk factors in addition to LDL-c levels, and family history according to published guidelines.
BP
T1D
A statement by the AHA in 2006 stratified diabetes patients by risk of atherosclerosis in order to guide management of comorbidities.39 T1D patients are in the high-risk Tier I secondary to the earlier age of diagnosis, the more severe degree of hyperlipidemia, and presence of symptoms. If the BP is ≥90th percentile, initial therapy consists of a reduced salt diet for 6 months with increased physical activity. If systolic BP remains >95%, angiotensin converting enzyme inhibitors are recommended to keep the BP <90th percentile or <130/80 mm Hg, whichever is lower. The ADA also recommended that systolic BP should be <130 mm Hg with initial change in diet and increase in activity to achieve this prior to considering medications.41,43
T2D
Per the AHA,39 T2D patients are classified in the moderate-risk Tier II. If the BP is between 90% and 95% or >120/80 mm Hg, a decrease in calories and an increase in activity level change are recommended. Pharmaceutical therapy is recommended if the BP remains >95% after 6 months. In these patients, obesity is an important consideration, and appropriate diet is recommended to keep the BMI <95%. However, if T2D adolescents have more than two additional CVD risk factors, which is common, they are considered to be in the high-risk Tier I. Additional research will help clarify the relative risks of CVD for T1D versus T2D in youth.
Other CVD risk factors
The National Heart, Lung, and Blood Institute Expert Panel published comprehensive guidelines in 2011 aimed at primary care providers to identify and manage cardiovascular risk factors in children and adolescents 0–21 years old.44 The two goals from these guidelines were (1) to prevent the development of risk factors and (2) to manage risk factors in order to prevent future CVD. Within these guidelines are specific recommendations for appropriate diets and activity level, tobacco exposure, lipid screening, and BP management.
Shifting paradigm
Two recent major intervention trials, the Action to Control Cardiovascular Risk in Diabetes (ACCORD study)45 and the Action in Diabetes and Vascular Disease (ADVANCE trial),46 question how intensive treatment of glycemia should be in T2D patients. Because these studies are in adults, how these data are extrapolated to youth with diabetes is uncertain. Tight glycemic control and meeting CVD targets remain consensus goals in pediatric diabetes.
In the ACCORD trial,45 10,251 subjects with a median T2D duration of 10 years and mean age of 62 years were enrolled to determine if intensive management would decrease the risk of cardiovascular events. The three goals for the intensive management group were (1) glycemia with HbA1c <6.0%, (2) hypertension with systolic BP <119 mm Hg, and (3) triglyceride and HDL, with goal triglyceride of 22 mg/dL lower and HDL 1 mg/dL higher than the control arm. The study showed an excess in the number of CVD deaths and nonfatal myocardial infarction in the intervention compared with the control group. This study suggests that the pathophysiology of CVD in T2D is not fully understood and that extrapolation of these data to pediatric patients is uncertain.
Cardiovascular Imaging
The recent explosion of advanced noninvasive imaging modalities has allowed further understanding of the early involvement of the heart and vasculature in the diabetes state with future potential clinical application. Direct evidence of disease even in the subclinical disease period is possible. These modalities used for determining vascular function include plethysmography of the brachial artery to determine flow-mediated dilation and measures of arterial stiffness including pulse-wave velocity, augmentation index, and brachial distensibility. Imaging surrogates of atherosclerosis include carotid intima-media thickness (CIMT) and CAC determination by CT.28 The DCCT/EDIC study has provided important data on the use of cardiovascular imaging, including CIMT47 and CAC,48 as surrogates for CVD in T1D.
Both cardiac and vascular dysfunctions are reported in T1D adults.28,49 Diabetic cardiomyopathy, defined as cardiac dysfunction in diabetes patients independent of coronary artery disease or hypertension, involves progression of diastolic to systolic dysfunction.50 Although systolic function is often not affected in adolescents, diastolic dysfunction was found to be evident in young insulin-dependent patients independent of disease duration.49 Vascular dysfunction also is present as early as adolescence in T1D and is in fact dependent on disease duration.51 Here, we define vascular dysfunction as increased vascular stiffness and endothelial dysfunction in which the endothelium changes towards a vasoconstrictor, pro-thrombotic, and pro-inflammatory state.52 Endothelial dysfunction therefore plays a critical role in the development of atherosclerotic disease.53
Plethysmography
Venous occlusion plethysmography involves external occlusion of an extremity and determining change in the local blood flow as a reflection of peripheral resistance. In both T1D and T2D adolescents, this was reduced compared with normal subjects.54,55 Further study demonstrated that abnormal plethysmography was an independent predictor of decreased maximal O2 uptake in T1D youths,55 indicating reduced exercise capacity in these patients as well.
Brachial artery ultrasound to determine flow-mediated dilation
Flow-mediated dilation studies such as brachial reactivity is performed by exposing local vasculature to ischemia and monitoring its nitric oxide-mediated vasodilation as reflected by increased flow. Studies show impaired endothelium-dependent flow-mediated dilation, indicating vascular dysfunction in T1D youths56 and specifically in children and adolescents with positive family history for CVD, familial hyperlipidemia, and obesity.57
Arterial stiffness
Arterial stiffness, defined as the reciprocal of distensibility, or the elasticity of a vessel,57 is an independent predictor of cardiovascular mortality in adults (Fig. 1).58 Factors that affect this property are arterial pressure, stroke volume, and vascular resistance. Imaging techniques used to measure vascular stiffness include arterial pressure waveforms, change in the diameter of an artery compared with the distending pressure, and pulse-wave velocity.57,58 This last technique is the reference for determining arterial stiffness in the adult population, but more data in young diabetes patients are needed.59 In a SEARCH substudy of 535 subjects with T1D (mean age, 17.8 years) there was lower brachial distensibility and higher augmentation index compared with patients with no diabetes, both suggesting increased arterial stiffness.59 It is interesting that in this group, males were more likely to have vascular abnormalities. This finding of increased augmentation index was found in two other studies of T1D youths60,61 and one of T2D youths.62
FIG. 1.
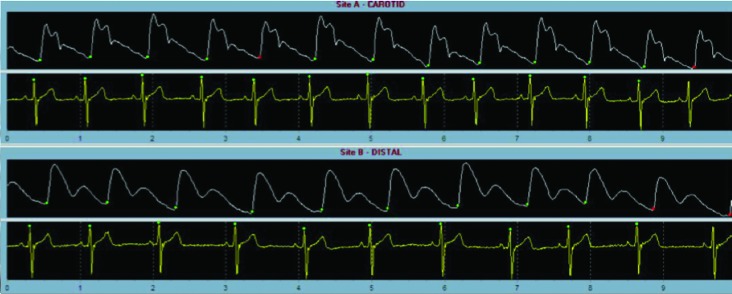
Pulse-wave velocity of the carotid and femoral arteries assessing arterial stiffness. This technique measures the speed of pressure propagation through a vessel. The speed is dependent on shape and elastic properties of the vessel.
CIMT
In adult studies, CIMT is a marker of preclinical atherosclerosis and correlates with cardiovascular risk factors. CIMT predicts CVD and is associated with the severity of CAD in adults.63–65 In a small study of 31 teenagers with T1D (mean age, 15 years), diabetes patients were found to have normal CIMT but significantly decreased endothelium-dependent vasodilation. This supports the idea that endothelial dysfunction occurs early after the diagnosis of T1D, even before any changes are seen in CIMT.66 In another study of 41 children with T1D (mean age, 11 years), 36% had evidence of endothelial dysfunction defined as total brachial artery flow-mediated response, significantly increased CIMT, and higher LDL-c concentration.67
Although these data demonstrate differences early in the course of T1D, several concerns for interpretation of CIMT in pediatrics have been raised. This includes understanding the effect of body size difference, age, ethnicity, sex, and the cost-effectiveness for identifying high-risk youths as well as methodologic challenges with reproducibility for longitudinal studies.57
Electron-beam CT or spiral or helical CT
Electron-beam CT or spiral or helical CT measures CAC in coronary vessels and predicts future coronary events (Fig. 2).56,68 In the Coronary Artery Calcification of Type 1 Diabetes (CACTI) study of 652 adults with T1D (mean age of 37 years, T1D duration of 23 years), T1D was associated with 3.5-fold increased odds of having positive CAC compared with controls without diabetes.27,69 In younger people with T1D, 17–28 years old, 11% had CAC.70 However, measuring CAC exposes patients to significant radiation and is not an appropriate screening test for pediatric patients. Unfortunately, there are no current biomarkers or imaging techniques that may serve as an alternative for such early signs of CAD in adolescents.71
FIG. 2.
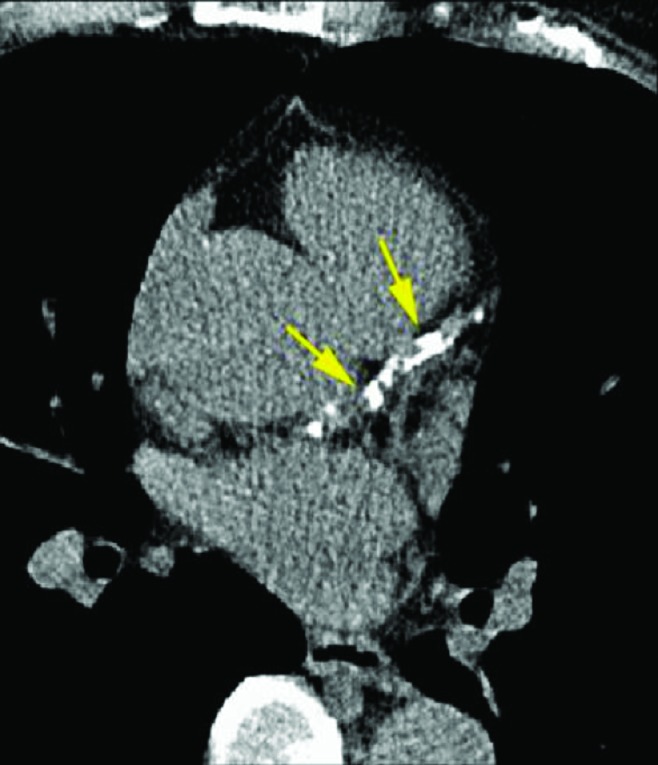
Coronary tomography in a patient with positive evidence of calcification in the coronary arteries (identified by the arrows).
Echocardiogram
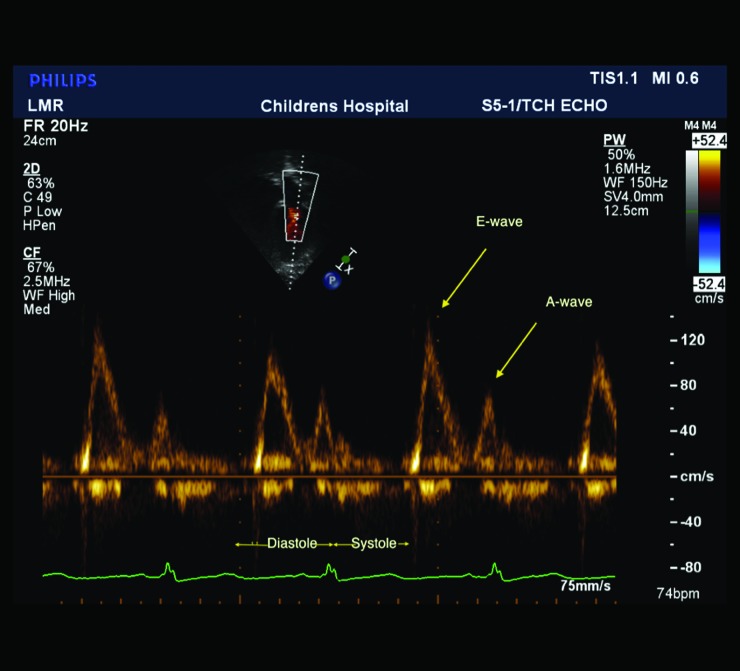
Cardiac dysfunction has been shown in adult diabetes patients by echocardiographic findings.72,73 A few studies have been published in children and adolescents that suggest early findings of cardiac dysfunction, although these studies have small numbers of patients, and these differences may not be clinically significant. T1D patients, 12–18 years old, had similar left ventricular dimensions but increased septal thickness, compared with control patients without diabetes.74 The mitral E-wave (early ventricular diastolic filling) velocity, a preload-dependent parameter, has been shown in adults to correlate with left ventricular filling pressures75 (Fig. 3). The E:A (A-wave represents atrial contraction) ratio is used to reflect relaxation impairment also. Comparison of T1D patients (mean age, 18.5 years) with subjects without diabetes showed no difference in cardiac systolic function.76 However, T1D patients with microalbuminuria and/or retinopathy showed a statistically lower E:A ratio and E-wave reflecting decreased diastolic function.76
FIG. 3.
Mitral inflow spectral Doppler in an obese adolescent female. The E-wave represents the early diastolic passive flow into the ventricle, and the A-wave represents atrial contraction. The E:A wave ratio is above 1.0 in patients with normal diastolic function.
In one study, normotensive, normoalbuminuric T1D adolescents (mean age of 17.3 years, T1D duration of 8.5 years) had decreased nocturnal BP change, decreased heart rate variability, and left ventricular end-diastolic and end-systolic diameter, suggesting compromised autonomic function and impaired left ventricular function.77 T1D children also have a larger Tei index, which is the addition of isovolumic contraction and isovolumic relaxation divided by the ejection time and is used to reflect global cardiac function.75
In obese children with BMI ≥25 kg/m2, diastolic dysfunction has been suggested with the E:A ratio being significantly lower in the obese children and inversely related to the BMI.78 Hyperinsulinemia and insulin resistance have been associated with abnormal myocardial collagen to muscle ratio and may contribute to the observed diastolic dysfunction.79 Support for this comes from rat models of T2D in which the prediabetes state is associated with left ventricular fibrosis and diastolic changes.80
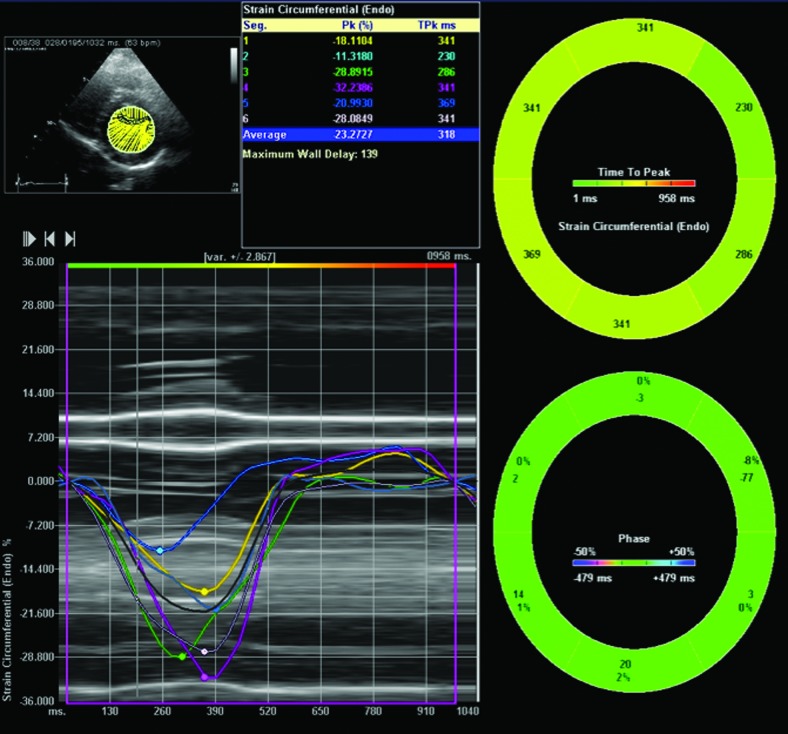
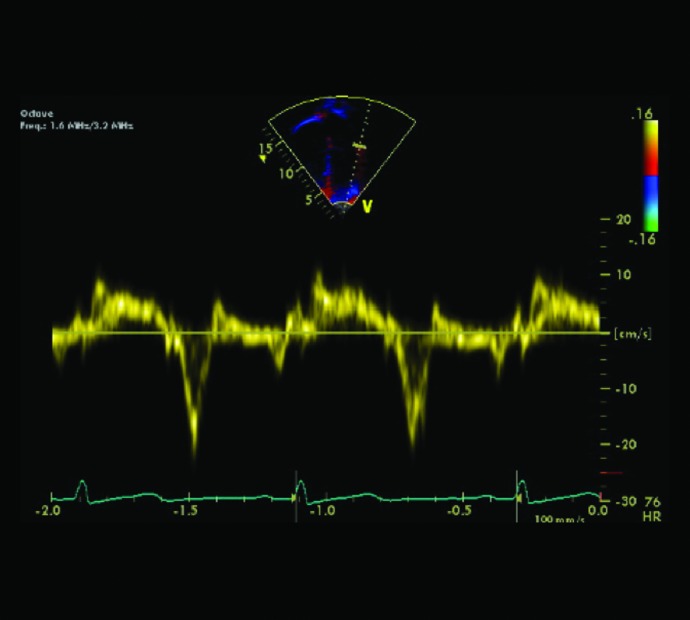
Speckle tracking imaging is an echocardiographic-based, angle-independent technique that tracks regions of myocardium over time to determine deformation (Fig. 4). Conventional echocardiogram, tissue Doppler imaging (Fig. 5), and speckle tracking imaging of 47 children and adolescents with T1D (mean age of 12.8 years, mean T1D duration of 3.4 years) were compared with those of pediatric subjects without diabetes.81 There were no differences in any of the speckle tracking parameters, including strain or strain rate (indicators of contractility). In addition, there were no differences in tissue Doppler indices of diastolic or systolic function, but there was correlation between diastolic parameters and HbA1c, suggesting that diastolic dysfunction in children with T1D is accelerated by poor glycemic control.81
FIG. 4.
Global and regional strain as determined by speckle tracking through a short axis view of the left ventricle at the level of the papillary muscle in an obese 15-year-old female.
FIG. 5.
Tissue Doppler of the mitral lateral wall to determine the E’:a’ ratio, which reflects diastolic function. HR, heart rate.
Novel imaging techniques
More recently studied imaging modalities have been used in diabetes patients, but with fewer data in the pediatric population. Intracoronary ultrasound in T1D patients (mean age, 43 years) showed that 71% of asymptomatic T1D patients had a mean plaque area of >40% compared with only 33% of age-matched heart transplant controls (P<0.0001).82 In the DCCT/EDIC study, 1,017 T1D patients (mean age, 49 years) underwent cardiac magnetic resonance imaging (MRI) with gadolinium. HbA1c and macroalbuminemia were associated with left ventricular structure abnormality as well as myocardial scar.83
MRI also enables assessment of the carotid arteries and the extent of plaques. In a small study of adult T2D patients, carotid MRI showed that T2D patients had higher risk of developing vulnerable carotid artery plaques (odds ratio, 2.59; 95% confidence interval, 1.15–5.81).84 Although neither intracoronary ultrasound nor MRI exposes subjects to radiation, which is a critical factor to consider in pediatric patients, these studies often require general anesthesia for pediatric patients. With the advent of faster MRI scan sequences, MRI application to children will become more practical.
Conclusions
Diabetes poses a significant risk, which starts as early as in adolescence, for lifelong cardiovascular complications. As the numbers of children and adolescents with T1D and T2D continue to rise at a dramatic rate, there is a tremendous potential economic and social burden of early CVD in these pediatric patients, who have an earlier onset and potentially longer duration of diabetes. Intensive control of hyperglycemia has been shown to decrease these risks; however, data on the treatment of CVD risk factors in childhood to improve health outcomes will require long-term follow-up into adulthood. Prevention of developing CVD risk factors will likely be a cost-saving approach and decrease morbidity and mortality.
Pediatric guidelines for management and treatment are often opinions from expert panels or extrapolated from adult studies because of insufficient data in youths with diabetes. In the interest of improving long-term health outcomes of these children, an aim of research is to provide pediatric-specific data to advance the understanding of the effect of diabetes on CVD risk in order to identify targets for early intervention. These may differ based on factors such as age, gender, and race. Advanced imaging technology may also help identify patients who are at particularly high risk early and, therefore, tailor the management approach. The noninvasive nature of such imaging modalities, as well as its immediate assessment of atherosclerosis, will be instrumental as we move forward in preventing early CVD in children and adolescents with diabetes. Rather than focusing on risk factors of CVD, noninvasive imaging may allow the treatment focus to be redirected to evidence of end-organ damage. Vascular imaging recommendations by the AHA are aimed at standardization of research in children at risk for atherosclerosis.57 As yet, there is no recommendation of cardiovascular imaging as part of the standard management of children with diabetes, and it remains a research tool. However, its full potential to play a critical role in determining risk factors and guiding treatment has yet to be recognized.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Meigs JB. Epidemiology of cardiovascular complications in type 2 diabetes mellitus. Acta Diabetol. 2003;40(Suppl 2):S358–S361. doi: 10.1007/s00592-003-0120-0. [DOI] [PubMed] [Google Scholar]
- 2.Donahue RP. Orchard TJ. Diabetes mellitus, macrovascular complications. An epidemiological perspective. Diabetes Care. 1992;15:1141–1155. doi: 10.2337/diacare.15.9.1141. [DOI] [PubMed] [Google Scholar]
- 3.Laing SP. Swerdlow AJ. Slater SD. Botha JL. Burden AC. Waugh NR. Smith AW. Hill RD. Bingley PJ. Patterson CC. Qiao Z. Keen H. The British Diabetic Association Cohort Study. II: Cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabet Med. 1999;16:466–471. doi: 10.1046/j.1464-5491.1999.00076.x. [DOI] [PubMed] [Google Scholar]
- 4.Mahgoub MA. Abd-Elfattah AS. Diabetes mellitus and cardiac function. Mol Cell Biochem. 1998;180:59–64. [PubMed] [Google Scholar]
- 5.Soltesz G. Patterson CC. Dahlquist G. Worldwide childhood type 1 diabetes incidence—what can we learn from epidemiology? Pediatr Diabetes. 2007;8(Suppl 6):6–14. doi: 10.1111/j.1399-5448.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 6.Liese AD. D'Agostino RB., Jr Hamman RF. Kilgo PD. Lawrence JM. Liu LL. Loots B. Linder B. Marcovina S. Rodriguez B. Standiford D. Williams DE. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL. Carroll MD. Curtin LR. McDowell MA. Tabak CJ. Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 8.Nadeau KJ. Maahs DM. Daniels SR. Eckel RH. Childhood obesity and cardiovascular disease: links and prevention strategies. Nat Rev Cardiol. 2011;8:513–525. doi: 10.1038/nrcardio.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enos WF. Holmes RH. Beyer J. Coronary disease among United States soldiers killed in action in Korea; preliminary report. JAMA. 1953;152:1090–1093. doi: 10.1001/jama.1953.03690120006002. [DOI] [PubMed] [Google Scholar]
- 10.Newman WP., 3rd Freedman DS. Voors AW. Gard PD. Srinivasan SR. Cresanta JL. Williamson GD. Webber LS. Berenson GS. Relation of serum lipoprotein levels, systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med. 1986;314:138–144. doi: 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- 11.Strong JP. Malcom GT. McMahan CA. Tracy RE. Newman WP., 3rd Herderick EE. Cornhill JF. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999;281:727–735. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- 12.Tardif JC. Heinonen T. Orloff D. Libby P. Vascular biomarkers and surrogates in cardiovascular disease. Circulation. 2006;113:2936–2942. doi: 10.1161/CIRCULATIONAHA.105.598987. [DOI] [PubMed] [Google Scholar]
- 13.Fleming TR. DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein JL. Hazzard WR. Schrott HG. Bierman EL. Motulsky AG. Hyperlipidemia in coronary heart disease. I. Lipid levels in 500 survivors of myocardial infarction. J Clin Invest. 1973;52:1533–1543. doi: 10.1172/JCI107331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castelli WP. Garrison RJ. Wilson PW. Abbott RD. Kalousdian S. Kannel WB. Incidence of coronary heart disease, lipoprotein cholesterol levels. The Framingham study. JAMA. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 16.Krolewski AS. Kosinski EJ. Warram JH. Leland OS. Busick EJ. Asmal AC. Rand LI. Christlieb AR. Bradley RF. Kahn CR. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–755. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 17.Laing SP. Swerdlow AJ. Slater SD. Burden AC. Morris A. Waugh NR. Gatling W. Bingley PJ. Patterson CC. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46:760–765. doi: 10.1007/s00125-003-1116-6. [DOI] [PubMed] [Google Scholar]
- 18.Dorman JS. Laporte RE. Kuller LH. Cruickshanks KJ. Orchard TJ. Wagener DK. Becker DJ. Cavender DE. Drash AL. The Pittsburgh Insulin-Dependent Diabetes Mellitus (IDDM) Morbidity, Mortality Study. Mortality results. Diabetes. 1984;33:271–276. doi: 10.2337/diab.33.3.271. [DOI] [PubMed] [Google Scholar]
- 19.Orchard TJ. From diagnosis, classification to complications, therapy. DCCT. Part II? Diabetes Control and Complications Trial. Diabetes Care. 1994;17:326–338. doi: 10.2337/diacare.17.4.326. [DOI] [PubMed] [Google Scholar]
- 20.Nathan DM. Cleary PA. Backlund JY. Genuth SM. Lachin JM. Orchard TJ. Raskin P. Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prince CT. Becker DJ. Costacou T. Miller RG. Orchard TJ. Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes mellitus: findings from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC) Diabetologia. 2007;50:2280–2288. doi: 10.1007/s00125-007-0797-7. [DOI] [PubMed] [Google Scholar]
- 22.Must A. Jacques PF. Dallal GE. Bajema CJ. Dietz WH. Long-term morbidity, mortality of overweight adolescents. A follow-up of the harvard growth study of 1922 to 1935. N Engl J Med. 1992;327:1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 23.Libby P. Plutzky J. Diabetic macrovascular disease: the glucose paradox? Circulation. 2002;106:2760–2763. doi: 10.1161/01.cir.0000037282.92395.ae. [DOI] [PubMed] [Google Scholar]
- 24.Guy J. Ogden L. Wadwa RP. Hamman RF. Mayer-Davis EJ. Liese AD. D'Agostino R., Jr Marcovina S. Dabelea D. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: The SEARCH for Diabetes in Youth case-control study. Diabetes Care. 2009;32:416–420. doi: 10.2337/dc08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orchard TJ. Costacou T. When are type 1 diabetic patients at risk for cardiovascular disease? Curr Diabetes Rep. 2010;10:48–54. doi: 10.1007/s11892-009-0089-3. [DOI] [PubMed] [Google Scholar]
- 26.Ebara T. Conde K. Kako Y. Liu Y. Xu Y. Ramakrishnan R. Goldberg IJ. Shachter NS. Delayed catabolism of apoB-48 lipoproteins due to decreased heparan sulfate proteoglycan production in diabetic mice. J Clin Invest. 2000;105:1807–1818. doi: 10.1172/JCI8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schauer IE. Snell-Bergeon JK. Bergman BC. Maahs DM. Kretowski A. Eckel RH. Rewers M. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011;60:306–314. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadeau KJ. Reusch JE. Cardiovascular function/dysfunction in adolescents with type 1 diabetes. Curr Diabetes Rep. 2011;11:185–192. doi: 10.1007/s11892-011-0180-4. [DOI] [PubMed] [Google Scholar]
- 29.Hassouna A. Loubani M. Matata BM. Fowler A. Standen NB. Galinanes M. Mitochondrial dysfunction as the cause of the failure to precondition the diabetic human myocardium. Cardiovasc Res. 2006;69:450–458. doi: 10.1016/j.cardiores.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Trost S. LeWinter M. Diabetic cardiomyopathy. Curr Treat Options Cardiovasc Med. 2001;3:481–492. doi: 10.1007/s11936-001-0022-9. [DOI] [PubMed] [Google Scholar]
- 31.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association: Management of dyslipidemia in children and adolescents with diabetes. Diabetes Care. 2003;26:2194–2197. doi: 10.2337/diacare.26.7.2194. [DOI] [PubMed] [Google Scholar]
- 33.Betteridge DJ. Diabetic dyslipidemia. Am J Med. 1994;96:25S–31S. doi: 10.1016/0002-9343(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 34.Siegel RD. Cupples A. Schaefer EJ. Wilson PW. Lipoproteins, apolipoproteins, and low-density lipoprotein size among diabetics in the Framingham Offspring Study. Metabolism. 1996;45:1267–1272. doi: 10.1016/s0026-0495(96)90246-2. [DOI] [PubMed] [Google Scholar]
- 35.Berenson GS. Srinivasan SR. Bao W. Newman WP., 3rd Tracy RE. Wattigney WA. Association between multiple cardiovascular risk factors, atherosclerosis in children, young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 36.Lauer RM. Clarke WR. Use of cholesterol measurements in childhood for the prediction of adult hypercholesterolemia. The Muscatine Study. JAMA. 1990;264:3034–3038. [PubMed] [Google Scholar]
- 37.Orchard TJ. Forrest KY. Kuller LH. Becker DJ. Lipid and blood pressure treatment goals for type 1 diabetes: 10-year incidence data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2001;24:1053–1059. doi: 10.2337/diacare.24.6.1053. [DOI] [PubMed] [Google Scholar]
- 38.Rosenbloom AL. Silverstein JH. Amemiya S. Zeitler P. Klingensmith GJ. Type 2 diabetes in children and adolescents. Pediatr Diabetes. 2009;10(Suppl 12):17–32. doi: 10.1111/j.1399-5448.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- 39.Kavey RE. Allada V. Daniels SR. Hayman LL. McCrindle BW. Newburger JW. Parekh RS. Steinberger J. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds K. Liese AD. Anderson AM. Dabelea D. Standiford D. Daniels SR. Waitzfelder B. Case D. Loots B. Imperatore G. Lawrence JM. Prevalence of tobacco use and association between cardiometabolic risk factors and cigarette smoking in youth with type 1 or type 2 diabetes mellitus. J Pediatr. 2011;158:594–601. doi: 10.1016/j.jpeds.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Diabetes Association: Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCrindle BW. Urbina EM. Dennison BA. Jacobson MS. Steinberger J. Rocchini AP. Hayman LL. Daniels SR. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation. 2007;115:1948–1967. doi: 10.1161/CIRCULATIONAHA.107.181946. [DOI] [PubMed] [Google Scholar]
- 43.Kendall DM. Blood pressure targets in diabetes: is this the time for change? J Clin Hypertens (Greenwich) 2011;13:258–262. doi: 10.1111/j.1751-7176.2011.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Summary report. Pediatrics. 2011;128(Suppl 5):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genuth S. Ismail-Beigi F. Clinical implications of the ACCORD trial. J Clin Endocrinol Metab. 2012;97:41–48. doi: 10.1210/jc.2011-1679. [DOI] [PubMed] [Google Scholar]
- 46.Hamet P. What matters in ADVANCE and ADVANCE-ON. Diabetes Obes Metab. 2012;14(Suppl 1):20–29. doi: 10.1111/j.1463-1326.2011.01509.x. [DOI] [PubMed] [Google Scholar]
- 47.Nathan DM. Lachin J. Cleary P. Orchard T. Brillon DJ. Backlund JY. O'Leary DH. Genuth S. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–2303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cleary PA. Orchard TJ. Genuth S. Wong ND. Detrano R. Backlund JY. Zinman B. Jacobson A. Sun W. Lachin JM. Nathan DM. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes. 2006;55:3556–3565. doi: 10.2337/db06-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zarich SW. Arbuckle BE. Cohen LR. Roberts M. Nesto RW. Diastolic abnormalities in young asymptomatic diabetic patients assessed by pulsed Doppler echocardiography. J Am Coll Cardiol. 1988;12:114–120. doi: 10.1016/0735-1097(88)90364-6. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X. Chen C. A new insight of mechanisms, diagnosis, treatment of diabetic cardiomyopathy. Endocrine. 2012 Feb 12; doi: 10.1007/s12020-012-9623-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Ce GV. Rohde LE. da Silva AM. Punales MK. de Castro AC. Bertoluci MC. Endothelial dysfunction is related to poor glycemic control in adolescents with type 1 diabetes under 5 years of disease: evidence of metabolic memory. J Clin Endocrinol Metab. 2011;96:1493–1499. doi: 10.1210/jc.2010-2363. [DOI] [PubMed] [Google Scholar]
- 52.Beckman JA. Creager MA. Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 53.Giannini C. Mohn A. Chiarelli F. Kelnar CJ. Macrovascular angiopathy in children and adolescents with type 1 diabetes. Diabetes Metab Res Rev. 2011;27:436–460. doi: 10.1002/dmrr.1195. [DOI] [PubMed] [Google Scholar]
- 54.Nadeau KJ. Zeitler PS. Bauer TA. Brown MS. Dorosz JL. Draznin B. Reusch JE. Regensteiner JG. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94:3687–3695. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nadeau KJ. Regensteiner JG. Bauer TA. Brown MS. Dorosz JL. Hull A. Zeitler P. Draznin B. Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95:513–521. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L. Jerosch-Herold M. Jacobs DR., Jr. Shahar E. Detrano R. Folsom AR. Coronary artery calcification and myocardial perfusion in asymptomatic adults: the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2006;48:1018–1026. doi: 10.1016/j.jacc.2006.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urbina EM. Williams RV. Alpert BS. Collins RT. Daniels SR. Hayman L. Jacobson M. Mahoney L. Mietus-Snyder M. Rocchini A. Steinberger J. McCrindle B. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–950. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 58.Sutton-Tyrrell K. Najjar SS. Boudreau RM. Venkitachalam L. Kupelian V. Simonsick EM. Havlik R. Lakatta EG. Spurgeon H. Kritchevsky S. Pahor M. Bauer D. Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 59.Urbina EM. Wadwa RP. Davis C. Snively BM. Dolan LM. Daniels SR. Hamman RF. Dabelea D. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site, sex: The SEARCH for Diabetes in Youth Study. J Pediatr. 2010;156:731–737. doi: 10.1016/j.jpeds.2009.11.011. 737.e1. [DOI] [PubMed] [Google Scholar]
- 60.Haller MJ. Samyn M. Nichols WW. Brusko T. Wasserfall C. Schwartz RF. Atkinson M. Shuster JJ. Pierce GL. Silverstein JH. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care. 2004;27:2911–2917. doi: 10.2337/diacare.27.12.2911. [DOI] [PubMed] [Google Scholar]
- 61.Brooks B. Molyneaux L. Yue DK. Augmentation of central arterial pressure in type 1 diabetes. Diabetes Care. 1999;22:1722–1727. doi: 10.2337/diacare.22.10.1722. [DOI] [PubMed] [Google Scholar]
- 62.Brooks BA. Molyneaux LM. Yue DK. Augmentation of central arterial pressure in type 2 diabetes. Diabet Med. 2001;18:374–380. doi: 10.1046/j.1464-5491.2001.00479.x. [DOI] [PubMed] [Google Scholar]
- 63.O'Leary DH. Polak JF. Kronmal RA. Manolio TA. Burke GL. Wolfson SK., Jr Carotid-artery intima, media thickness as a risk factor for myocardial infarction, stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 64.Azen SP. Mack WJ. Cashin-Hemphill L. LaBree L. Shircore AM. Selzer RH. Blankenhorn DH. Hodis HN. Progression of coronary artery disease predicts clinical coronary events. Long-term follow-up from the Cholesterol Lowering Atherosclerosis Study. Circulation. 1996;93:34–41. doi: 10.1161/01.cir.93.1.34. [DOI] [PubMed] [Google Scholar]
- 65.Chambless LE. Heiss G. Folsom AR. Rosamond W. Szklo M. Sharrett AR. Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 66.Singh TP. Groehn H. Kazmers A. Vascular function and carotid intimal-medial thickness in children with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 2003;41:661–665. doi: 10.1016/s0735-1097(02)02894-2. [DOI] [PubMed] [Google Scholar]
- 67.Jarvisalo MJ. Raitakari M. Toikka JO. Putto-Laurila A. Rontu R. Laine S. Lehtimaki T. Ronnemaa T. Viikari J. Raitakari OT. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109:1750–1755. doi: 10.1161/01.CIR.0000124725.46165.2C. [DOI] [PubMed] [Google Scholar]
- 68.Margolis JR. Chen JT. Kong Y. Peter RH. Behar VS. Kisslo JA. The diagnostic, prognostic significance of coronary artery calcification. A report of 800 cases. Radiology. 1980;137:609–616. doi: 10.1148/radiology.137.3.7444045. [DOI] [PubMed] [Google Scholar]
- 69.Dabelea D. Kinney G. Snell-Bergeon JK. Hokanson JE. Eckel RH. Ehrlich J. Garg S. Hamman RF. Rewers M. Effect of type 1 diabetes on the gender difference in coronary artery calcification: A role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52:2833–2839. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 70.Starkman HS. Cable G. Hala V. Hecht H. Donnelly CM. Delineation of prevalence and risk factors for early coronary artery disease by electron beam computed tomography in young adults with type 1 diabetes. Diabetes Care. 2003;26:433–436. doi: 10.2337/diacare.26.2.433. [DOI] [PubMed] [Google Scholar]
- 71.Godsland IF. Pavitt D. Okoturo O. Edwards RJ. Rubens MB. Feher MD. Flather MD. Elkeles RS. Can protein biomarkers provide an index of coronary artery calcification in patients with type 2 diabetes? Atherosclerosis. 2010;213:570–572. doi: 10.1016/j.atherosclerosis.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Di Bello V. Talarico L. Picano E. Di Muro C. Landini L. Paterni M. Matteucci E. Giusti C. Giampietro O. Increased echodensity of myocardial wall in the diabetic heart: an ultrasound tissue characterization study. J Am Coll Cardiol. 1995;25:1408–1415. doi: 10.1016/0735-1097(95)00026-Z. [DOI] [PubMed] [Google Scholar]
- 73.Fraser GE. Luke R. Thompson S. Smith H. Carter S. Sharpe N. Comparison of echocardiographic variables between type I diabetics and normal controls. Am J Cardiol. 1995;75:141–145. doi: 10.1016/s0002-9149(00)80063-6. [DOI] [PubMed] [Google Scholar]
- 74.Salem M. El Behery S. Adly A. Khalil D. El Hadidi E. Early predictors of myocardial disease in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2009;10:513–521. doi: 10.1111/j.1399-5448.2009.00517.x. [DOI] [PubMed] [Google Scholar]
- 75.Nagueh SF. Middleton KJ. Kopelen HA. Zoghbi WA. Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 76.Vazeou A. Papadopoulou A. Miha M. Drakatos A. Georgacopoulos D. Cardiovascular impairment in children, adolescents, and young adults with type 1 diabetes mellitus (T1DM) Eur J Pediatr. 2008;167:877–884. doi: 10.1007/s00431-007-0603-z. [DOI] [PubMed] [Google Scholar]
- 77.Karavanaki K. Kazianis G. Konstantopoulos I. Tsouvalas E. Karayianni C. Early signs of left ventricular dysfunction in adolescents with type 1 diabetes mellitus: the importance of impaired circadian modulation of blood pressure and heart rate. J Endocrinol Invest. 2008;31:289–296. doi: 10.1007/BF03346360. [DOI] [PubMed] [Google Scholar]
- 78.Mehta SK. Holliday C. Hayduk L. Wiersma L. Richards N. Younoszai A. Comparison of myocardial function in children with body mass indexes ≥25 versus those <25 kg/m2. Am J Cardiol. 2004;93:1567–1569. doi: 10.1016/j.amjcard.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 79.Type 2 diabetes in children and adolescents. American Diabetes Association. Pediatrics. 2000;105:671–680. doi: 10.1542/peds.105.3.671. [DOI] [PubMed] [Google Scholar]
- 80.Mizushige K. Yao L. Noma T. Kiyomoto H. Yu Y. Hosomi N. Ohmori K. Matsuo H. Alteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin-resistant prediabetic stage of a type II diabetic rat model. Circulation. 2000;101:899–907. doi: 10.1161/01.cir.101.8.899. [DOI] [PubMed] [Google Scholar]
- 81.Kim EH. Kim YH. Left ventricular function in children and adolescents with type 1 diabetes mellitus. Korean Circ J. 2010;40:125–130. doi: 10.4070/kcj.2010.40.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Larsen JR. Tsunoda T. Tuzcu EM. Schoenhagen P. Brekke M. Arnesen H. Hanssen KF. Nissen SE. Dahl-Jorgensen K. Intracoronary ultrasound examinations reveal significantly more advanced coronary atherosclerosis in people with type 1 diabetes than in age- and sex-matched non-diabetic controls. Diabetes Vasc Dis Res. 2007;4:62–65. doi: 10.3132/dvdr.2007.009. [DOI] [PubMed] [Google Scholar]
- 83.Turkbey EB. Backlund JY. Genuth S. Jain A. Miao C. Cleary PA. Lachin JM. Nathan DM. van der Geest RJ. Soliman EZ. Liu CY. Lima JA. Bluemke DA. Myocardial structure, function, and scar in patients with type 1 diabetes mellitus. Circulation. 2011;124:1737–1746. doi: 10.1161/CIRCULATIONAHA.111.022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esposito L. Saam T. Heider P. Bockelbrink A. Pelisek J. Sepp D. Feurer R. Winkler C. Liebig T. Holzer K. Pauly O. Sadikovic S. Hemmer B. Poppert H. MRI plaque imaging reveals high-risk carotid plaques especially in diabetic patients irrespective of the degree of stenosis. BMC Med Imaging. 2010;10:27. doi: 10.1186/1471-2342-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]