Abstract
The glossopharyngeal and vagus nerves mediate the complex interplay between the many functions of the upper aerodigestive tract. Defects may occur anywhere from the brainstem to the peripheral nerve and can result in significant impairment in speech, swallowing, and breathing. Multiple etiologies can produce symptoms. This review will broadly examine the normal functions, clinical examination, and various pathologies of cranial nerves IX and X.
Keywords: Glossopharyngeal, Vagus, cranial nerve disorders, dysphonia, dysphagia
INTRODUCTION
The glossopharyngeal (IX) and vagus (X) nerves mediate the complex interplay between the many functions of the upper aerodigestive tract. Swallowing, breathing, and communicating all rely on delicate interactions between the sensory inputs and motor outputs of many of the cranial nerves. These processes are individually crucial to a patient’s basic vital functions; that each must work in perfect concert in a narrow anatomical space speaks to the important roles of cranial nerves IX and X.
Patients with deficits in the glossopharyngeal or vagus nerves may present with dysphagia, dysphonia, dyspnea, or a combination of these symptoms. Many symptoms are subtle and overlap with other disease processes, sometimes making diagnoses elusive. Diagnoses are further complicated by difficulties in locating the causative lesions and the subjective nature of functional assessments. Neurologists, otolaryngologists, and speech pathologists work together to diagnose and treat these difficult disorders.
ANATOMY
Cranial nerve IX – Glossopharyngeal nerve
The following summary gives a broad overview of the clinically relevant anatomy of cranial nerves IX and X. A detailed analysis of anatomical pathways is beyond the scope of this review, and we refer the reader to Snell’s Clinical Neuroanatomy and Parent’s Carpenter’s Human Neuroanatomy for a complete discussion.
The glossopharyngeal nerve is comprised of general somatic afferent, general visceral efferent, special visceral afferent, and parasympathetic fibers that supply the tongue and pharynx. The efferent motor fibers of cranial nerve IX supply the stylopharyngeus muscle,1 which helps elevate the larynx and expand the pharynx during swallowing.2 General somatic afferent fibers convey pain, temperature, and tactile sensation from the posterior third of the tongue, tonsils, medial aspect of the tympanic membrane, and Eustachian tube to both the superior and inferior glossopharyngeal ganglia. Stimulation of tactile fibers in the upper pharynx elicits swallowing, gagging, and vomiting reflexes. Special afferent fibers convey taste from the posterior one third of the tongue. Afferent impulses from chemoreceptors in the carotid body and baroreceptors in the carotid sinus travel via general visceral afferent fibers within the glossopharyngeal nerve to the nucleus solitarius. The carotid sinus reflex involving the glossopharyngeal and vagus nerves assists in the regulation of arterial blood pressure.1 Efferent parasympathetic fibers exit the medulla, then travel along Jacobson’s nerve to the tympanic plexus, through the lesser petrosal nerve and ultimately to the otic ganglion.2 Postganglionic parasympathetic fibers terminate on secretory cells in the parotid gland.
Cranial nerve X – Vagus nerve
The vagus nerve contains visceral efferent and afferent fibers and innervates the head, neck, thorax, and abdomen.3 The efferent motor fibers of the vagus nerve supply all striated muscles of the larynx and pharyx, except the stylopharyngeus (supplied by IX) and the tensor veli palatini (supplied by V3). Motor fibers leave the vagus nerve as three main branches.1 The pharyngeal branch travels between the internal and external carotid arteries and enters the pharynx at the upper border of the middle constrictor muscle, supplying levator veli palatini, salpingopharyngeus, palatopharyngeus and the uvula.3 Palatoglossus is the only striated tongue muscle innervated by the vagus nerve and acts to elevate the posterior portion of the tongue.4 The superior laryngeal nerve branches distal to the pharyngeal branch and descends lateral to the pharynx. The external branch of the superior laryngeal nerve supplies motor innervation to the cricothyroid muscle. The third motor branch off the vagus nerve is the recurrent laryngeal nerve. The right recurrent laryngeal nerve descends anterior to the subclavian artery, then turns posteriorly under the artery. The left recurrent laryngeal nerve turns posteriorly around the aortic arch and ascends through the superior mediastinum. The two then return to the base of the neck and travel superiorly in their respective tracheoesophageal grooves.5 Both recurrent branches enter the larynx below the inferior constrictor and supply all intrinsic muscles of larynx excluding the cricothyroid muscle (supplied by the external branch of the superior laryngeal nerve).
The vagus nerve receives sensory input from the larynx, pharynx, external auditory canal, lateral aspect of the tympanic membrane, and the meninges of the posterior fossa.6 Subglottic sensation is mediated by the recurrent laryngeal nerve, whereas the internal branch of the superior laryngeal nerve mediates sensation from the supraglottis. Glottic sensation likely involves both nerves. The vagus nerve also receives general visceral afferent information from the trachea, bronchi, lungs, heart, aortic arch, esophagus, and abdominal viscera.3 The vagus nerve also supplies parasympathetic innervation to smooth muscle and glands of the pharynx, larynx, and thoracic and abdominal viscera.
NORMAL FUNCTIONS OF THE PHARYNX AND LARYNX
The upper aerodigestive tract controls speech, swallowing, and breathing. These functions must act in perfect coordination as they are anatomically intertwined. During swallowing, the larynx must close to prevent liquids and solids from entering the airway. During speech production and respiration, the larynx and pharynx must remain patent to allow adequate airflow. The glossopharyngeal and vagus nerves control this dynamic interplay.
Swallowing
Numerous cranial nerves are involved in the swallowing reflex (Table 1). Normal swallowing function requires a strict interplay with the palate, tongue, and larynx. The tongue and muscles of mastication prepare the food into a compact bolus in the oral phase. The pharyngeal phase is initiated as the tongue moves the bolus posteriorly and the base of tongue contacts the posterior pharyngeal wall. The soft palate elevates to prevent nasopharyngeal reflux and the pharynx constricts, propelling the bolus further towards the esophagus. Swallowing requires sensory input from the superior laryngeal nerve for a synchronized motor response. While topical anesthesia of the laryngeal mucosa does not seem to affect swallowing,7 formal superior laryngeal nerve block increases the rate of laryngeal penetration and frank tracheal aspiration.8 At the initiation of deglutition, the larynx elevates, and the entire upper airway closes in response to stimulation of the superior laryngeal nerve. From superior to inferior, the aryepiglottic folds, false vocal folds, and true vocal folds approximate to midline as a response to stimulation. This effectively closes off the larynx and helps direct a food bolus towards the esophagus.9
Table 1.
Cranial Nerves Involved in Swallowing
| Cranial Nerve | Swallowing Function | |
|---|---|---|
|
|
||
| V- | Trigeminal | Muscles of mastication, sensation to the face including oral mucosa and anterior 1=3 of tongue |
| VII- | Facial | Taste to anterior 1=3 of tongue, motor function to lips (oral competence) |
| IX- | Glossopharyngeal | Sensation and taste to posterior 1=3 of tongue |
| X- | Vagus | Sensation to larynx, motor function to soft palate, pharynx, larynx, and esophagus |
| XII- | Hypoglossal | Motor innervation to intrinsic and extrinsic tongue musculature |
Airway Protection and Modulation
The larynx has evolved different mechanisms to protect the airway, the most important of which is glottic closure. Stimulation of the larynx also triggers reflexes producing cough, apnea, and cardiovascular effects such as bradycardia and hypotension.
During respiration, the larynx remains patent with the vocal folds in an open position to allow for adequate airflow. The pharynx must maintain some basal tone to provide structure to the upper airway and prevent collapse. Airflow receptors within the larynx help fine tune respiratory drive and are mediated by respiratory centers in the medulla. The vocal folds adduct and abduct with respiratory cycles, and the posterior cricoarytenoid muscle contracts with each inspiration before active contraction of the diaphragm.9,10
Phonation
Sound production is a highly evolved and specialized process. Theories of voice production have evolved over time, underlining the complex relationship between vocal fold vibration, airflow, and resonance.10 Phonation requires several intact mechanisms.11 Adequate airflow from the lungs must move over the vocal folds to induce oscillation. The induced mucosal wave depends on the pliability, tension, and bulk of the vocal fold itself. The laryngeal musculature must have adequate tone and synchronized movement to approximate the vocal folds in the midline for sound production. Furthermore, muscular control of vocal fold length and tension modulates vocal pitch. Neurological impairment may affect any of the above factors, thus disrupting normal sound production.
The vocal folds produce raw sound which is then shaped by the resonant space of the head, neck, and chest. Actual speech production relies on intact cognitive function, and articulation mediated by the tongue, lips, and palate. Dysphonia is the abnormal production of sound, and is often caused by a laryngeal problem. Dysarthria, or deficient speech articulation, may result from a wide variety of disorders disrupting coordination between the brain and musculature of the mouth, tongue, and larynx.12
CLINICAL HISTORY
Patients with lesions of the glossopharyngeal or vagal nerves may present with a variety of symptoms, centering around problems with speech, swallowing, or breathing. Patients with dysfunction of the vagus nerve may present with dysphagia, hoarseness, or dyspnea, while patients with an isolated glossopharyngeal lesion may remain asymptomatic because of redundancy in the motor output of the nerve. Often, the two nerves are affected in concert given their anatomic proximity. Other factors in the patient history may point the clinician to a specific etiology of disease such as time course of disease onset, recent head and neck or thoracic surgery, symptoms associated with thyroid dysfunction, other cranial nerve abnormalities, or systemic neurologic symptoms.
Specific characteristics of the voice history are important in discerning laryngeal pathologies.13 Patients may describe their voice as “hoarse” though their symptom is more accurately described as “breathiness.” Hoarseness usually refers to a scratchy or rough sound, clinically aligned with a disturbance in the vibratory dynamics of the vocal fold as in mass lesions or common variety viral laryngitis. Breathiness, however, refers to abnormal escape of air throughout the voice production. Patients may also describe vocal fatigue, or “tiring out” after prolonged speaking. Vocal fatigue may occur at multiple levels, including pathologic changes in the neuromuscular junction as in myasthenia gravis, muscular fatigue in compensated unilateral vocal fold paralysis, or at the neuronal level in multiple sclerosis or amyotrophic lateral sclerosis. Specific clinical findings for various disease manifestations will be addressed later in this article.
NEUROLOGIC EXAMINATION
Patients suspected of having neurologic disease should undergo a complete neurological examination, including a detailed cranial nerve examination. In addition, patients with laryngeal or pharyngeal symptoms should undergo a complete head and neck examination by an otolaryngologist, including indirect or direct examination of the larynx.
The vocal examination may take place during the patient interview. A brief vocal capabilities battery noting habitual voice patterns, loudness, and articulation are particularly important when evaluating for neurological disease. Maximal phonation time is a straightforward test to estimate vocal fold approximation or glottic closure. The patient attempts to say a prolonged “ee” sound; patients with vocal fold paralysis will often have marked air escape and will be unable to sustain phonation for longer than a few seconds.
Differentiating between dysphonia and dysarthria is important in distinguishing neurological disease. Asking the patient to recite passages that are highly difficult to articulate, such as “lips teeth tip of the tongue”, can differentiate between dysphonic and dysarthric problems. Examination of the oral cavity, including anatomical features and movement patterns of the lips, palate, and tongue will also guide diagnosis for patients presenting with dysarthria.10
Referral to an otolaryngologist is essential for patients suspected of laryngeal manifestations of disease. Traditionally, indirect laryngoscopy is performed in the clinic with a mirror placed in the oropharynx. This affords the physician a gross view of the larynx, including the mucosal surfaces and generalized movement of the true and false vocal folds. Further detail is ascertained by flexible laryngoscopy, in which a flexible scope is passed through the nasal cavity. The physician can view the status of velopharyngeal closure, as well as the oropharynx, hypopharynx and endolarynx. Valuable clinical information is garnered from observation during quiet respiration and phonation. Videostroboscopy augments standard endoscopy by allowing for an assessment of the vocal fold mucosal wave. Using a flexible or rigid endoscope, the physician directs a strobe light toward the vocal folds during phonation. This type of examination is an essential tool for assessment of vocal fold vibration and can often provide critical functional information.14
DISORDERS OF THE GLOSSOPHARYNGEAL AND VAGUS NERVES
Many systemic neurological diseases will first present with speech or swallowing disorders. Etiologies vary widely for cranial nerve disorders of the glossopharyngeal and vagus nerves. In this section we will broadly examine pathologies based on site of the lesion.
Pyramidal Lesions
The larynx and pharynx are diffusely supplied by different areas of the cortex, and lesions may cause a variety of symptoms. Although classically thought to occur exclusively in brainstem strokes, dysphagia may also be caused by isolated cerebral lesions. The incidence of dysphagia after an acute stroke has been reported from 37% to 78%, increasing the risk of aspiration events and subsequent pneumonia.15 Cortical lesions may cause a range of dysphonic symptoms from abnormal vocal fold movement with stridor to complete aphasia.16
The nuclei of cranial nerves IX and X receive bilateral input from the cortex, and unilateral lesions are often asymptomatic. Bilateral lesions of the corticobulbar tracts result in pseudobulbar palsy. Symptoms include slow, dysarthric speech and variable dysphagia, while pharyngeal and palatal reflexes remain intact. Patients also present with emotional lability marked by inappropriate crying or laughing.16
Extrapyramidal Movement Disorders
Extrapyramidal contributions to cranial nerves IX and X are poorly understood, but can be significant causes of laryngeal and pharyngeal dysfunction. Movement disorders of the larynx include Parkinson’s, essential tremor, and spasmodic dysphonia. They are defined by inadequate motor control of the laryngeal musculature, resulting in irregular muscle tension, spasmodic contractions, or tremor. Patients experience irregular vocal production including strained voice, tremor, or pitch variability.
Parkinson’s disease manifests as bradykinesia, a loss of postural reflexes, rigidity, and resting tremor. Nearly 70% of patients with Parkinson’s experience difficulty with speech production with 29% of those patients reporting it as their most debilitating symptom.17 Patients have a hypokinetic dysarthria as well as decreased airflow and breath support to the vocal folds. Speech fluency is uneven marked by short bursts of monotone, quiet voice production interspersed with long, inappropriately placed pauses. Although physiologic swallowing function is often intact, patients may drool due to the inability to initiate a voluntary swallow.18 On laryngeal examination, patients have vocal fold bowing and a large glottic gap.19,20 Treatments focused on reducing the glottic aperture by either vocal fold augmentation or specific speech therapy have been successful.
Essential tremor is a common movement disorder most often affecting the hands and the head. Voice tremor, caused by tremor in the laryngeal or pharyngeal muscles, can occur as a singular symptom or may be associated with tremor elsewhere in the body. Patients have rhythmic alterations of the voice including pitch and loudness, best demonstrated during vowel prolongation.10 Treatment with oral medications or surgery has variable success. Botulinum toxin injections of the thyroarytenoid muscle have also been used to treat vocal tremor with some success.21
Dystonias are characterized by uncontrolled, involuntary muscle contractions. They may involve any singular voluntary muscle or groups of muscles. Spasmodic dysphonia is considered an idiopathic focal dystonia involving either the adductor or abductor muscles of the larynx. Patients with adductor dysphonia present with a strangled or strained voice, while abductor dysphonia produces breathy interruptions secondary to contraction of the posterior cricoarytenoid muscle.22 Injection of botulinum toxin into affected muscles is accepted as a safe and very effective treatment for spasmodic dypshonia.23,24
Medullary Lesions
The nuclei to cranial nerves IX and X lie in the medulla oblongata, and lesions within the brainstem can cause both upper and lower motor neuron signs and symptoms. Postpolio syndrome, syringomyelia, and Arnold-Chiari malformation can result in both laryngeal paralysis and dysphagia. Motor neuron diseases including primary lateral sclerosis, amyotrophic lateral sclerosis, progressive bulbar palsy, and progressive spinal muscular atrophy can also lead to laryngeal pathology.
Amyotrophic lateral sclerosis (ALS) is a devastating motor neuron disease defined by degeneration of upper and lower motor neurons. Patients experience progressive muscle weakness, fasciculations, and atrophy. Worldwide incidence is 1 to 2 in 100,000, with a slightly higher predominance in men.25 Thirty percent of patients have bulbar symptoms at the time of disease onset, and nearly all patients manifest bulbar involvement at the late stages of the disease.26
As ALS affects both upper and lower motor neurons, laryngopharyngeal paresis may be flaccid or spastic. Dysarthria, marked by slurred or “thick” speech, may be caused by an atrophic or spastic tongue, with fasiculations and a classic “bag of worms” appearance. Vocal quality varies but is usually flat, raspy, and weak. Examination of the vocal folds often reveals intact adduction but poor abduction. Patients may also present with inspiratory stridor due to passive in-drawing of the vocal folds. Weakened abductor muscles are unable to resist the Venturi effect of inhalation, causing paradoxical adduction.12 Palatal weakness with resultant velopharyngeal insufficiency results in hypernasal voice and nasopharyngeal regurgitation of liquids. Dysphagia becomes progressively worse, with some patients unable to manage their own secretions. Increasing aspiration events are coupled with a decreased cough reflex, and these factors contribute significantly to pneumonia. Patients may benefit from tracheostomy placement in cases of bilateral abductory vocal fold paresis where the glottic airway becomes inadequate, to increase pulmonary toilet, or for mechanical respiration in the end-stages of the disease.
Peripheral Nerve Lesions
The glossopharyngeal and vagus nerves pass through the cerebellopontine angle (CPA) before exiting the skull. Mass lesions within the CPA may cause lower cranial nerve involvement. Vestibular schwannomas account for greater than 90% of all CPA tumors, and usually present with sensorineural hearing loss and disequilibrium.27 While some patients present with facial nerve weakness due to involvement of cranial nerve VII, involvement of the lower cranial nerves is exceedingly rare.
The glossopharyngeal, vagus, and accessory nerves exit the skull base together through the jugular foramen. Infection such as skull base osteomylitis, skull base fractures, or neoplasms may affect the three nerves in concert, resulting in ipsilateral vocal fold, palate, and shoulder weakness. In addition to the above symptoms, lesions within the retropharyngeal space may also involve the hypoglossal nerve and the ascending sympathetic chain, resulting in tongue weakness and Horner’s syndrome respectively. Specific lesions and associated syndromes can be found in Table 2.28
Table 2.
Neurological Syndromes Associated with Disorders of Cranial Nerves IX and X
| Syndrome | Lesion | CN IX and X Manifestations | Additional Neurologic Manifestations | Etiology |
|---|---|---|---|---|
| Wallenberg | Lateral medulla | Ipsilateral paralysis and anesthesia of the pharynx, and larynx. Results in dysphonia and dysphagia. | Ipsilateral loss of corneal reflex, loss of pain and temperature sensation. Ipsilateral Horner’s Syndrome. Contralateral loss of pain and temperature sensation on trunk and extremities. | Infarction, neoplasm |
| Cestan-Chenais | Lateral/medial medulla | Ipsilateral paralysis and anesthesia of the pharynx, and larynx. Results in dysphonia and dysphagia. | Manifestations associated with Wallenberg’s Syndrome, with the addition of contralateral hemiplegia. | Thrombosis of the vertebral artery proximal to PICA origin |
| Vernet | Jugular foramen | Ipsilateral paralysis and anesthesia of the pharynx, and larynx. Results in dysphonia and dysphagia. | Ipsilateral paralysis of CN XI resulting in paralysis of the sternocleidomastoid and trapezius muscles. | Neoplasm, infection, skull base fractures |
| Collet-Sicard | Extra cranial at the jugular foramen | |||
| Garcin | Jugular foramen, skull base | Ipsilateral paralysis and anesthesia of the pharynx, and larynx. Results in dysphonia and dysphagia. | Progressive involvement of multiple cranial nerves. | Neoplasm, infection, trauma. |
| Villaret | Parapharyngeal space | Ipsilateral paralysis and anesthesia of the pharynx, and larynx. Results in dysphonia and dysphagia. | Ipsilateral paralysis of CN XI, XII resulting in paralysis of sternocleidomastoid, trapezius, and muscles of the tongue. | Neoplasm, infection |
| Tapia | Intersection of CN X and XII below the nodose ganglia | Ipsilateral paralysis and anesthesia of the pharynx, and larynx. Results in dysphonia and dysphagia. | Ipsilateral paralysis of CN XII, resulting in paralysis of the muscles of the tongue. | Neoplasm, trauma |
Adapted from Gorlin RJ, Cohen MM, Hennekam RCM. Syndromes of the head and neck. 4th ed. Oxford [England]; New York: Oxford University Press; 2001:xiv, 1283 p.
Isolated lesions of the glosspharyngeal nerve are difficult to detect both for the clinician and the patient. Paralysis of the stylopharyngeus muscle will be undetected by the patient if the vagus nerve is intact. Likewise, a unilateral decrease in saliva production from the parotid gland will go unnoticed if the patient’s other salivary glands are functioning. Glossopharyngeal neuralgia is a rare disease characterized by brief episodes of pain in the base of tongue and deep in the neck, usually in response to chewing or swallowing. Patients have stereotyped, unilateral pain lasting seconds to minutes. Pain may be idiopathic, but may also indicate other pathology such as a mass lesion, infection, or glossopharyngeal neuroma. Eagle’s syndrome has also been implicated, in which elongation of the styloid process is hypothesized to irritate the glossopharyngeal nerve.29
Though more common than isolated glossopharyngeal nerve lesions, the exact incidence of vagus nerve lesions is unknown.30 Many patients with vocal fold immobility are asymptomatic and do not seek medical attention. Furthermore, some patients with lung cancer or other thoracic malignancies have other, more pressing medical problems, and hoarseness may be temporarily ignored.
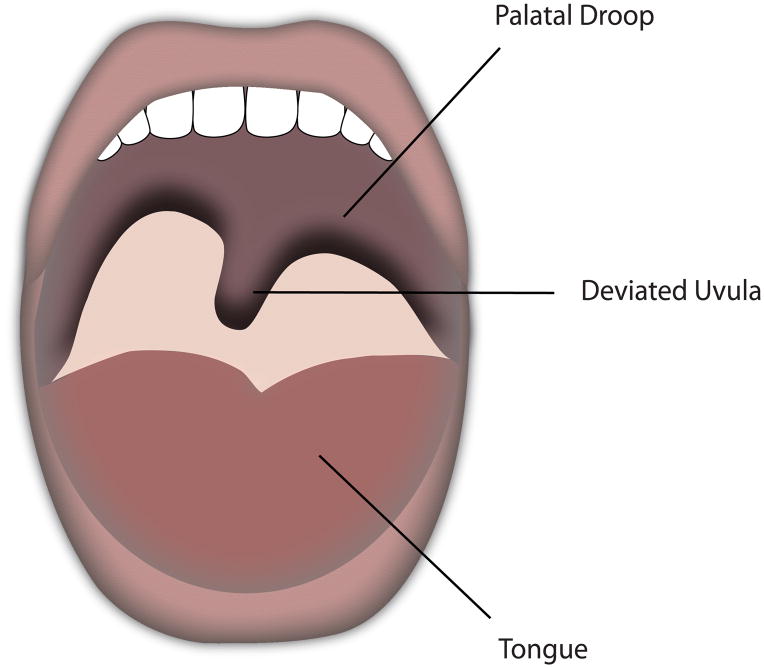
Vagus nerve injuries classically present with hoarseness, and the patient’s additional symptoms will often indicate the site of the lesion within the vagus nerve. Lesions of the vagus nerve distal to the take-off of the pharyngeal branches or lesions to the recurrent laryngeal nerve will present with hoarseness alone due to paralysis of the vocal fold (Figure 1). Patients with high vagal lesions also experience dysphagia from palatal weakness, paralysis of the constrictor muscles, and loss of sensation from the superior laryngeal nerve.31 High vagal lesions also cause deviation of the uvula to the contralateral side due to intact palatal muscle pull, and a palatal droop on the ipsilateral side of the lesion (Figure 2). Historically, the position of the paralyzed vocal fold has been described as either “paramedian” or “cadaveric”. It was previously thought that a high vagal lesion would lead to the paralyzed fold in the cadaveric position, but an isolated injury of the recurrent laryngeal nerve would remain in the paramedian position due to preservation of the cricothyroid innervation by the superior laryngeal nerve. These terms have fallen out of favor in recent years given the clinical variety of vocal fold positions seen in patients with vocal fold paralysis.32 Depending on the position of the paralyzed vocal fold, not all patients will exhibit signs of hoarseness. In some cases, the contralateral vocal fold can move past midline to reach the paralyzed fold. Patients may compensate over a period of months, and may complain of vocal fatigue after prolonged use but no frank hoarseness or raspy quality to the voice.
Figure 1.
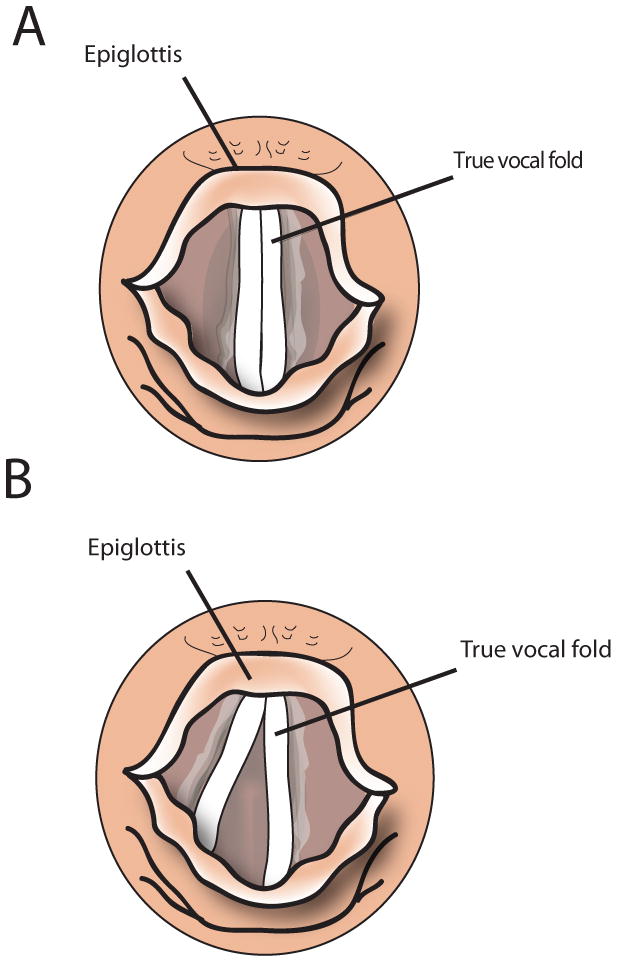
Position of vocal folds on phonation in a) normal state and b) left vocal fold paralysis.
Figure 2.
Left vagal lesion with contralateral uvular deviation and ipsilateral palatal droop.
Multiple etiologies cause vocal fold immobility including infectious agents, neurotoxic drugs, neoplasms, and iatrogenic injury. Surgical injury accounts for approximately one third of vocal fold paralysis. Thyroid surgery has long been implicated in recurrent laryngeal nerve paralysis, though other non-thyroid surgeries such as carotid endarterectomy and anterior cervical approaches to the spine have likely surpassed thyroid surgery as the most common causes for vocal fold immobility.33
The recurrent laryngeal nerves travel through the mediastinum before entering the larynx, and they are susceptible to injury throughout their course. The left recurrent laryngeal nerve is more often injured than the right, as it has a longer course through the mediastinum back into the larynx. Specific etiologies in the mediastinum include lung or thoracic malignancies and metastatic lesions. Cardiovocal syndrome, also known as Ortner’s syndrome, is the association of hoarseness due to a left recurrent laryngeal nerve palsy caused by cardiovascular pathology. First described in patients with left atrial enlargement secondary to mitral valve stenosis, it is now recognized that a host of cardiovascular pathologies may cause impingement of the left recurrent laryngeal nerve between the aorta and pulmonary artery.34
Many patients remain asymptomatic or compensate over a number of months, avoiding the need for treatment. Patients who do remain symptomatic, however, have difficulty swallowing and communicating, which can have a drastic impact on quality of life.35 Specific voice-related questionnaires can be helpful in determining a patient’s need for treatment.36 Treatment options include voice therapy, injection laryngoplasty, and laryngeal framework surgery.
Neuromuscular Junction Disorders
Myasthenia gravis (MG) is an autoimmune disorder causing rapid muscle fatigue due to destruction of the acetylcholine receptor. Patients most often present with diplopia, ptosis, or generalized weakness. Symptoms may also be limited to the larynx, and most often include hoarseness and vocal fatigue. Laryngeal examination reveals mobility defects, and may also include phase asymmetry or muscle tension dysphonia.37 Treatment is with systemic pyridostigmine.
CONCLUSION
Speech, swallowing, and breathing disorders can be devastating to the patient, and prompt diagnosis and treatment of disorders of the glossopharyngeal and vagus nerves is paramount. Multiple etiologies can produce symptoms. Close cooperation among neurologists, otolaryngologists, and speech therapists is vital in the evaluation and treatment of these patients.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) U54-DA021519 (ELF), DK07610 (ELF), NS04765 (ELF) and the Program for Neurology Research and Discovery (ABE, AEK, NDH, ELF).
References
- 1.Snell RS. Clinical Neuroanatomy. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 2.Remley KBHH, Smoker WRK, Osborn AG. CT and MRI in the evaluation of glossopharyngeal, vagal, and spinal accessory neuropathy. Semin Ultrasound CT MR. 1987;(8):284–300. [Google Scholar]
- 3.Afifi AKBR. Functional Neuroanatomy. 2. New York City, NY: McGraw-Hill Professional; 2005. [Google Scholar]
- 4.Goetz CG. Textbook of Clinical Neurology. 3. Philadelphia, PA: Saunders; 2007. [Google Scholar]
- 5.Castillo M, Mukherji SK. Magnetic resonance imaging of cranial nerves IX, X, XI, and XII. Topics in magnetic resonance imaging. 1996;8(3):180–186. [PubMed] [Google Scholar]
- 6.Parent A. Carpenter’s Human Neuroanatomy. 9. Baltimore, MD: Williams & Wilkins; 1996. pp. 442–451. [Google Scholar]
- 7.Bastian RW, Riggs LC. Role of sensation in swallowing function. The Laryngoscope. 1999;109(12):1974–1977. doi: 10.1097/00005537-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Sulica L, Hembree A, Blitzer A. Swallowing and sensation: evaluation of deglutition in the anesthetized larynx. The Annals of otology, rhinology, and laryngology. 2002;111(4):291–294. doi: 10.1177/000348940211100402. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki CT. Electrophysiology of the Larynx. In: Blitzer A, Brin MF, Sasaki CT, Fahn S, Harris KS, editors. Neurologic disorders of the larynx. New York: Thieme Medical Publishers; 1992. pp. 45–53. [Google Scholar]
- 10.Aronson AE. Clinical voice disorders : an interdisciplinary approach. 2. New York: Thieme; 1985. p. xii.p. 417. [Google Scholar]
- 11.Jiang J, Lin E, Hanson DG. Vocal fold physiology. Otolaryngologic clinics of North America. 2000;33(4):699–718. doi: 10.1016/s0030-6665(05)70238-3. [DOI] [PubMed] [Google Scholar]
- 12.Sataloff RT, Mandel S, Gupta R, Mandel H. Neurologic Disorders Affecting the Voice in Performance. In: Sataloff RT, editor. Clinical assessment of voice. San Diego: Plural Publishing; 2005. pp. 201–223. [Google Scholar]
- 13.Sataloff RT, Hawkshaw M, Anticaglia J. Patient History. In: Sataloff RT, editor. Clinical assessment of voice. San Diego: Plural Publishing; 2005. pp. 1–16. [Google Scholar]
- 14.Woodson GE, Blitzer A. Neurologic Evaluation of the Larynx and the Pharynx. In: Cummings CW, Flint PW, Harker LA, et al., editors. Cummings otolaryngology head & neck surgery. 4. Philadelphia: Elsevier Mosby; 2005. pp. 2054–2064. [Google Scholar]
- 15.Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke; a journal of cerebral circulation. 2005;36(12):2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 16.Tatemichi TK, Freddo L, Mohr JP, Blitzer A. Pyramidal Disease. In: Blitzer A, Brin MF, Sasaki CT, Fahn S, Harris KS, editors. Neurologic disorders of the larynx. New York: Thieme Medical Publishers; 1992. pp. 229–239. [Google Scholar]
- 17.Hartelius L, Svensson P. Speech and swallowing symptoms associated with Parkinson’s disease and multiple sclerosis: a survey. Folia Phoniatr Logop. 1994;46(1):9–17. doi: 10.1159/000266286. [DOI] [PubMed] [Google Scholar]
- 18.Brin MF, Fahn S, Blitzer A, Ramig LO, Stewart C. Movement Disorders of the Larynx. In: Blitzer A, Brin MF, Sasaki CT, Fahn S, Harris KS, editors. Neurologic disorders of the larynx. New York: Thieme Medical Publishers; 1992. pp. 248–278. [Google Scholar]
- 19.Blumin JH, Pcolinsky DE, Atkins JP. Laryngeal findings in advanced Parkinson’s disease. The Annals of otology, rhinology, and laryngology. 2004;113(4):253–258. doi: 10.1177/000348940411300401. [DOI] [PubMed] [Google Scholar]
- 20.Hanson DG, Gerratt BR, Ward PH. Cinegraphic observations of laryngeal function in Parkinson’s disease. The Laryngoscope. 1984;94(3):348–353. doi: 10.1288/00005537-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Adler CH, Bansberg SF, Hentz JG, et al. Botulinum toxin type A for treating voice tremor. Archives of neurology. 2004;61(9):1416–1420. doi: 10.1001/archneur.61.9.1416. [DOI] [PubMed] [Google Scholar]
- 22.Sataloff RT, Deems DA. Spasmodic Dysphonia. In: Sataloff RT, editor. Clinical assessment of voice. San Diego: Plural Publishing; 2005. pp. 241–256. [Google Scholar]
- 23.Watts CR, Truong DD, Nye C. Evidence for the effectiveness of botulinum toxin for spasmodic dysphonia from high-quality research designs. J Neural Transm. 2008;115(4):625–630. doi: 10.1007/s00702-007-0757-x. [DOI] [PubMed] [Google Scholar]
- 24.Rubin AD, Wodchis WP, Spak C, Kileny PR, Hogikyan ND. Longitudinal effects of Botox injections on voice-related quality of life (V-RQOL) for patients with adductory spasmodic dysphonia: part II. Archives of otolaryngology--head & neck surgery. 2004;130(4):415–420. doi: 10.1001/archotol.130.4.415. [DOI] [PubMed] [Google Scholar]
- 25.Siddique N, Siddique T, Sufit R. Degenerative Motor, Sensory, and Autonomic Disorders. In: Goetz CG, editor. Textbook of Clinical Neurology. Philadelphia: Saunders Elsevier; 2007. [Google Scholar]
- 26.Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118 ( Pt 3):707–719. doi: 10.1093/brain/118.3.707. [DOI] [PubMed] [Google Scholar]
- 27.Brackmann DE, Arriaga M. Extra-Axial Neoplasms of the Posterior Fossa. In: Cummings CW, Flint PW, Harker LA, et al., editors. Cummings otolaryngology head & neck surgery. 4. Philadelphia: Elsevier Mosby; 2005. pp. 3803–3844. [Google Scholar]
- 28.Gorlin RJ, Cohen MM, Hennekam RCM. Syndromes of the head and neck. 4. Oxford [England] ; New York: Oxford University Press; 2001. p. xiv.p. 1283. [Google Scholar]
- 29.De Simone R, Ranieri A, Bilo L, Fiorillo C, Bonavita V. Cranial neuralgias: from physiopathology to pharmacological treatment. Neurol Sci. 2008;29 (Suppl 1):S69–78. doi: 10.1007/s10072-008-0892-7. [DOI] [PubMed] [Google Scholar]
- 30.Myssiorek D. Recurrent laryngeal nerve paralysis: anatomy and etiology. Otolaryngologic clinics of North America. 2004;37(1):25–44. v. doi: 10.1016/S0030-6665(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 31.Richardson BE, Bastian RW. Clinical evaluation of vocal fold paralysis. Otolaryngologic clinics of North America. 2004;37(1):45–58. doi: 10.1016/S0030-6665(03)00179-8. [DOI] [PubMed] [Google Scholar]
- 32.Woodson GE. Configuration of the glottis in laryngeal paralysis I & II. The Laryngoscope. 1993;103(11 Pt 1):1227–1241. doi: 10.1288/00005537-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal LH, Benninger MS, Deeb RH. Vocal fold immobility: a longitudinal analysis of etiology over 20 years. The Laryngoscope. 2007;117(10):1864–1870. doi: 10.1097/MLG.0b013e3180de4d49. [DOI] [PubMed] [Google Scholar]
- 34.Mulpuru SK, Vasavada BC, Punukollu GK, Patel AG. Cardiovocal syndrome: a systematic review. Heart, lung & circulation. 2008;17(1):1–4. doi: 10.1016/j.hlc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Fang TJ, Li HY, Gliklich RE, et al. Quality of life measures and predictors for adults with unilateral vocal cord paralysis. The Laryngoscope. 2008;118(10):1837–1841. doi: 10.1097/MLG.0b013e31817e7431. [DOI] [PubMed] [Google Scholar]
- 36.Hogikyan ND, Sethuraman G. Validation of an instrument to measure voice-related quality of life (V-RQOL) J Voice. 1999;13(4):557–569. doi: 10.1016/s0892-1997(99)80010-1. [DOI] [PubMed] [Google Scholar]
- 37.Mao VH, Abaza M, Spiegel JR, et al. Laryngeal myasthenia gravis: report of 40 cases. J Voice. 2001;15(1):122–130. doi: 10.1016/S0892-1997(01)00012-1. [DOI] [PubMed] [Google Scholar]