Abstract
Mice deficient in group 1b phospholipase A2 have decreased plasma lysophosphatidylcholine and increased hepatic oxidation that is inhibited by intraperitoneal lysophosphatidylcholine injection. This study sought to identify a mechanism for lysophosphatidylcholine-mediated inhibition of hepatic oxidative function. Results showed that in vitro incubation of isolated mitochondria with 40-200 μM lysophosphatidylcholine caused cyclosporine A-resistant swelling in a concentration-dependent manner. However, when mitochondria were challenged with 220 μM CaCl2, cyclosporine A protected against permeability transition induced by 40 μM, but not 80 μM lysophosphatidylcholine. Incubation with 40-120 μM lysophosphatidylcholine also increased mitochondrial permeability to 75 μM CaCl2 in a concentration-dependent manner. Interestingly, despite incubation with 80 μM lysophosphatidylcholine, the mitochondrial membrane potential was steady in the presence of succinate, and oxidation rates and respiratory controls indices were similar to controls in the presence of succinate, glutamate/malate, and palmitoyl-carnitine. However, mitochondrial oxidation rates were inhibited by 30-50% at 100 μM lysophosphatidylcholine. Finally, while 40 μM lysophosphatidylcholine has no effect on fatty acid oxidation and mitochondria remained impermeable in intact hepatocytes, 100 μM lysophosphatidylcholine inhibited fatty-acid stimulated oxidation and caused intracellular mitochondrial permeability. Taken together, these present data demonstrated that LPC concentration-dependently modulates mitochondrial microenvironment, with low micromolar concentrations of lysophosphatidylcholine sufficient to change hepatic oxidation rate whereas higher concentrations are required to disrupt mitochondrial integrity.
Keywords: Lysophosphatidylcholine, Oxidation, Hepatocytes, Mitochondria
1. Introduction
Phospholipase A2 (PLA2) are enzymes that hydrolyze phospholipids at the sn-2 position to release a lysophospholipid and a fatty acid [1]. Types of PLA2 include secreted, cytoplasmic, lysosomal, calcium-independent (iPLA2), and platelet activating factor acetylhydrolase [2]. These enzymes are important in a variety of cellular and systemic processes such as digestion, inflammation, metabolism, cell signaling, and immunity [1, 2]. The group 1b enzyme (PLA2G1B) is a secreted PLA2, produced mainly in the pancreas, and is responsible for the absorption of lysophospholipids, which are taken up into the bloodstream through the portal circulation subsequent to their conversion from dietary and biliary phospholipids [3-5]. Plasma concentrations of lysophospholipids in humans range from ~150 μM in normal subjects to ~200-250 μM in diabetic patients [6]. Most if not all of the lysophospholipids transported in plasma are albumin-bound [7]. Though other secreted PLA2 enzymes are present in the blood (e.g. group IIA, group V, group X, etc.), mice that are deficient in Pla2g1b activity have decreased plasma levels of lysophosphatidylcholine (LPC) compared to wild type animals, a difference which is exacerbated with high fat diet challenge [4].
Mice deficient in PLA2G1B (Pla2g1b−/− mice) have increased postprandial hepatic fatty acid oxidation rates compared to Pla2g1b+/+ mice after exposure to high fat diet, leading to protection against diet-induced obesity [4, 8]. Systemic supplementation of LPC prior to oral lipid load decreases hepatic fatty acid oxidation to levels similar to those observed in Pla2g1b+/+ mice, as well as cause stimulation of triglyceride production in fasting Pla2g1b−/− and Pla2g1b+/+ mice [9, 10]. These observations suggest a direct and acute effect of LPC on hepatocytes and that Pla2g1b-mediated LPC absorption may play a role in postprandial partitioning of dietary fatty acids to triglyceride (TG) production instead of β-oxidation.
While the cellular mechanisms responsible for LPC-stimulated very low density lipoprotein (VLDL) production have been investigated in several reports [11-14], the potential effects of LPC on fatty acid oxidation has been studied less extensively. Whether the reduced fatty acid oxidation observed in Pla2g1b−/− mice and increased TG production in both Pla2g1b−/− and Pla2g1b+/+ mice subsequent to LPC injection is due to direct or indirect inhibition of hepatic oxidative mechanisms has not been established. This study aims to further characterize the effect of LPC on oxidation rates by interrogating murine hepatocytes directly. We isolated mitochondria in order to determine the effects of exogenous LPC on mitochondrial permeability and oxidative function. The data showed that levels of LPC need to be delicately balanced in order to control mitochondrial and cellular respiration.
2. Methods
2.1 Mice
Wild type C57BL/6J mice were originally purchased from Jackson Laboratories and a breeding colony was established in our institutional facility. Mice were maintained in accordance to protocols approved by the Institutional Animal Care and Use Committee at the University of Cincinnati. Access to food and water was ad libitum, except as indicated. Mice had a 12 hour light/dark cycle. Male mice of at least 10 weeks of age were used in each experiment for isolation of primary hepatocytes and liver mitochondria.
2.2 Mitochondria isolation
Hepatic mitochondria were isolated from mice using a method based on a published protocol [15]. Briefly, freshly isolated mouse livers were kept at 4°C, diced into <1 mm pieces, homogenized in Isolation Media (220 mM D-mannitol (Sigma), 70 mM sucrose (Fisher), 1 mM EDTA (Fisher), 10 mM 3-(N-Morpholino)propanesulfonic acid (MOPS, Sigma), 0.5% BSA (Sigma), pH 7.2 with KOH (Fisher)), and centrifuged at 500 × g for 15 min. The supernatant was strained through gauze and then centrifuged again at 10,000 × g for 15 min to produce a mitochondrial pellet. This pellet was resuspended two times in Wash Media (250 mM sucrose, 10 mM MOPS, pH 7.2 with KOH) and recovered by centrifugation at 10,000 × g. The final pellet was suspended in 250-500 μL of wash medium and stored on ice until used for experiments.
2.3 Mitochondrial swelling and permeability
Mitochondrial protein concentration was determined by bicinchoninic acid assay (BCA, Thermo Scientific). Isolated mitochondria (0.7 mg protein/mL) were incubated in Assay Media (250 mM sucrose, 2.5 mM MgCl2 (Fisher), 0.5 mM EDTA (Fisher), 10 mM MOPS, 0.72 mM K2HPO4(Fisher), 0.28 mM KH2PO4(Sigma), to pH 7.2 with KOH) supplemented with 5 mM glutamate/5 mM malate as respiratory substrates and egg LPC (Sigma) with or without 2 μM cyclosporine A (CsA, Sigma) at room temperature (~22°C). Since LPC has detergent properties and is amphipathic, 1.7 mM SDS was used as a negative control to achieve total mitochondrial membrane solubilization [16]. Mitochondria were incubated in the indicated concentrations of LPC for 2 min prior to the addition of 75 μM or 220 μM CaCl2 (Fisher). Absorbance at 530 nm was recorded initially after exposure to LPC and after 10 min of incubation with CaCl2. In other experiments, mitochondria were incubated with LPC for 3 min and centrifutged at 4°C at 12,000 × g. Cytochrome C released into the media was determined by enzyme-linked immunoabsorbent assay (Abcam).
2.4 Membrane potential
Mitochondria were incubated in Assay Media containing 25 μM safranin O (Sigma) and LPC for 2 min at room temperature. Safranin was used as a spectrophotometric indicator of mitochondrial voltage since there is a tight positive correlation between absorbance and membrane potential. Thus, absorbance values were measured at its peak absorption at wavelength of 530 nm [17]. Baseline absorbance at 530 nm was recorded prior to the addition of 5 mM succinate. Negative control for total mitochondrial membrane solubilization was achieved by incubation with 1.7 mM SDS. After a 3 min equilibration period, 5 mM succinate was then added by multichannel pipette to each well. Absorbance at 530 nm was measured every 30 s for 10 min after succinate addition.
2.5 Mitochondrial calcium uptake
Mitochondria were incubated in Assay Media containing 5 mM glutamate, 5 mM malate, 1 μM Calcium Green-5N (Invitrogen), which is a fluorescent probe that binds Ca2+ and is impermeable to membranes, and varying concentrations of LPC. Fluorescence intensity was determined at 538 nm after excitation at 485 nm at baseline and every 2.5 s for 10 min after the addition of 75 μM CaCl2. In negative controls, the mitochondrial membrane was solubilized by incubation with SDS.
2.6 Mitochondrial oxygen consumption
In order to determine the effect of intracellular LPC on mitochondrial fatty acid oxidation, O2 consumption was measured at 37°C with a Gilson oxymeter. Isolated mitochondria (1 mg protein/mL) were added to Assay Media containing one of the following sets of substrates: 5 mM succinate, 5 mM glutamate/5 mM malate, or 10 μM palmitoyl-carnitine/1 mM malate. After 2 min, 441 nmol ADP was added and state 3 and 4 respiration rates, ADP/O ratio, and respiratory control index (RCI) were determined.
2.7 Cellular oxygen consumption
Chow-fed C57BL/6J mice were anesthetized with inhaled isofluorane and primary hepatocytes were isolated as previously described by perfusion with 100 U/mL collagenase [18]. Primary mouse hepatocytes were then plated on a collagen-coated 96-well Seahorse (Seahorse Bioscience) plate at a density of 5000 cells/well. The cells were allowed to adhere overnight and then treated with 50 μM oleate and indicated leels of LPC, 10 μg/mL oligomycin, 3 μM carbonyl cyanide 4-(trifluoromethoxy) phenyhydrazone (FCCP) and 4 μM antimycin A/1 μM rotenone to measure oxidation changes and construct mitochondrial bioenergetics profiles [19-21]. Basal oxygen consumption rate (OCR) was established prior to injection of oleate and LPC.
2.8 Hepatocyte viability and mitochondrial permeability transition
Primary hepatocytes were plated in dark-walled microtiter plates for overnight incubation. Cells were then washed with phosphate-buffered saline (PBS), acclimated in Hepatozyme-SFM (Invitrogen) for 1 hr, treated with 100 μM oleate complexed to BSA at a ratio of 5:1 and indicated concentrations of LPC for 10 min, washed three times with PBS, and then incubated in 2 μM calcein-acetoxymethylester (AM, BD Biosciences) for 30 min at 37°C in the dark [22, 23]. Fluorescence intensity after excitation at 485 nm and emission at 538 nm was measured in a microplate fluorimeter to determine hepatocyte viability. Viability was also determined by measuring oxygen consumption during experiments using the XF96 Analyzer. In order to measure mitochondrial permeability, 8 mM CoCl2 (Sigma), which quenches cytosolic fluorescence of calcein for visualization of mitochondria when the mitochondrial membrane is impermeable [22, 23], was added to isolated hepatocytes prior to treatment with oleate, BSA, and LPC. Images of fluorescence intensity after excitation at 485 nm and emission at 538 nm were captured at baseline and after 10 min using Image-Pro Plus on an Olympus IX71 with a RETIGA EXi FAST camera (QImaging).
2.9 Statistics
Results are shown as mean ± SEM. Student's t test was used for comparisons between groups. Differences at p<0.05 were considered significant.
3. Results
3.1 LPC induces concentration-dependent mitochondrial swelling
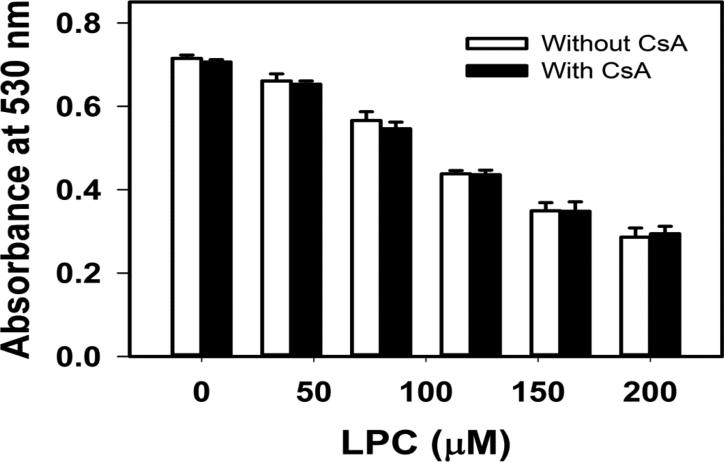
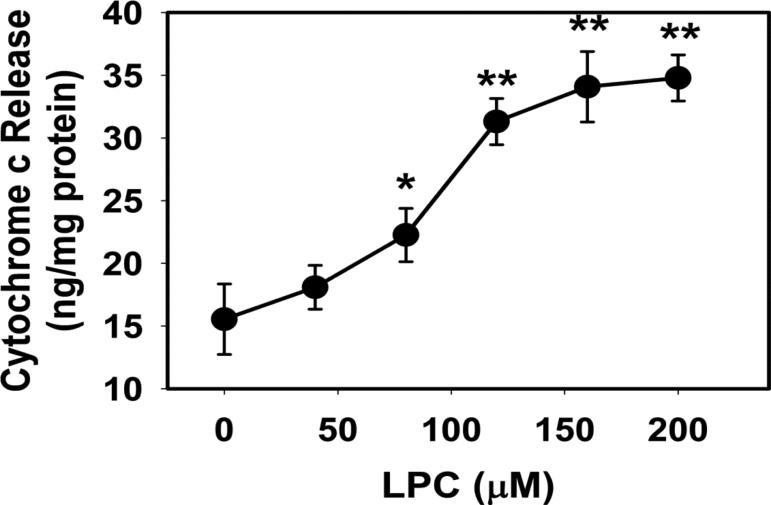
Exogenous LPC in the low micromolar range easily incorporates into the mitochondrial membrane and changes its permeability [24]. At concentrations as low as 20 μM, LPC can form micelles [25], and concentrations from 50 to 200 μM have been used to permeabilize cell membranes [26]. For initial characterization, the effect of LPC exposure on mitochondrial permeability was assessed. Isolated mitochondria was incubated with LPC in the presence of albumin to mimic intracellular environment where LPC is found to be transported by fatty acid binding proteins [27]. LPC induced mitochondrial swelling in a concentration-dependent manner from 50 μM to 200 μM (Fig. 1). This response was not inhibited by 2 μM CsA, indicating that LPC did not open the Ca2+-sensitive membrane transition permeability pore in the absence of Ca2+ ion. The increase in mitochondrial swelling coincided with cytochrome c release into the media. Whereas the levels of cytochrome c in the media of mitochondria incubated with 50 μM LPC was similar to controls, incubation with 80 to 200 μM LPC resulted in a concentration-dependent increase of cytochrome c release (Fig. 2). The amount of cytochrome c released by incubation with 200 μM was 2-fold greater than controls, but was at least 50-fold less than the amount of cytochrome c released after incubation and solublization of mitochondria with SDS.
Fig. 1.
Effects of LPC on mitochondrial swelling. Mitochondria (0.7 mg protein/mL) were incubated with 5 mM glutamate and 5 mM malate as respiratory substrates and the indicated concentrations of LPC in the absence (open bars) or presence (filled bars) of CsA. Mitochondrial swelling was assessed by measuring absorbance at 530 mm one min after exposure to LPC. The absorbance of mitochondria incubated with respiratory substrates and SDS is signified by the dotted line. Error bars denote mean ± standard deviations from 3 determinations.
Fig. 2.
LPC induced cytochrome c release from mitochondria. Mitochondria (0.7 mg protein/mL) were incubated with 5 mM glutamate and 5 mM malate and the indicated concentrations of LPC. Cytochrome c released into the incubation media was determined by ELISA assay. The data represent mean ± standard deviations from 3 determinations. * and ** denote significant differences from incubation without LPC at P = 0.01 and P ≤ 0.001, respectively.
3.2 Mitochondria exposed to LPC remained responsive to calcium ion
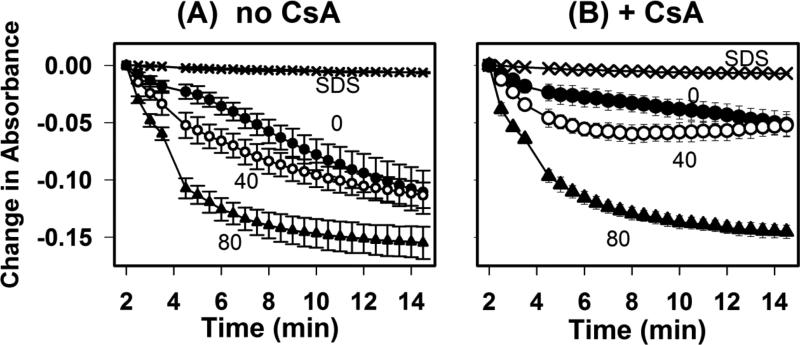
Exogenous administration of Ca2+ causes membrane permeability transition (MPT) in isolated mitochondria, which can lead to cell death and apoptosis in whole cells [28, 29]. Ablation of the membrane-bound iPLA2γ, which produces LPC, has also been shown to suppress mitochondrial swelling and MPT [30]. Calcium-loaded mitochondria also became more permeable after exposed to LPC. Therefore, we determined if LPC may modify the mitochondrial response to Ca2+ [24, 31]. To test this possibility, we examined the concentration-dependent influence of LPC on Ca2+-induced MPT. Kinetic studies showed that the mitochondrial swelling response to 220 μM CaCl2 increased with LPC addition in a time- and concentration-dependent manner (Fig. 3A). To determine whether the effect was due to massive dissolution of the mitochondrial membrane, mitochondria were solubilized using SDS as a negative control (confirmed by microscopy). Such preparations did not exhibit a decrease in absorbance after addition of either concentration of calcium (Fig. 3A). Moreover, the LPC concentration-dependent modulation of calcium-induced mitochondrial swelling was partially suppressed by CsA (comparing Figs. 3A and 3B), thus confirming that mitochondrial membrane was still maintained in the presence of LPC.
Fig. 3.
Effects of LPC on Ca2+-induced mitochondrial swelling. Mitochondria (0.7 mg protein/mL) were incubated with 5 mM glutamate and 5 mM malate respiratory substrates and LPC at 0 (filled circles), 40 (open circles), or 80 (filled triangles) or with SDS (X) in the absence (A) or presence (B) of 2 μM CsA for 2 min prior to the addition of 220 μM CaCl2. Kinetics of mitochondrial swelling was determined based on changes in absorbance very 2.5 min. Data represent mean ± standard deviations from 3 separate determinations.
3.3 LPC causes increased mitochondrial permeability to calcium ion
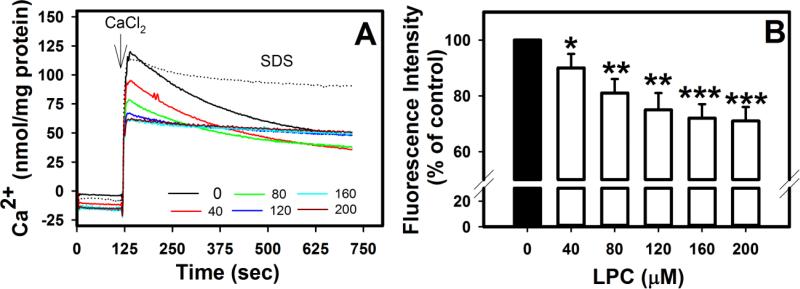
Previous studies have shown that when mitochondria were added to media containing 10 μM Ca2+ and 50-100 μM LPC, Ca2+ uptake was inhibited [31]. Addition of 50 μM LPC to preparations containing mitochondria preloaded with Ca2+ resulted in temporary release of Ca2+ into the media [31]. In contrast, this study examined the effect of LPC on Ca2+ homeostasis by briefly exposing mitochondria to LPC prior to addition 75 μM CaCl2 in order to determine the response of the mitochondrial membrane. To examine this, extramitochondrial Ca2+ was measured by a membrane impermeable fluorescent indicator. In the absence of LPC, Ca2+ uptake occurred gradually in a nonlinear manner within 10 min (Fig. 4A). Surprisingly, brief exposure to LPC resulted in more rapid Ca2+ uptake as shown by decreased equilibration times (Fig. 4A) and decreased peaks in fluorescence intensity (Fig. 4B). With 40 μM and 80 μM LPC, uptake curves were shifted to the left in a concentration-dependent manner. Moreover, with ≥120 μM LPC, fluorescence intensity equilibrated within 20 s, suggesting greater permeability to Ca2+. As a negative control, mitochondria solubilized with SDS showed no decrease in fluorescence intensity following CaCl2 addition. Thus the order of exposure of LPC and Ca2+ may have different effects upon membrane permeability and equilibration according to electrochemical and/or osmotic gradients.
Fig. 4.
Effects of LPC on mitochondrial Ca2+ uptake. Mitochondria (0.7 mg/mL) were incubated with 1 μM Calcium Green 5N and the indicated concentrations of LPC or SDS. Extramembranous Ca2+ was calculated from fluorescence intensity measured at 538 nm after excitation at 485 nm prior to and after addition of 75 μM CaCl2. A shows representative tracings from 3 separate determinations and B shows the mean ± standard deviations of peak fluorescence intensity measured at 35 sec after CaCl2 addition. Differences from control samples without LPC addition are indicated as * P < 0.05, ** P < 0.01, *** P < 0.001.
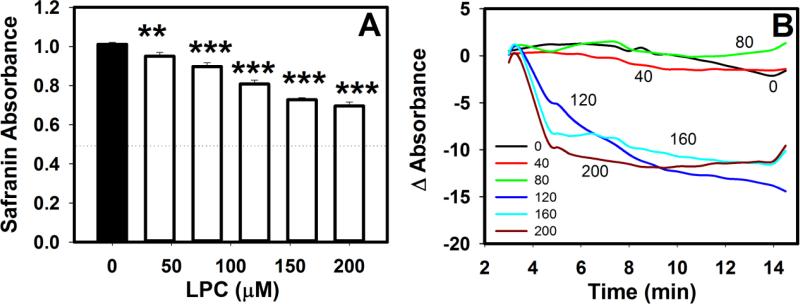
3.4 Mitochondrial membrane potential intact with low micromolar concentrations of LPC
Maintenance of mitochondrial membrane potential is vital to maintaining the proton gradient for coupled oxidative phosphorylation. Since LPC caused increased permeability to Ca2+, we investigated whether LPC also compromised the mitochondrial membrane potential. Safranin absorbance was used to indirectly measure membrane potential since a tight positive correlation exists between this parameter and the mitochondrial membrane potential [17]. A 2 min exposure of mitochondria to LPC in the absence of respiratory substrate caused a linear (R2=0.99) concentration-dependent decrease in safranin absorbance (Fig. 5A). When succinate was added to preparations in order to activate the electron transport chain, the membrane potential remained at initial levels with LPC concentrations up to 80 μM (Fig. 5B). However, with ≥120 μM LPC, respiration caused an additional loss of membrane potential below the already depolarized baseline levels. In contrast, mitochondria solubilized with SDS showed no change in absorbance with succinate addition.
Fig. 5.
Effects of LPC on mitochondrial membrane potential. Mitochondria (0.7 mg protein/mL) were incubated with 25 μM safranin O and LPC at the indicated concentrations. A. Mitochondrial membrane potential was assessed after 2 min incubation based on absorbance at 530 nm. The absorbance of mitochondria incubated with SDS is indicated by the dotted line. The data represent mean ± standard deviations from 4 separate determinations. ** and *** denote differences from no LPC samples at P < 0.01 and P < 0.001. B. Kinetics of absorbance changes after incubating mitochondria with various concentrations of LPC as indicated. Standard deviations are similar to that in panel A but error bars are omitted from the graph for better visualization.
3.5 Maintenance of mitochondrial respiration in the presence of LPC
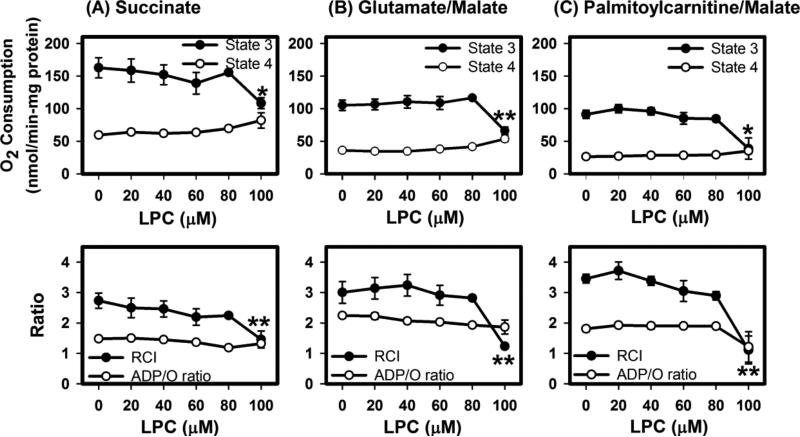
To determine if isolated mitochondria incubated with LPC would maintain optimal oxidative function after2 min exposure to LPC, respiration was measured in the presence of 40-80 μM LPC, concentrations at which the membrane potential remained constant after substrate addition (Fig. 5) but swelling and increased permeability to Ca2+ had occurred (Figs. 1 and 4). Results showed that in the presence of up to 80 μM LPC, mitochondria incubated with succinate to activate complex II maintained maximal state 3 (with ADP) oxygen uptake similar to levels observed in controls. This occurred with no major change in state 4 (without ADP) respiration rates. After brief exposure to 100 μM LPC, state 3 respiration rate and respiratory control index (RCI) decreased dramatically (Fig. 6A).
Fig. 6.
Effects of LPC on mitochondrial respiration. Mitochondria (1 mg protein/mL) were incubated with (A) 5 μM succinate, (B) 5 μM glutamate/5 μM malate, or (C) 10 μM palmitoyl-carnitine/1 μM malate as respiratory substrates. Oxygen levels in the media were measured prior to and after addition of 441 nmol ADP. State 3 respiration rates were determined by slope of oxygen levels over time subsequent to ADP addition. State 4 respiration rates were determined by the slope of oxygen levels after state 3 respiration was completed. The data represent mean ± standard deviations from 4-5 determinations. * and ** denote differences from no LPC addition at P < 0.05 and P < 0.01, respectively.
Respiration rates were also determined in the using glutamate and malate as complex I substrates. Mitochondria briefly exposed to 20-80 μM LPC displayed state 3 respiration rates similar to controls and consistent RCI (Fig. 6B). State 3 oxygen consumption rate and RCI were decreased following brief incubation with 100 μM LPC.
Deficiencies in transport of fatty acid across the mitochondrial membrane (e.g. CPT1 deficiency) can slow the oxidation rate [32]. Since LPC alters the membrane, transport of fatty acids may be affected. To test this possibility, oxidation was stimulated by addition of palmitoyl-carnitine as a respiratory substrate. Again, state 3 respiration rate was similar to controls with incubations of ≤80 μM LPC. However, 100 μM LPC inhibited state 3 respiration rate without affecting state 4 respiration rate. The decrease in oxygen uptake correlated with a decrease in respiratory control index (RCI) but without a significant decrease in phosphorylation efficiency as measured by ADP/O ratio (Fig. 6C).
With all substrates tested, a difference of 20 μM LPC caused a great effect on respiration rates. Taken together, these results suggest that small fluctuations in LPC concentration even with brief exposure within the mitochondrial microenvironment can have a profound effect on mitochondrial function.
3.6 LPC regulates fatty acid-stimulated hepatic oxidation
The concentration of LPC in the intracellular microenvironment of mitochondria is modified in part by intracellular phospholipases and lysophospholipases, fatty acid binding proteins, and equilibration with mitochondrial and cellular membranes [24, 33, 34]. The liver encounters a Pla2g1b-dependent increase in portal lysophospholipids as well as an increase in plasma non-esterified fatty acids in the postprandial state [9]. Intracellular LPC concentration could possibly be modified by exogenous lysophospholipids delivered to the cell via the plasma. In order to determine the effect of extracellular LPC on whole cell oxidative function, murine primary hepatocytes were isolated and mitochondrial bioenergetics measurements were taken after incubation with fatty acid, LPC, and mitochondrial inhibitors.. Albumin was added to preparations in order to better simulate plasma conditions and to decrease the membrane-permeabilizing characteristics of LPC [26].
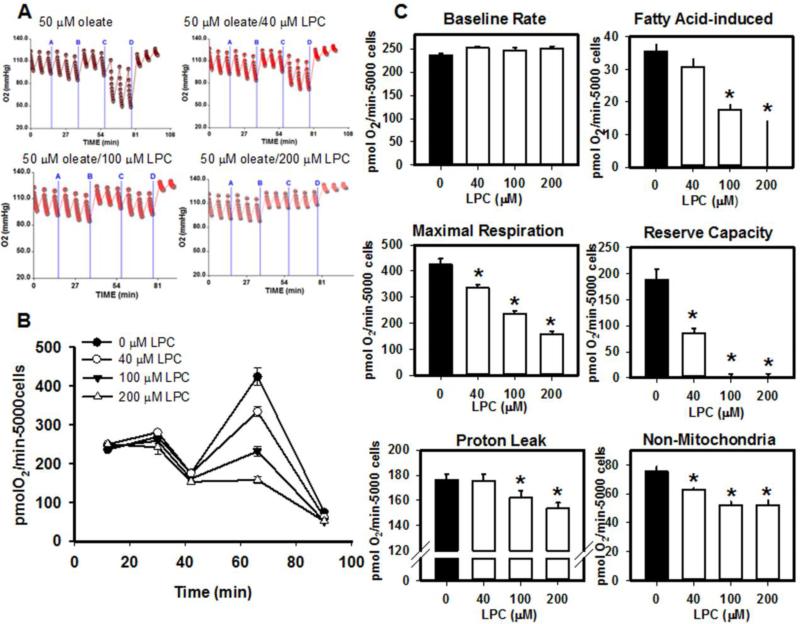
Results showed that incubation of hepatocytes with fatty acid alone resulted in a 10-20% increase in oxygen consumption rate above baseline (Fig. 7). When LPC was co-administered with fatty acid, a concentration-dependent decrease in fatty acid-stimulated oxidation and a reduction in maximal respiration were observed. A decrease in oxidation reserve capacity was also observed with LPC and fatty acid co-administration (Fig. 7). The addition of LPC also also resulted in decreased proton leak and non-mitochondrial respiration (Fig. 7). Incubation of hepatocytes with 500-1500 μM LPC and fatty acid resulted in OCR decreasing below baseline levels and further decline in OCR with FCCP stimulation (not shown).
Fig. 7.
Effects of extracellular LPC on fatty acid-stimulated oxidation in isolated hepatocytes. Primary mouse hepatocytes were plated at 5000 cells/well in a collagen-coated 96-well Seahorse plates and treated with 50 μM oleate with or without the indicated concentrations of LPC at 15 min (A). Oligomycin (10 μg/mL) was added at 30 min (B), 3 μM FCCP was added at 40 min (C), and 4 μM antimycin A/1 μM rotenone were added at 65 min (D). Panel A shows representative tracings at each LPC concentration. Panel B shows mean data ± standard deviations from 3-6 separate determinations. Panel C shows baseline oxygen consumption rate (OCR) prior to addition of oleate and LPC. Fatty acid induced rate was determined by subtracting basal OCR from the increasein OCR following addition of oleate or oleate/LPC. Maximal respiration was determined following addition of FCCP. Reserve capacity was determined by the remaining OCR following administration of oligomycin. Non-mitochondrial respiration was determined from the respiration following administration of antimycin A and rotenone. Data represent mean ± standard error. * denotes significant differences from samples without LPC addition at P < 0.05.
3.7 Extracellular LPC induces mitochondrial permeability
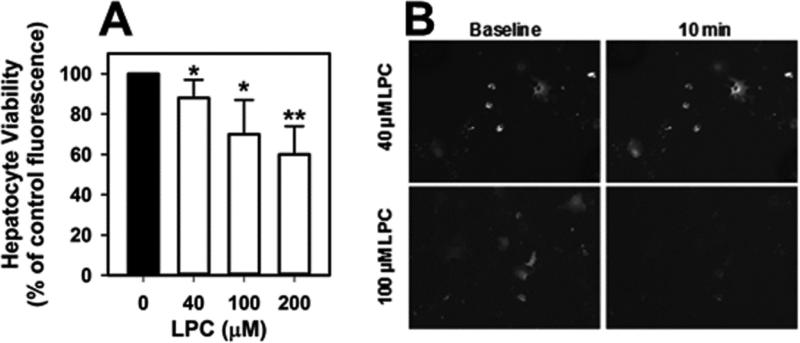
Hepatocyte viability was assessed by two methods. Firstly, during mitochondrial bioenergetics studies, O2 tension within the wells was measured using the Seahorse XF96. With each concentration of LPC/oleate added, O2 decreased sharply, and while the O2 tension change declined with increasing concentrations of LPC, the recovery of O2 tension demonstrated that the LPC did not cause cell death (Fig. 7). Secondly, hepatocytes were exposed to a longer incubation with LPC/oleate and then labeled with calcein-AM and CoCl2 prior to the addition of fatty acid to visualize mitochondrial permeability [22, 23]. The addition of Co2+ is expected to quench the fluorescence signal of calcein in the cytosol but not the mitochondria when the mitochondrial membrane is impermeable to small molecules. Mitochondrial fluorescence in hepatocytes incubated with 40 μM LPC and fatty acid was comparable to that observed in controls, indicative of mitochondrial impermeability. However, hepatocytes incubated with and above 100 μM LPC showed decreased mitochondrial fluorescence, which is consistent with increased mitochondrial permeability (Fig. 8). Pretreatment with 2 μM CsA did not prevent the decrease in mitochondrial fluorescence observed in the presence of 100 μM or 200 μM LPC (not shown), suggesting a Ca2+-independent mechanism. The influence of exogenous LPC on cellular metabolic activity was determined by labeling cells with calcein-AM after their incubation with LPC. A concentration-dependent decrease in fluorescence compared to control cells incubated in the absence of LPC was observed (Fig. 8). Thus while low extracellular levels of LPC caused some cytosolic dysfunction, oxidation remained intact. However, higher concentrations of LPC caused mitochondrial permeability and decreased oxidative function. Mitochondrial permeability coincided with a reduced catabolic activity in cells (Fig 8).
Fig. 8.
Concentration-dependent effects of LPC on hepatocyte viability. A. Primary mouse hepatocytes were incubated with 100 μM oleate complexed with albumin at a 5:1 ratio and LPC for 10 min prior to incubation with 2 μM calcein-AM and 8 mM CoCl2 at 37°C in the dark. Cell viability was determined based on cytoplasmic activity assessed by measuring fluorescence intensity after excitation at 485 nm and emission at 538 nm. The data represent mean ± standard deviations from 5 different experiments. * and ** denote differences from cells incubated without LPC at P < 0.05 and P < 0.01, respectively. B. Images of hepatocytes before and after incubation for 10 min with oleate/BSA in the presence or40 or 100 μM LPC. Intact mitochondria show bright punctate appearances.
4. Discussion
The current study showed the ability of isolated mitochondria to sustain maximal oxidative function with a variety of respiratory substrates (succinate, glutamate/malate, and acyl-carnitine) in the presence of micromolar (≤80 μM) concentrations of LPC despite the presence of increased membrane permeability to water and Ca2+ , release of cytochrome c,and reduced membrane potential. When the concentration of LPC increased to ≥100 μM, further mitochondrial membrane permeability, increased susceptibility to Ca2+-induced damage, release of cytochrome c, exacerbated impairment of mitochondrial membrane potential, and decreased in oxidative function were observed. Our data also showed that exogenous addition of low micromolar concentrations of LPC to primary hepatocytes had minimal impact on fatty acid-stimulated oxidation. However, increasing the extracellular LPC concentration by 60 μM resulted in hepatocytes that displayed increased mitochondrial permeability and reduced oxidative function. Interestingly, the threshold concentration of LPC required to induce functional deficits in primary hepatocytes and isolated mitochondria converged at approximately 80-100 μM LPC.
The in vitro data collected in this study has direct physiological relevance, adding mechanistic information to our previous observations that PLA2G1B-mediated phospholipid digestion in the intestinal lumen after meal consumption contributes LPC to the liver to inhibit fatty acid oxidation and promote triglyceride synthesis for storage and/or VLDL secretion [8-10]. Herein we showed that meal-induced repression of fatty acid oxidation is likely caused by LPC-mediated suppression of mitochondrial function, thereby partitioning fatty acids absorbed from the meal to intracellular sites for triglyceride biosynthesis. Although the effective concentrations used in the current in vitro studies were lower than plasma LPC levels in vivo, plasma LPC binds tightly to albumin and albumin stimulates the release of hepatic LPC [5, 10, 13, 35, 36]. In addition, absorbed LPC from the gut is directed to the liver by the portal vein prior to accessing the systemic circulation [4]. Thus, the effective concentration of LPC that causes inhibition of hepatic mitochondrial function may be much less than LPC levels in the plasma. In any event, the current study highlights that small changes in the effective LPC concentration could alter hepatocyte function. Others have shown that extracellular administration of LPC leads to increases in cytoplasmic LPC [37, 38]. Alternatively, hepatocytes in situ may be acclimated to the prevailing plasma LPC concentration and the small changes in postprandial LPC are sufficient to inhibit hepatocyte fatty acid-stimulated oxidation.
In addition to the direct biophysical effect, LPC is also known to have a number of signaling effects that may play a role in postprandial hepatic oxidative functions. LPC promotes postprandial hyperglycemia by decreasing insulin sensivity [4]. LPC also increases JNK acdtivation and promotes insulin resistance in myocytes and may also play a large role in insulin resistance in hepatocytes [39, 40]. Extracellular administration of LPC results in an increase in JNK phosphorylation within 15-30 min [37, 39, 41]. Activated JNK phosphorylates peroxisome proliferator-activated receptor-γ (PPARγ) within 30 min, which decreases its transcriptional activity and reduces the effects of PPARγ ligands, such as fatty acids [42]. LPC also causes activation of the extracellular signal-regulated kinase mitogen-activated protein kinase and causes the production of reactive oxygen species through altering cytosolic Ca2+ levels [43]. Interestingly, these LPC effects on cell signaling may be a direct consequence of LPC-induced changes in mitochondrial permeability and generation of reactive oxygen species as demonstrated in endothelial cells [44].
The current study also showed that alterations in hepatic mitochondrial integrity and function occurred during a short incubation period with LPC. These observations are also consistent with previous reports showing Pla2g1b inactivation resulted in increased fatty acid oxidation in mouse liver after lipid meal consumption and that acute treatment with LPC abolished this effect [9]. Interestingly, chronic feeding of high fat diet to mice have been shown to promote hepatic steatosis, which is characterized not only by increased hepatic triglyceride content, but also increased hepatic LPC [38, 43]. Diet-induced hepatomegaly can also be reduced by Pla2g1b inactivation [8]. Furthermore, and consistent with our data showing the loss of mitochondrial integrity and increased release of cytochrome c with hepatocyte toxicity at high concentrations of LPC, extended exposure of hepatocytes to LPC can cause mitochondrial release of Bax to trigger apoptosis, and LPC is an important player in palmitate-induced lipoapoptosis of hepatocytes [37, 45]. However, coincubation of Chang cells with LPC and oleate attenuated the apoptosis observed, suggesting that a prolonged mismatch in lipid digestive products may be necessary to precipitate disease [38]. The action of other digestive enzymes also contribute dietary and biliary fatty acids to the gut milieu from the digestion of phospholipids, triglycerides, and cholesteryl esters.
Taken together, these results showed that LPC may participate in both acute and chronic responses of hepatocytes to high fat diet by modulating mitochondrial function and integrity in a concentration-dependent manner. Acute exposures lead to decreased fatty acid oxidation, which is beneficial in the postprandial state so sufficient fatty acid substrates are available for VLDL production. However, prolonged exposures lead to decreased energy production, cell injury, and hepatic disease. Thus, pharmacological intervention to lower LPC levels and/or absorption in response to high fat diet may be a viable strategy to reduce high fat diet-induced hepatosteatosis and its accompanying risk of metabolic diseases. Preliminary studies showing efficacy of the generic PLA2 inhibitor methyl indoxam in improving diet-induced glucose intolerance, which can be mediated by absorbed LPC, are consistent with this possibility [4, 46].
Highlights.
Lysophosphatidylcholine (LPC) increases mitochondrial permeability to Ca2+.
Mitochondria have steady, but lowered membrane potential at low micromolar LPC.
Mitochondria retain maximum oxidation rates in low micromolar LPC.
A 20 μM LPC difference in mitochondrial microenvironment decreases oxidation by 30%.
Exogenous LPC causes mitochondrial dysfunction in primary hepatocytes.
Acknowledgements
This work was supported by Grant DK069967 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. N.I. Hollie was a fellowship recipient of grants F31HL110527 from the National Heart, Lung, And Blood Institute, Grant Number T32GM063483 from the National Institute of General Medical Sciences, and Grant Number 11PRE7310047 from the American Heart Association during the course of this study.
List of abbreviations
- AM
acetoxymethylester
- CsA
cyclosporine A
- FCCP
carbonyl cyanide 4-(trifluoromethoxy) phenyhydrazone
- iPLA2
calcium independent phospholipase A2
- LPC
lysophosphatidylcholine
- MOPS
3-(N-Morpholino)propanesulfonic acid
- MPT
membrane permeability transition
- OCR
oxygen consumption rate
- PLA2
phospholipase A2
- PPAR
peroxisome proliferator-activated receptor
- RCI
respiratory control index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 2.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Richmond BL, Hui DY. Molecular structure and tissue-specific expression of the mouse pancreatic phospholipase A2 gene. Gene. 2000;244:65–72. doi: 10.1016/s0378-1119(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 4.Labonté ED, Kirby RJ, Schildmeyer NM, Cannon AM, Huggins KW, Hui DY. Group 1B phospholipase A2-mediated lysophospholipid absorption directly contributes to postprandial hyperglycemia. Diabetes. 2006;55:935–941. doi: 10.2337/diabetes.55.04.06.db05-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portman OW, Soltys P, Alexander M, Osuga T. Metabolism of lysolecithin in vivo: effects of hyperlipemia and atherosclerosis in squirrel monkeys. J. Lipid Res. 1970;11:596–604. [PubMed] [Google Scholar]
- 6.Rabini RA, Galassi R, Fumelli P, Dousset N, Solera ML, Valdiguie P, Curatola G, Ferretti G, Taus M, Mazzanti L. Reduced Na+ -K+ ATPase activity and plasma lysophosphatidylcholine concentrations in diabetic patients. Diabetes. 1994;43:915–919. doi: 10.2337/diab.43.7.915. [DOI] [PubMed] [Google Scholar]
- 7.Guo S, Shi X, Yang F, Chen L, Meehan EJ, Bian C, Huang M. Structural basis of transport of lysophospholipids by human serum albumin. Biochem. J. 2009;423:23–30. doi: 10.1042/BJ20090913. [DOI] [PubMed] [Google Scholar]
- 8.Huggins KW, Boileau AC, Hui DY. Protection against diet-induced obesity and obesity-related insulin resistance in Group 1B PLA2-deficient mice. Am. J. Physiol. 2002;283:E994–E1001. doi: 10.1152/ajpendo.00110.2002. [DOI] [PubMed] [Google Scholar]
- 9.Labonté ED, Pfluger PT, Cash JG, Kuhel DG, Roja JC, Magness DP, Jandacek RJ, Tschöp MH, Hui DY. Postprandial lysophospholipid suppresses hepatic fatty acid oxidation: the molecular link between group 1B phospholipase A2 and diet-induced obesity. FASEB J. 2010;24:2516–2524. doi: 10.1096/fj.09-144436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollie NI, Hui DY. Group 1B phospholipase A2 deficiency protects against diet-induced hyperlipidemia in mice. J. Lipid Res. 2011;52:2005–2011. doi: 10.1194/jlr.M019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z, Luchoomun J, Bakillah A, Hussain MM. Lysophosphatidylcholine increases apolipoprotein B secretion by enhancing lipid synthesis and decreasing its intracellular degradation in HepG2 cells. Biochim. Biophys. Acta. 1998;1391:13–24. doi: 10.1016/s0005-2760(97)00200-2. [DOI] [PubMed] [Google Scholar]
- 12.Wanninger J, Neumeier M, Weigert J, Liebisch G, Weiss TS, Schaffler A, Aslanidis C, Schmitz G, Scholmerich J, Buechler C. Metformin reduces cellular lysophosphatidylcholine and thereby may lower apolipoprotein B secretion in primary human hepatocytes. Biochim. Biophys. Acta. 2008;1781:321–325. doi: 10.1016/j.bbalip.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Robinson BS, Yao Z, Baisted DJ, Vance DE. Lysophosphatidylcholine metabolism and lipoprotein secretion by cultured rat hepatocytes deficient in choline. Biochem. J. 1989;260:207–214. doi: 10.1042/bj2600207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran K, Wang Y, DeLong CJ, Cui Z, Yao Z. The assembly of very low density lipoproteins in rat hepatoma McA-RH7777 cells is inhibited by phospholipase A2 antagonists. J. Biol. Chem. 2000;275:25023–25030. doi: 10.1074/jbc.M908971199. [DOI] [PubMed] [Google Scholar]
- 15.Matlib MA, Shannon WA, Srere PA. Measurement of matrix enzyme activity in situ in isolated mitochondria made permeable with toluene. Methods Enzymol. 1979;56:544–550. doi: 10.1016/0076-6879(79)56052-2. [DOI] [PubMed] [Google Scholar]
- 16.Henriksen JR, Andresen TL, Feldborg LN, Duelund L, Ipsen JH. Understanding detergent effects on lipid membranes: a model study of lysolipids. Biophys. J. 2010;98:2199–2205. doi: 10.1016/j.bpj.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akerman KE, Wikstrom MK. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976;68:191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- 18.Basford JE, Wancata L, Hofmann SM, Silva RA, Davidson WS, Howles PN, Hui DY. Hepatic deficiency of low density lipoprotein receptor related protein-1 reduces high density lipoprotein secretion and plasma levels in mice. J. Biol. Chem. 2011;286:13079–13087. doi: 10.1074/jbc.M111.229369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill BG, Benavides GA, Lancaster JR, Jr, Ballinger S, Dell'Italia L, Jianhua Z, Darley-Usmar VM. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelickson BR, Benavides GA, Johnson MS, Chacko BK, Venkatraman A, Landar A, Betancourt AM, Bailey SM, Darley-Usmar VM. Nitric oxide and hypoxia exacerbate alcohol-induced mitochondrial dysfunction in hepatocytes. Biochim. Biophys. Acta. 2011;1807:1573–1582. doi: 10.1016/j.bbabio.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woollacott AJ, Simpson PB. High throughput fluorescence assays for the measurement of mitochondrial activity in intact human neuroblastoma cells. J. Biomol. Screen. 2001;6:413–420. doi: 10.1177/108705710100600607. [DOI] [PubMed] [Google Scholar]
- 23.Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa F. Transient and long lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys. J. 1999;76:725–734. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rustenbeck I, Munster W, Lenzen S. Relationship between accumulation of phospholipase A2 reaction products and Ca2+ release in isolated liver mitochondria. Biochim. Biophys. Acta. 1996;1304:129–138. doi: 10.1016/s0005-2760(96)00113-0. [DOI] [PubMed] [Google Scholar]
- 25.Robinson N, Saunders L. The physical properties of lysolecithin and its sols. I. Solubilities, surface and interfacial tensions. J. Pharm. Pharmacol. 1958;10:384–391. doi: 10.1111/j.2042-7158.1958.tb10320.x. [DOI] [PubMed] [Google Scholar]
- 26.Schroff RW, Bucana CD, Klein RA, Farrell MM, Morgan ACJ. Detection of intracytoplasmic antigens by flow cytometry. J. Immunol. Methods. 1984;70:167–177. doi: 10.1016/0022-1759(84)90182-0. [DOI] [PubMed] [Google Scholar]
- 27.Thumser AE, Wilton DC. The binding of natural and fluorescent lysophospholipids to wild type and mutant rat liver FA-binding protein and albumin. Biochem. J. 1995;307:305–311. doi: 10.1042/bj3070305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyanagi E, Yano H, Kato Y, Fujita H, Utsumi K, Sasaki J. L-carnitine suppresses oleic-acid induced membrane permeability transition of mitochondria. Cell Biochem. Funct. 2008;26:778–786. doi: 10.1002/cbf.1506. [DOI] [PubMed] [Google Scholar]
- 29.Garcia N, Correa F, Chavez E. On the role of the respiratory complex I on membrane permeability transition. J. Bioenerg. Biomembr. 2005;37:17–23. doi: 10.1007/s10863-005-4119-9. [DOI] [PubMed] [Google Scholar]
- 30.Moon SH, Jenkins CM, Kiebish MA, Sims HF, Mancuso DJ, Gross RW. Genetic ablation of calcium-independent phospholipase A2γ (iPLA2γ) attenuates calcium-induced opening of the mitochondrial permeability transition pore and resultant cytochrome c release. J. Biol. Chem. 2012;287:29837–29850. doi: 10.1074/jbc.M112.373654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenzen S, Gorlich J-K, Rustenbeck I. Regulation of transmembrane ion transport by reaction products of phospholipase A2. I. Effects of lysophospholipids on mitochondrial Ca2+ transport. Biochim. Biophys. Acta. 1989;982:140–146. doi: 10.1016/0005-2736(89)90184-3. [DOI] [PubMed] [Google Scholar]
- 32.Fontaine M, Dessein AF, Douillard C, Dobbelaere D, Brivet M, Boutron A, Zater M, Mention-Mulliez K, Martin-Ponthieu A, Vianey-Saban C, Briand G, Porchet N, Vamecq J. A novel mutation in CPT1a resulting in hepatic CPT deficiency. JIMD Rep. 2012;6:7–14. doi: 10.1007/8904_2011_94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancuso DJ, Sims HF, Yang K, Kiebish MA, Su X, Jenkins CM, Guan S, Moon SH, Pietka T, Nassir F, Schappe T, Moore K, Han X, Abumrad NA, Gross RW. Genetic ablation of calcium-independent phospholipase A2γ prevents obesity and insulin resistance during high fat feeding by mitochondrial uncoupling and increased adipocyte fatty acid oxidation. J. Biol. Chem. 2010;285:36495–36510. doi: 10.1074/jbc.M110.115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagakos WS, Gajda AM, Agellon L, Binas B, Choi V, Mandap B, Russnak T, Zhou YX, Storch J. Different functions of intestinal and liver-type fatty acid binding proteins in intestine and in whole body energy homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G803–G814. doi: 10.1152/ajpgi.00229.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YL, Im YJ, Ha NC, Im DS. Albumin inhibits cytotoxic activity of lysophosphatidylcholine by direct binding. Prostaglandins Other Lipid Mediat. 2007;83:130–138. doi: 10.1016/j.prostaglandins.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Robinson BS, Baisted DJ, Vance DE. Comparison of albuin-mediated release of lysophosphatidylcholine and lysophosphatidylethanolamine from cultured rat hepatocytes. Biochem. J. 1989;264:125–131. doi: 10.1042/bj2640125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakisaka K, Cazanave SC, Fingas CD, Guicciardi ME, Bronk SF, Werneburg NW, Mott JL, Gores GJ. Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G77–G84. doi: 10.1152/ajpgi.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han MS, Park SY, Shinzawa K, Kim S, Chung KW, Lee J-H, Kwon CH, Lee K-W, Lee J-H, Park CK, Chung WJ, Hwang JS, Yan J-J, Song D-K, Tsujimoto Y, Lee M-S. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J. Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Han MS, Lim Y-M, Quan W, Kim JR, Chung KW, Kang M, Kim S, Park SY, Han J-S, Park S-Y, Cheon HG, Dal Rhee S, Park T-S, Lee M-S. Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance. J. Lipid Res. 2011;52:1234–1246. doi: 10.1194/jlr.M014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaneto H, Nakatani Y, Kawamori D, Miyatsuka T, Matsuoka TA. Involvement of oxidative stress and the JNK pathway in glucose toxicity. Rev. Diabet. Stud. 2004;1:165–174. doi: 10.1900/RDS.2004.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang X, Gibson S, Flowers M, Furui T, Best RCJ, Mills GB. Lysophosphatidylcholine stimulates activator protein 1 and the c-Jun N-terminal kinase activity. J. Biol. Chem. 1997;272:13683–13689. doi: 10.1074/jbc.272.21.13683. [DOI] [PubMed] [Google Scholar]
- 42.Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology. 1999;140:392–397. doi: 10.1210/endo.140.1.6457. [DOI] [PubMed] [Google Scholar]
- 43.Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, Lutjohann D, Kerksiek A, van Kruchten R, Maeda N, Staels B, van Bilsen M, Shiri-Sverdlov R, Hofker MH. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474–486. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe N, Zmijewski JW, Takabe W, Umezu-Goto M, Le Goffe C, Sekine A, Landar A, Watanabe A, Aoki J, Arai H, Kodama t., Murphy MP, Kalyanaraman R, Darley-Usmar VM, Noguchi N. Activation of mitogen-activated protein kinases by lysophosphatidylcholine-induced mitochondrial reactive oxygen species generation in endothelial cells. Am. J. Pathol. 2006;168:1737–1748. doi: 10.2353/ajpath.2006.050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han MS, Park SY, Shinzawa K, Kim S, Chung KW, Lee J-H, Kwon CH, Lee K-W, Lee J-H, Park CK, Chung WJ, Hwang JS, Yan J-J, Song D-K, Tsujimoto Y, Lee M-S. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J. Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Hui DY, Cope MJ, Labonte ED, Chang H-T, Shao J, Goka E, Abousalham A, Charmot D, Buysse J. The Phospholipase A2 inhibitor methyl indoxam suppresses diet-induced obesity and glucose intolerance in mice. Br. J. Pharmacol. 2009;157:1263–1269. doi: 10.1111/j.1476-5381.2009.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]