Abstract
QRFP, a member of the RFamide-related peptide family, is a strongly conserved hypothalamic neuropeptide that has been characterized in various species. Prepro-QRFP mRNA expression is localized to select regions of the hypothalamus, which are involved in the regulation of feeding behavior. The localization of the peptide precursor has led to the assessment of QRFP on feeding behaviors and the orexigenic effects of QRFP have been detected in mice, rats, and birds. QRFP acts in a macronutrient specific manner in satiated rats to increase the intake of a high fat diet, but not the intake of a low fat diet, and increases the intake of chow in food-restricted rats. Studies suggest that QRFP’s effects on food intake are mediated by the adiposity signal, leptin, and hypothalamic neuropeptides. Additionally, QRFP regulates the expression and release of hypothalamic Neuropeptide Y and proopiomelanocortin/α-Melanocyte-Stimulating Hormone. QRFP binds to receptors throughout the brain, including regions associated with food intake and reward. Taken together, these data suggest that QRFP is a mediator of motivated behaviors, particularly the drive to ingest high fat food. The present review discusses the role of QRFP in the regulation of feeding behavior, with emphasis on the intake of dietary fat.
Keywords: high fat diet, neuropeptide Y, agouti-related peptide, arcuate nucleus, ventromedial hypothalamus
Introduction
Within the last decade, a 26-amino acid peptide exhibiting the Arg-Phe-NH2 signature was isolated from the brains of frogs [1]. This peptide, named QRFP-26 or 26RFa (pyroglutamylated argininephenylalanineamide peptide) is strongly conserved across vertebrates and is a member of the RFamide-related peptide family. This family of peptides was first discovered in the venus clam, is biologically active, and has the motif Arg-Phe-NH2 at the C-terminal end. Several RFamide-related peptides have been characterized in vertebrates and invertebrates and affect a variety of physiological systems. These peptides affect analgesia, food intake, locomotor activity, blood pressure, hormone regulation, and the reproductive axis [2 – 12]. Recently, the cDNA encoding QRFP-26 has been characterized in chicks and quail [13], goldfish [14], zebra finch [15], bovine [16], rat [1, 16], mouse [16, 17] and humans [1, 16, 17]. Several studies have been conducted with QRFP-26 or the N elongated form, QRFP-43, which have indicated that these peptides increase food intake and locomotor activity, reduce core temperature and enhance luteinizing hormone release, follicle stimulating hormone release, and gonadotropin releasing hormone release [1, 18 – 25].
The effects of QRFP-26 and QRFP-43 on feeding behavior have been investigated in several studies, which report an orexigenic effect of these peptides. In male and female rats, QRFP-26 and QRFP-43 administration alters macronutrient selection and specifically increases the intake of a calorically dense high fat diet [23, 24]. Increases in the intake of high fat diets have been linked to an overconsumption of calories, which increases the propensity for weight gain and subsequent risk of obesity. The regulation of feeding behavior is complex and involves the consolidation of peripheral and central signals and the ability to sense and detect specific nutrients [26 – 31]. Studies investigating factors contributing to high fat food intake, weight gain, and obesity have employed both human and animal models and have been instrumental in the development of strategies to combat increasing rates of obesity and its cormorbidities [32 – 41]. In the present review, we will focus on the role of QRFP-26 and QRFP-43 on feeding behavior, with an emphasis on the relationship between QRFP-26 and QRFP-43 and high fat food.
QRFP and GPR103: Distribution in the Hypothalamus
The structure of QRFP-26 is strongly conserved across vertebrates, in particular the C-terminal octapeptide, suggesting that this region is crucial for the biological activity of the peptide. Based on a comparison of the amino acid sequence of QRFP-26, the primary structure of QRFP has a similarity ranging from 74–85 % across mammals, birds and amphibians, and an 80 % homology between humans and rats/mice [1, 2, 42, 43]. The QRFP precursor has been shown to generate a 26-amino acid peptide (QRFP-26) and an N-terminal extended form of 43-amino acids (QRFP-43), both of which exert behavioral effects in rodents [1, 18 – 20, 23 – 25].
The expression and localization of prepro-QRFP mRNA in the central nervous systems of a variety of species including mice, rats, chicks, goldfish, zebra finch, and quail have been assessed using in situ hybridization and PCR. In these species, prepro-QRFP mRNA is almost exclusively expressed in the hypothalamus, a region of the brain that is important in the regulation of feeding behavior. In the rat hypothalamus, expression of prepro-QRFP mRNA is limited to the arcuate nucleus (ARC), retrochiasmatic area, lateral hypothalamus (LH), and ventromedial hypothalamus (VMH) [1, 24, 44]. In mice, prepro-QRFP mRNA is limited to the LH and periventricular hypothalamic nucleus [4, 17, 25]. Outside of the forebrain, prepro-QRFP mRNA is expressed in the cerebellum and spinal cord in mice [17]. In the bird, prepro-QRFP mRNA is expressed in anterior hypothalamic nuclei [13]. The site-specific expression of the QRFP gene in the hypothalamus, which is a prominent in feeding neurocircuitry, initiated studies to evaluate the effects of QRFP-26 and QRFP-43 on feeding behavior.
QRFP-26 and QRFP-43 are potent ligands for GPR103, a G protein-coupled receptor. In humans, one receptor for QRFP-26 and QRFP-43 has been identified, GPR103, also referred to as SP9155 or AQ27 [4, 17]. Two GPR103 receptors, GPR103a and GPR103b, have been characterized in rodents and are differentially expressed throughout the brain [4, 17, 25, 44, 45]. The human GPR103 receptor exhibits 85 % amino acid similarity with the mouse GPR103a and 79 % amino acid identity with the mouse GPR103b [25]. In the rat, GPR103a (QRFP-r1) has an 84 % similarity and GPR103b (QRFP-r2) has a 82 % amino acid identity to human GPR103 [44]. In the mouse, GPR103a and GPR103b exhibit a 75 % amino acid similarity [25], while in rat the 2 receptors exhibit a 78 % similarity [44]. While both QRFP-26 and QRFP-43 bind to GPR103a, QRFP-43 exhibits a higher affinity for this receptor than QRFP-26, which exhibits a higher affinity for GPR103a than smaller fragments of the QRFP peptide [16]. In rodents, QRFP-43 and QRFP-26 have a similar efficacy for both GPR103 receptors [25, 44].
These receptors are more widely expressed than the peptide precursor and are found in the various regions of the central nervous system. Based on in situ hybridization analyses, GPR103a (QRFP-r1) mRNA is expressed in regions of the brain which include, but are not limited to, the olfactory bulbs, nucleus of the solitary tract, VMH, and cortex, [4, 25, 44]. The second receptor, GPR103b (QRFP-r2) mRNA is detected in numerous brain regions including, but not limited to, sub-regions of the septum, hypothalamus, amygdala, thalamus, hippocampus, midbrain, pons, cerebellum, and medulla. Within the hypothalamus, GPR103b mRNA expression was detected in the paraventricular hypothalamus, VMH, anterior hypothalamus, and the medial preoptic area [25, 44]. The brain regions, which express these receptors, provide insight into the sites of actions of QRFP and allow hypotheses to be made regarding the behavioral effects of QRFP-26 and QRFP-43. Further investigations on the relationship between QRFP-26 and QRFP-43 and the GPR130a and GPR103b receptors have used cell culture models to determine the effects of QRFP on intracellular Ca++ levels. These studies have determined that QRFP-26 and QRFP-43 exert its actions by binding to GPR103a and GPR103b receptors and increasing intracellular Ca++ levels, which is suggestive of an excitatory response [25].
The localization of QRFP precursor expression and the expression of its receptors provides insight into the behavioral effects of the QRFP peptides and the physiological systems which may be affected by the release and binding of QRFP-26 and QRFP-43. The expression of prepro-QRFP mRNA is primarily limited to the hypothalamus, however, due to the more widespread expression of the 2 QRFP receptors in the rodent brain, it is hypothesized that QRFP-26 and QRFP-43 exert actions in various brain regions within the hypothalamus and beyond the hypothalamus. A detailed analysis of the distribution of QRFP-26 binding sites in the central nervous system using radio-ligand binding has been conducted to further assess the neuronal sites of action of QRFP-26. In addition, these QRFP-26 binding sites have been colocalized with the expression of the receptor, GPR103a, using in situ hybridization [45]. The results from this study allowed for the assessment of the relationship between the QRFP-26 and GPR103a. Several brain regions associated with feeding behavior were considered QRFP-26 binding sites and many also expressed GPR103a. These binding sites included the nucleus accumbens and ventral tegmental area, which are important in reward processing and may regulate QRFP’s effects on high fat food intake. Other QRFP-26 binding regions involved in feeding behavior were the entire amygdalar complex, the locus coeruleus, which is important for arousal, the parabrachial nucleus, which is important for taste processing, the raphe nucleus, and the nucleus of the solitary tract, which is an important modulator of nutrient intake [28 – 31, 46]. In the hypothalamus, QRFP-26 binding sites were reported in many subregions and colocalized with GPR103 in the VMH, LH, ARC, paraventricular nucleus, and dorsomedial hypothalamus. The data gathered from this study provided evidence for the binding of QRFP-26 throughout the brain and suggested that the wide distribution of binding sites meant that QRFP-26 has multiple functions throughout the central nervous system. In some brain regions, QRFP binding and GPR103a receptor mRNA was not colocalized, leaving the possibility for another receptor. This study did not assess the co-localization of GPR103b mRNA and QRFP-26 binding, which could account for some of their findings. Additionally, binding studies have indicated that QRFP-26 can also bind to the NPFF-2 receptor with moderate affinity and selectivity [47]. In the rat, NPFF-2 binding sites are located in the nucleus accumbens, caudate putamen and hypothalamus, which may be relevant sites for QRFP-26 actions on feeding and motivation [48].
Control of Food Intake: QRFP
The hypothalamus is the master regulator of feeding behavior and receives input from peripheral hormones (e. g., glucagon like peptide-1, peptide YY, ghrelin) and adipose signals (e. g., insulin, leptin) and integrates information from multiple brain regions. Hypothalamic subregions are important in the regulation of food intake and are abundant in neurotransmitters, neuropeptides, and receptor systems which regulate food intake, meal size and meal pattern, and the intake of specific macronutrients (i. e., fat). The interaction of circulating signals with neurons in the ARC, including those that express neuropeptide Y (NPY) and agouti-related peptide (AgRP) and/or proopiomelanocortin (POMC) regulate food intake. Activation of NPY/AgRP neurons leads to an increase in food intake, while activation of POMC neurons leads to a decrease in food intake [26, 29 – 31, 49 – 55]. The amount of fat in the diet has profound effects on hypothalamic circuitry and several neuropeptides and receptor systems have been associated with the intake of dietary fat. In particular, various studies have reported that AgRP, galanin, QRFP-26, QPFP-43, and mu opioid receptor agonists selectively increase fat intake in rodents [23, 24, 56 – 60].
Histological studies demonstrating that prepro-QRFP mRNA expression was restricted to the hypothalamus in rodents and birds has provided the basis for the investigation of QRFP’s effects of feeding behavior. The earliest studies assessed the effects of central administration of QRFP on food intake in mice, while more recent studies have examined the effects of centrally administered QRFP in rats, chicks and the zebra finch (Table 1). The majority of these studies have reported an orexigenic effect of QRFP in these animal models. However, there have been conflicting studies on the effects of QRFP on food intake in rats, which will be discussed in detail in this review.
Table 1.
Feeding effects of QRFP in various species.
| Species | QRFP | Diet | Food intake | Reference |
|---|---|---|---|---|
| Mouse | QRFP-26 | Standard chow | Increase | Chartrel et al., 2003 [1] |
| Mouse | QRFP-43 | Moderately fat diet (32.6 %) Standard chow diet |
Increase (acute) Increase (chronic) Transient increase |
Moriya et al., 2006 [20] |
| Mouse | QRFP-26 QRFP-43 26RFa1–16 26RFa8–16 26RFa20–26 9Rfa |
Standard chow diet | Increase Increase No change No change Increase Increase |
do Rego et al., 2006 [18] |
| Mouse | QRFP-26 QRFP-43 |
Standard chow diet | Increase Increase |
Takayasu et al., 2006 [25] |
| Rat | QRFP-26 | Standard chow diet | No change | Kampe et al., 2006 [44] |
| Rat | QRFP-43 | Standard chow diet | No change | Patel et al., 2008 [22] |
| Rat | QRFP-26 QRFP-43 QRFP-26 QRFP-43 |
High fat diet (55 %) Low fat diet (10 %) |
Increase Increase No change No change |
Primeaux et al., 2008 [24] |
| Rat | QRFP-26 | Standard chow diet | Increase | Lectez et al., 2009 [19] |
| Rat (female) | QRFP-26 QRFP-43 QRFP-26 QRFP-43 |
High fat diet (60 %) Low fat diet (10 %) |
Increase Increase No change No change |
Primeaux, 2011 [23] |
| Broiler chicks Layer chicks |
26RFa-27 26RFa-8 26RFa-27 26RFa-8 |
Standard diet | Increase Increase No change No change |
Ukena et al., 2010 [13] |
| Zebra finch | QRFP-26 | Standard diet | Increase | Tobari et al., 2011 [15] |
Chartrel and colleagues [1] were the first to investigate the effects of centrally administered QRFP-26 on food intake in mice. In this study, food restricted mice received intracerebroventricular injections of QRFP-26 and chow intake was measured at various intervals. The highest dose of QRFP-26 increased chow intake by approximately 60 % within 2 h. Subsequently, several reports supported these initial findings in mice using QRFP-43 and QRFP-26 and expanded the original findings by investigating the effects of QRFP on food intake in fully satiated mice and following chronic administration of QRFP [18, 20, 25]. Studies in mice consuming a chow diet also indicated that a fragment of QRFP-26 (26RFa20–26) and a 9 amino acid peptide sequence (9RFa) increased food intake [18]. Chronic administration of QRFP-43 produced a transient increase in chow intake, increased body weight change, and adiposity [20]. Chronic intracerebroventricular administration of QRFP-43 was also investigated in mice fed a moderately fat diet (32.6 % kcal from fat) and the highest dose of QRFP-43 led to a 6.5 ± 0.5 g increase in body weight compared to a 2.0 ± 0.4 g increase following vehicle administration. Cumulative intake of the moderately fat diet over the 14 day period was significantly increased in the mice receiving the highest doses of QRFP-43 and adiposity was increased by all doses of QRFP-43 [20]. This study in mice was the first study in rodents to assess the effects of QRFP on the intake of dietary fat. Central administration of QRFP-26 and QRFP-43 in mice has consistently increased the intake of standard chow and even a moderately fat diet. However, in rats, there have been some inconsistencies regarding the orexigenic effects of QRFP. Kampe and colleagues were the first to assess the effects of QRFP-26 on standard chow intake in rats [44]. QRFP-26 administered into the third ventricle prior to the dark phase, did not alter chow intake as measured over the next 24 h. When QRFP-26 was administered prior to the beginning of the light phase, there was a moderate but transient increase in chow intake, which did not reach statistical significance. Therefore, these data, combined with locomotor activity and energy expenditure data, suggested that QRFP-26 had a limited impact on the central regulation of energy balance in rats [44]. Subsequently, a study investigating the effects of centrally administered QRFP-43 in chow fed rats also failed to detect a significant increase in chow intake at 1 h and 24 h following QRFP-43 administration [22]. Combined these studies suggested that QRFP did not play a significant role in the intake of a standard low fat chow diet.
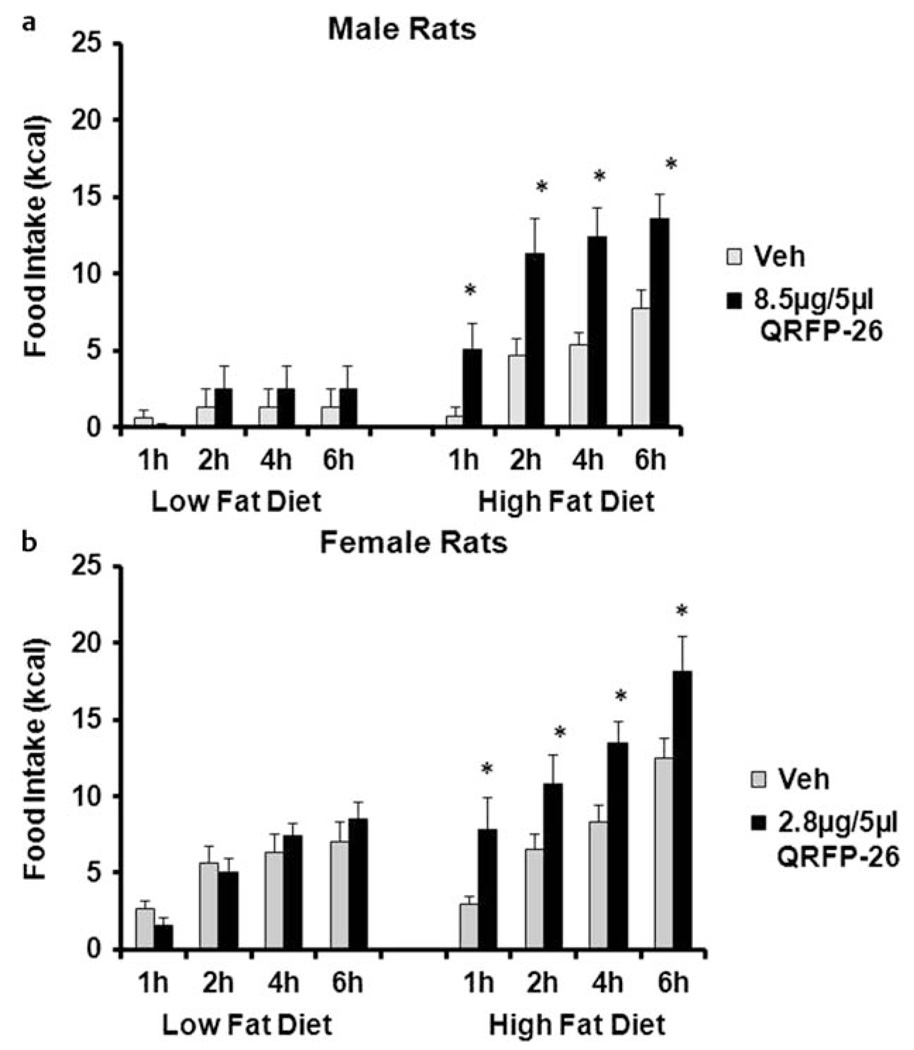
In mice, QRFP administration increased the intake of a moderately fat diet [20] as well as a standard chow diet. A standard chow diet is relatively low in fat (approximately 13 % kcal from fat); therefore, Primeaux et al. [24] hypothesized that the amount of fat in the diet may be an important factor mediating the orexigenic actions of QRFP in rats. In these studies, rats were habituated to either a high fat diet (55 % kcal from fat) or a low fat diet (10 % kcal from fat). QRFP-26 or QRFP-43 was administered into the lateral ventricles and the intake of either the high fat or the low fat diet was measured 1 h, 2 h, 4 h and 24 h following QRFP administration. As hypothesized, both QRFP-26 and QRFP-43 produced dose-dependent orexigenic effects in rats consuming the high fat diet, but not in rats consuming the low fat diet. A subsequent experiment demonstrated that central QRFP-26 administration also selectively produced a 7-fold increase in the intake of a high fat diet with a slightly higher fat content (60 % kcal from fat; Primeaux, unpublished results) (Fig. 1a). These data supported the hypothesis that in rats, QRFP produces a macronutrient specific effect. A subsequent study in female rats reported a macronutrient specific effect of QRFP-26 on high fat food intake [23]. In this study, random cycling female rats were habituated to high fat diet (60 % kcal from fat) or a low fat diet (10 % kcal from fat) and QRFP-26 was administered into the lateral ventricles. QRFP-26 produced a 2.6-fold increase in high fat food intake at the 1 h time point, without altering low fat diet (Fig. 1b). In both of these studies, the orexigenic effects of QRFP were transient, with the largest increase in high fat food intake occurring during the first hour and no differences detected at 24 h.
Fig. 1.
The effects of intracerebroventricular administration of QRFP-26 on low fat (10 % kcal from fat) and high fat food intake (60 % kcal from fat). a QRFP-26 selectively increased high fat food intake at 1 h, 2 h, 4 h, and 6 h following administration in male rats. b QRFP-26 administration increased high fat food intake, but not low fat food intake at each time in random cycling female rats. *p < 0.05, compared to vehicle treated, data is expressed as mean ± SEM.
To date, there has only been one report of an increase in the intake of a low fat chow diet following QRFP-26 administration in rats. In this study, rats were food-restricted and deprived of half of their daily chow intake for 18 h prior to testing [19]. Following food restriction, central administration of QRFP-26 increased chow intake by approximately 1.6-fold at 2 h following QRFP-26 administration. The orexigenic effects of QRFP in rats and in many of the mouse experiments appear to be related to the motivation associated with the feeding behavior. Many of the studies investigating the effects of QRFP in mice used a food-restricted model, while in rats the studies that supported an orexigenic effect of QRFP were conducted in rats that were either fed a high fat diet, which increases the motivation to eat, or conducted in food-restricted rats, which were motivated to eat.
The increase in high fat food intake following QRFP administration, the increase in chow intake following food restriction, and localization of prepro-QRFP mRNA expression, and the location of QRFP binding sites suggests that QRFP regulates homeostatic and non-homeostatic mechanisms associated with feeding behavior in rodents, particularly rats. It can be hypothesized that QRFP regulates motivation and may activate reward circuitry in the brain. In rats, there are a few neuropeptide/neurotransmitter systems which selectively regulate high fat food intake (e. g., AgRP, mu opioid receptors, galanin). Therefore, the addition of QRFP to that select list will be important for future research investigating high fat diet-induced obesity and has the potential to influence the development of therapies to combat the rising rates of obesity across the world. Recent evidence suggests that QRFP also has an orexigenic effect in birds (e. g., broiler chicks, zebra finch) [13, 15]. The significance of these findings and the importance of motivation in these models have yet to be determined.
Regulation of QRFP by Energy Status, Nutritional Status and QRFP’s Role in Hypothalamic Feeding Circuitry
In order to fully understand the role of QRFP in food intake regulation, particularly high fat food intake, it is necessary to assess the effects of nutritional status on QRFP expression, the regulation of QRFP by peripheral signals regulating energy status, and the ability of QRFP to regulate and interact with hypothalamic feeding circuitry. The effects of energy (i. e., fasted vs. fed) and nutritional status (i. e., high fat diet) on hypothalamic QRFP expression has been evaluated [24, 25, 61].
Many of the studies investigating the effects of nutritional or energy status on QRFP levels have been conducted in rats and mice, however, a recent study was conducted in young women. This study was designed to determine if circulating QRFP-26 plasma levels were subject to circadian variations and whether circulating QRFP-26 was altered by the presence of an eating disorders [62]. The results from this study indicated that circulating QRFP-26 levels were highest in the morning and fall in the afternoon. Additionally, circulating QRFP-26 levels were elevated in women diagnosed with anorexia. Anorectic women also had lower circulating leptin levels and 17-β-estradiol levels, than women in the control group. Another group of women with constitutional thinness, also had lower circulating levels QRFP-26 levels than those in the control group, though these differences did not reach significance [62]. Since QRFP is associated with increased motivation to eat, these results suggest an adaptive response to promote energy intake and increase fat stores in response to undernutrition.
In mice, a 48-h fast significantly increased hypothalamic prepro-QRFP mRNA, suggesting a role for QRFP in the motivation to eat [25]. The effects of specific macronutrients on the expression of hypothalamic prepro-QRFP mRNA levels have not been assessed in mice, but have been assessed in rats [24, 61]. In rats, the effects of diet on hypothalamic QRFP expression have been assessed using multiple diets and multiple time points. An assessment of body weight, adiposity and hypothalamic prepro-QRFP mRNA levels was conducted following 3 weeks of high fat diet (55 % kcal from fat) consumption. Rats consuming the high fat diet gained more weight and more body fat than the control rats, which consumed a low fat diet. The consumption of the high fat diet significantly increased the expression of prepro-QRFP mRNA in the VMH/ARC region of the hypothalamus, but not the LH region, suggesting that nutritional status regulated the expression of prepro-QRFP mRNA in regions associated with feeding behavior [24]. In another study, 8 week consumption of a moderately fat diet (40 % kcal from fat), a control diet (30 % kcal from fat) or a low fat diet (5 % kcal from fat) led to a significant increase in body weight, energy intake and adiposity in the moderately fat and the control fed rats compared to the low fat group. Though prepro-QRFP mRNA expression was not assessed in this study, QRFP-43 in the VMH was assessed by radioimmunoassay. QRFP-43 levels were highest in the low fat fed group, while the rats fed the moderately fat diet expressed the lowest levels of QRFP-43 in the VMH (levels were undetectable). The authors hypothesized that the undetectable levels of QRFP-43 in the VMH may decrease the orexigenic drive associated with the presence of a dietary fat, thereby leading to “normalization” of the feeding response [61]. Adiposity factors arise in the periphery and interact with the brain to alter feeding behavior. The regulation of QRFP by the peripheral adiposity signal, leptin, has been assessed in mice and rats and the role of QRFP’s receptor, GPR103, in regulating peripheral metabolic pathways has been assessed in cell culture. 3T3L1 adipocyte cells express prepro-QRFP and GPR103b, but not GPR103a, in a time dependent manner following differentiation [63]. Incubation with QRFP-26 and QRFP-43 increased intracellular triglyceride content, as shown by increased Oil Red O staining by 1.4- and 1.5-fold, respectively. QRFP-26 (10 nM, 1 000 nM) increased lipoprotein lipase activity by 2- and 2.4- fold, respectively, which served as a marker for the generation of fatty acids from circulating triglyceride-rich lipoproteins. Fatty acid uptake in the 3T3L1 cells was increased by incubation with QRFP-26 (31 %) and QRFP-43 (27 %), and fatty acid uptake increased with higher concentrations of QRFP. Knockdown of GPR103b in these cells abolished the increase in fatty acid uptake following incubation with QRFP-26 and QRFP-43. Incubation of QRFP-26 and QRFP-43 also increased gene expression of several genes associated with lipid metabolism (i. e., CD36). In the mouse, consumption of a high fat diet for 24 weeks significantly increased GPR103b mRNA in epididymal fat depots, and decreased prepro-QRFP mRNA in epididymal, perirenal and inguinal fat depots. These data supported a role for QRFP and its receptor, GPR130b in regulating fatty acid uptake, and promoting fat storage [63].
Increased adiposity is associated with increased levels of circulating leptin and peripheral and central administration of leptin decreases food intake [26, 30]. Genetically-altered mouse models have been used to assess the hypothesis that circulating leptin regulates the orexigenic effects of QRFP. In these studies, prepro-QRFP mRNA levels in the hypothalamus of ob/ob mice, which are leptin deficient and db/db mice, which are leptin resistant, were measured using Real Time PCR. In both mouse models, hypothalamic prepro-QRFP mRNA was upregulated, suggesting that circulating leptin regulates QRFP expression [25]. In rats, body adiposity and circulating leptin levels were increased following 8 weeks consumption of a moderately fat diet while QRFP-43 levels in the hypothalamus were decreased. A correlational analysis revealed a significant negative correlation between plasma leptin levels and hypothalamic QRFP-43 levels, but no correlation between circulating insulin, triglycerides or glucose with hypothalamic QRFP-43 [61]. These data further support a role for circulating leptin in the regulation of hypothalamic QRFP-43. Additionally, the co-administration of leptin with QRFP-26 significantly attenuated the orexigenic effects of QRFP-26 in food-deprived rats, though not to the same level as leptin alone [19]. The regulation of hypothalamic QRFP-26, QRFP-43, or prepro-QRFP levels by other adiposity signals or peripheral signals has not been investigated.
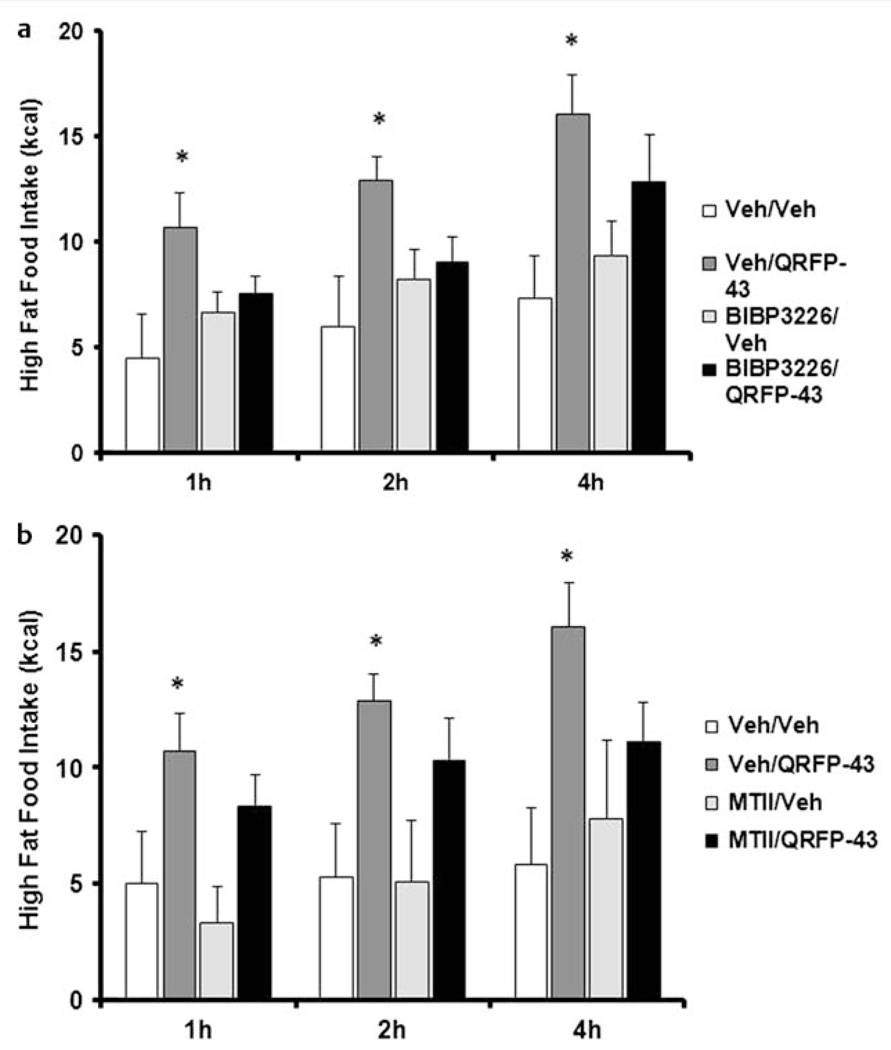
Prepro-QRFP mRNA is expressed in hypothalamic regions that are known to contain integral neural systems in the control of food intake. Determining the interaction of QRFP with existing feeding neural circuits is important for understanding the mechanisms by which QRFP controls food intake, particularly high fat food intake. To determine the hypothalamic mechanism involved in the control of QRFP’s feeding effects, the neuropeptide Y Y1 receptor antagonist, BIBP3226, was administered in combination with QRFP-43 in mice. Pretreatment of BIBP3226 attenuated the effects of QRFP-43 on the intake of a standard diet, suggesting that QRFP-induced feeding behavior requires the activation of the NPY Y1 receptor [25]. To evaluate the interaction of QRFP with the orexin system, which is localized to the LH, QRFP-43 was administered to orexin knockout mice. QRFP-43 was able to stimulate food intake in orexin knockout mice, suggesting that the orexigenic effects of QRFP-43 were independent of orexin [25]. In rats, co-administration of QRFP-26 with either a NPY Y1 receptor antagonist or a NPY Y5 receptor antagonist attenuated the orexigenic effects of QRFP-26 on chow intake in food-restricted rats [19], supporting a role for an interaction between QRFP and NPY in the regulation of feeding behavior. Our lab has investigated the effects of the QRFP-43 on high fat food intake in rats following the administration of the NPY Y1 receptor antagonist BIBP3226 and the MC3/MC4 receptor agonist, MTII (unpublished data; Fig. 2). Neither BIBP3226 nor MTII alone significantly decreased high fat food intake, while QRFP-43 significantly increased high fat food intake at each time point assessed. When co-administered with BIBP3226, the effects of QRFP-43 on high fat food intake were partially attenuated and did not reach vehicle-treated levels (Fig. 2a). Co-administration of the MC3/MC4 agonist, which activates the anorexigenic melanocortin system, with QRFP-43 also produced a partial attenuation of the orexigenic effects of QRFP on high fat food intake (Fig. 2b). The dose of BIBP3226 and particularly MTII were chosen because they did not have effects on food intake when given alone. AgRP is an inverse agonist at the MC3/MC4 receptors and increases fat intake through this mechanism. These data suggest that QRFP-43 is at least partially dependent on NPY and AgRP actions on feeding behavior, since pharmacological blockade of their receptors partially attenuated the increased high fat food intake seen following administration of QRFP-43 alone (unpublished results).
Fig. 2.
a Intracerebroventricular administration of QRFP-43 (22.5 µg/5 µl) and the neuropeptide Y Y1 receptor antagonist, BIBP3226 (13.3 nM/5 µl), on high fat food intake (55 % kcal from fat). QRFP-43 increased high fat food intake. Administration of BIBP3226, 30 min prior to administration of QRFP-43, partially attenuated the effects of QRFP-43 (n = 6–8/group). b Intracerebroventricular administration of the MC3/MC4 agonist, MTII (1 nM/5 µl), and QRFP-43 (22.5 µg/5 µl) on high fat food intake (55 % kcal from fat). QRFP-43 increased high fat food intake. Administration of MTII, 30 min prior to administration of QRFP-43, partially attenuated the effects of QRFP-43 (n = 5–7/group). Data were analyzed by a 2 × 2 ANOVA and Bonferroni post-hoc analyses. *p < 0.05 compared to vehicle treated, data is expressed as mean ± SEM.
Very few studies have investigated the colocalization of QRFP with neural systems in the hypothalamus that regulate food intake. Double-labeled in situ hybridization revealed that the receptor, GPR103, is present in NPY neurons of the ARC but not on POMC neurons. Further analyses indicated that central administration of QRFP-26 stimulated prepro-NPY mRNA expression in the ventral hypothalamus (e. g., ARC) and decreased POMC mRNA expression. The effects of central QRFP-26 administration on prepro-NPY and POMC mRNA levels were attenuated by the co-administration of NPY Y1 and Y5 receptor antagonists. In ventral hypothalamic explants, the addition of QRFP-26 led to approximately a 37-fold increase in NPY release and approximately a 50 % decrease in α-MSH release [19]. These data demonstrate that QRFP likely exerts its orexigenic effects by increasing the release of hypothalamic NPY and decreasing the release of hypothalamic α-MSH.
Future of QRFP and the Regulation of Fat Intake
QRFP26- and QRFP-43 are orexigenic peptides with localized mRNA expression in the hypothalamus, a region important in the regulation of food intake. Studies in rodents have shown that in the hypothalamus, QRFP’s effects on food intake are mediated by both NPY and AgRP and regulated by circulating leptin levels [19, 25]. In rats, the orexigenic effects of centrally administered QRFP-26 and QRFP-43 are mediated by factors associated with an increased motivation to eat [19, 23, 24]. In these studies, the consumption of a high fat diet or a 50 % food restriction were sufficient to lead to QRFP-induced increases in food intake, however ad libitum access to low fat diets were not. Recent investigations into the structure activity relationship of QRFP-26 and its analogues have led to the development of analogues which bind to the GPR103 receptor with high potency. Analogues of QRFP-26 (20–26) have been developed which bind with high potency to GPR103, are more stable than QRFP (20–26), more potent in mobilizing intracellular Ca + + and exert a long-lasting orexigenic effect in mice [64, 65]. The development of these analogues increases the potential for therapeutic applications in feeding-related disorders and future emphasis should be placed on determining the mechanisms by which the QRFP peptides and analogues alter the motivation to eat and other motivated behaviors. Several neural systems are involved in the intake of dietary fat. In the hypothalamus, AgRP, mu opioid receptors, and galanin have been shown to selectively increase in the intake of high fat diet, and the expression of AgRP, galanin, and mu opioid receptors are regulated by the intake of high fat diet [24, 56, 66 –68]. In rats, QRFP-26 and QRFP-43 also selectively increase the intake of a high fat diet and the expression of hypothalamic prepro-QRFP mRNA is regulated by the consumption of a high fat diet [23, 24]. The effects of QRFP on high fat diet intake are partially attenuated by NPY Y1 antagonist and a melanocortin MC3/MC4 receptor agonist, suggesting a partial dependence on the NPY and melanocortin systems (Fig. 2). These data are in congruence with reports that QRFP administration induces the release of NPY in hypothalamic explants and GPR103 receptors are located on NPY neurons in the ARC [19]. QRFP may be exerting its effects of high fat diet intake via AgRP release in the hypothalamus, since many of the NPY expressing neurons in the ARC also express AgRP.
In addition to the hypothalamus, several other brain regions are associated with fat intake, nucleus accumbens and the amygdala and neuropeptides regulating fat intake activate these brain regions [66, 67, 69 – 72]. These regions are involved in motivated behaviors and the nucleus accumbens is involved in the rewarding aspects of consuming a high fat diet. Studies have shown that QRFP binds to regions of the brain outside of the hypothalamus, which may be relevant for feeding behaviors (i. e., amygdala, nucleus accumbens). To date, QRFP-26 and QRFP-43 have been administered intracerbroventricularly; therefore, the direct actions of these peptides in specific brain regions have not been determined. However, based on the information provided by QRFP-26 binding assays and receptor localization assays, QRFP-26 likely exerts its effects on feeding behavior via the hypothalalmus, and also potentially through projections to the nucleus of the solitary tract in the brainstem, the ventral tegmental area and nucleus accumbens. These regions are involved in reward processes and influence motivated behaviors. More studies are needed to assess the role of QRFP-26 and QRFP-43, and its analogues in reward-associated behaviors and to examine the role of these peptides in the neural circuitry of reward.
Studies investigating QRFP have indicated that this peptide is strongly conserved across vertebrates and invertebrates, has localized expression in the hypothalamus, is regulated by adiposity signals and nutrient status, and has an orexigenic effect in multiple species. Due to the effects of the QRFP peptides on feeding behavior (i. e., high fat intake, intake following food-restriction) and evidence that QRFP-26 binds to brain regions associated with feeding and reward behaviors, future investigations should be directed toward determining the role of QRFP on motivated behaviors. Additionally, determining the mechanisms by which QRFP-26 and QRFP-43 and their analogues increase the intake of dietary fat has the potential to influence the development of therapies to treat the ever increasing prevalence of obesity.
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest in the authorship or publication of this contribution.
References
- 1.Chartrel N, Dujardin C, Anouar Y, Leprince J, Decker A, Clerens S, Do-Rego JC, Vandesande F, Llorens-Cortes C, Costentin J, Beauvillain JC, Vaudry H. Identification of 26RFa, a hypothalamic neuropeptide of the RFamide peptide family with orexigenic activity. Proc Natl Acad Sci USA. 2003;100:15247–15252. doi: 10.1073/pnas.2434676100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chartrel N, Alonzeau J, Alexandre D, Jeandel L, Alvear-Perez R, Leprince J, Boutin J, Vaudry H, Anouar Y, Llorens-Cortes C. The RFamide neuropeptide 26RFa and its role in teh control of neuroendocrine functions. Front Neuroendocrinol. 2011;32:387–397. doi: 10.1016/j.yfrne.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Dockray GJ. The expanding family of RFamide peptides and their effects on feeding behaviour. Exp Physiol. 2004;89:229–235. doi: 10.1113/expphysiol.2004.027169. [DOI] [PubMed] [Google Scholar]
- 4.Fukusumi S, Fujii R, Hinuma S. Recent advances in mammalian RFamide peptides: the discovery and functional analyses of PrRP, RFRPs and QRFP. Peptides. 2006;27:1073–1086. doi: 10.1016/j.peptides.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs A, Laszlo K, Galosi R, Toth K, Ollmann T, Peczely L, Lenard L. Microinjection of RFRP-1 in the central nucleus of amygdala decreases food intake in the rat. Brain Res Bull. 2012;88:589–595. doi: 10.1016/j.brainresbull.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Kutzlep C, Busmann A, Wendland M, Maronde E. Discovery of novel regulatory peptides by reverse pharmacology: spotlight on chemerin and the RF-amide peptides metastin and QRFP. Curr Protein Pept Sci. 2005;6:265–278. doi: 10.2174/1389203054065419. [DOI] [PubMed] [Google Scholar]
- 7.Losa-Ward SM, Todd KL, McCaffrey KA, Tsutsui K, Patisaul HB. Disrupted organization of RFamide pathways in teh hypothalmus is associated with advanced puberty in female rats neonatally exposed to bisphenol A. Biol Reprod. 2012;87:28. doi: 10.1095/biolreprod.112.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocrine Reviews. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parhar I, Ogawa S, Kitahashi T. RFamide peptides as mediators in environmental control of GnRH neurons. Prog Neurobiol. 2013;98:176–196. doi: 10.1016/j.pneurobio.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Poling MC, Kim J, Dhamija S, Kauffman AS. Develoopment, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology. 2012;153:1827–1840. doi: 10.1210/en.2011-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmoneaux V, Ancel C, Poirel VJ, Gauer F. Kisspeptins and RFRP-3 act in concert to synchronize rodent reproduction with seasons. Front Neurosci. 2013;7:22. doi: 10.3389/fnins.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umatani C, Abe H, Oka Y. Neuropeptide RFRP inhibites the pacemaker activity of terminal nerve GnRH neurons. J Neurophysiol. 2013;109:2354–2363. doi: 10.1152/jn.00712.2012. [DOI] [PubMed] [Google Scholar]
- 13.Ukena K, Tachibana T, Iwakoshi-Ukena E, Saito Y, Minakata H, Kawaguchi R, Osugi T, Tobari Y, Leprince J, Vaudry H, Tsutsui K. Identification, localization, and fucntion of a novel avian hypothalamic neuropeptide, 26RFa, and its cognate receptor, G protein-couple receptor 103. Endocrinology. 2010;151:2255–2264. doi: 10.1210/en.2009-1478. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Zhang Y, Li S, Huang W, Liu X, Lu D, Meng Z, Lin H. Molecular cloning and functional characterization of the first non-mammalian 26RFa/QRFP orthologue in Goldfish, carassius auratus. Mol Cell Endocrinol. 2009;303:82–90. doi: 10.1016/j.mce.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Tobari Y, Iijima N, Tsunekawa K, Osugi T, Haraguchi S, Ubuka T, Ukena K, Okanoya K, Tsutsui K, Ozawa H. Identification, localization and functional implication of 26RFa ortholog peptide in the brain of zebra finch (taeniopygia guttata) J Neuroendocrinol. 2011;23:791–803. doi: 10.1111/j.1365-2826.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 16.Fukusumi S, Yoshida H, Fujii R, Maruyama M, Komatsu H, Habata Y, Shintani Y, Hinuma S, Fujino M. A new peptidic ligand and its receptor regulating adrenal function in rats. J Biol Chem. 2003;278:46387–46395. doi: 10.1074/jbc.M305270200. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Luo L, Gustafson EL, Yadav D, Laverty M, Murgolo N, Vassileva G, Zeng M, Laz TM, Behan J, Qiu P, Wang L, Wang S, Bayne M, Greene J, Monsma F, Zhang FL. Identification and characterization of a novel RFamide peptide ligand for orphan G-protein-coupled receptor SP9155. J Biol Chem. 2003;278:27652–27657. doi: 10.1074/jbc.M302945200. [DOI] [PubMed] [Google Scholar]
- 18.do Rego JC, Leprince J, Chartrel N, Vaudry H, Costentin J. Behavioral effects of 26RFamide and related peptides. Peptides. 2006;27:2715–2721. doi: 10.1016/j.peptides.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Lectez B, Jeandel L, El-Yamani FZ, Arthaud S, Alexandre D, Mardargent A, Jegou S, Mounien L, Bizet P, Magoul R, Anouar Y, Chartrel N. The orexigenic activity of the hypothalamic neuropeptide 26RFa is mediated by the neuropeptide Y and proopiomelanocortin neurons of the arcuate nucleus. Endocrinology. 2009;150:2342–2350. doi: 10.1210/en.2008-1432. [DOI] [PubMed] [Google Scholar]
- 20.Moriya R, Sano H, Umeda T, Ito M, Takahashi Y, Matsuda M, Ishihara A, Kanatani A, Iwaasa H. RFamide peptide QRFP43 causes obesity with hyperphagia and reduced thermogenesis in mice. Endocrinology. 2006;147:2916–2922. doi: 10.1210/en.2005-1580. [DOI] [PubMed] [Google Scholar]
- 21.Navarro VM, Fernandez-Fernandez R, Nogueiras R, Vigo E, Tovar S, Chartrel N, Le MO, Leprince J, Aguilar E, Pinilla L, Dieguez C, Vaudry H, Tena-Sempere M. Novel role of 26RFa, a hypothalamic RFamide orexigenic peptide, as putative regulator of the gonadotropic axis. J Physiol. 2006;573:237–249. doi: 10.1113/jphysiol.2006.106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel SR, Murphy KG, Thompson EL, Patterson M, Curtis AE, Ghatei MA, Bloom SR. Pyroglutamylated RFamide peptide 43 stimulates the hypothalamic-pituitary-gonadal axis via gonadotrophin-releasing hormone in rats. Endocrinology. 2008;149:4747–4754. doi: 10.1210/en.2007-1562. [DOI] [PubMed] [Google Scholar]
- 23.Primeaux SD. QRFP in female rats: effects on high fat food intake and hypothalamic gene expression across the estrous cycle. Peptides. 2011;32:1270–1275. doi: 10.1016/j.peptides.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Primeaux SD, Blackmon C, Barnes MJ, Braymer HD, Bray GA. Central administration of the RFamide peptides, QRFP-26 and QRFP-43, increases high fat food intake in rats. Peptides. 2008;29:1994–2000. doi: 10.1016/j.peptides.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takayasu S, Sakurai T, Iwasaki S, Teranishi H, Yamanaka A, Williams SC, Iguchi H, Kawasawa YI, Ikeda Y, Sakakibara I, Ohno K, Ioka RX, Murakami S, Dohmae N, Xie J, Suda T, Motoike T, Ohuchi T, Yanagisawa M, Sakai J. A neuropeptide ligand of the G protein-coupled receptor GPR103 regulates feeding, behavioral arousal, and blood pressure in mice. Proc Natl Acad Sci USA. 2006;103:7438–7443. doi: 10.1073/pnas.0602371103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3:589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- 27.Berthoud HR. Interactions between the “cognitive“ and “metabolic” brain in the control of food intake. Physiol Behav. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Berthoud HR, Munzberg H, Richards BK, Morrison CD. Neural and metabolic regulation of macronutrient intake and selection. Proc Nutr Soc. 2012;71:390–400. doi: 10.1017/S0029665112000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin AC, Zheng H, Berthoud HR. An expanded view of energy homeostatis: Neural integration of metabolic, cognitive, and emotional drives to eat. Physiol Behav. 2009;97:572–580. doi: 10.1016/j.physbeh.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz MW, Woods SC, Porte JD, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 31.Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol. 2005;184:291–318. doi: 10.1677/joe.1.05866. [DOI] [PubMed] [Google Scholar]
- 32.Bouret SG. Crossing the border: developmental regulation of leptin transport to the brain. Endocrinology. 2008;149:875–876. doi: 10.1210/en.2007-1698. [DOI] [PubMed] [Google Scholar]
- 33.Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr. 1998;68:1157–1173. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- 34.Bray GA, Popkin BM. Dietary fat affects obesity rate. Am J Clin Nutr. 1999;70:572–573. doi: 10.1093/ajcn/70.4.572. [DOI] [PubMed] [Google Scholar]
- 35.Bray GA, Paeratakul S, Popkin BM. Dietary fat and obesity: a review of animal, clinical and epidemiological studies. Physiol Behav. 2004;83:549–555. doi: 10.1016/j.physbeh.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 36.Lissner L, Heitmann BL. Dietary fat and obesity: evidence from epidemiology. Eur J Clin Nutr. 1995;49:79–90. [PubMed] [Google Scholar]
- 37.Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, Levin BE, Larsen PJ, Knudsen LB, Fosgerau K, Vrang N. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol. 2010;206:287–296. doi: 10.1677/JOE-10-0004. [DOI] [PubMed] [Google Scholar]
- 38.Primeaux SD, Braymer HD, Bray GA. High fat diet differentially regulates the expression of olfactory receptors in teh duodenum of obesity-prone and obesity-resistant rats. Dig Dis Sci. 2013;58:72–76. doi: 10.1007/s10620-012-2421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Primeaux SD, Braymer HD, Bray GA. CD36 mRNA in the gastrointestinal tract is differentially regulated by dietary fat intake in obesity-prone and obesity-resistant rats. Dig Dis Sci. 2013;58:369–370. doi: 10.1007/s10620-012-2364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Primeaux SD, Barnes MJ, Braymer HD, Bray GA. Sensitivity to the satiating effects of Exendin 4 is decreased in obesity-prone Osborne-Mendel rats compared to obesity-resistant S5B/Pl rats. Int J Obes. 2010;34:1427–1433. doi: 10.1038/ijo.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricci MR, Levin BE. Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R610–R618. doi: 10.1152/ajpregu.00235.2003. [DOI] [PubMed] [Google Scholar]
- 42.Chartrel N, Bruzzone F, Leprince J, Tollemer H, Anouar Y, Do-Rego JC, Segalas-Milazzo I, Guilhaudis L, Cosette P, Jouenne T, Simonnet G, Vallarino M, Beauvillain JC, Costentin J, Vaudry H. Structure and functions of the novel hypothalamic RFamide neuropeptides R-RFa and 26RFa in vertebrates. Peptides. 2006;27:1110–1120. doi: 10.1016/j.peptides.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 43.Thuau R, Guilhaudis L, Segalas-Milazzo I, Chartrel N, Oulyadi H, Boivin S, Fournier A, Leprince J, Davoust D, Vaudry H. Structural studies on 26RFa, a novel human RFamide-related peptide with orexigenic activity. Peptides. 2005;26:779–789. doi: 10.1016/j.peptides.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Kampe J, Wiedmer P, Pfluger PT, Castaneda TR, Burget L, Mondala H, Kerr J, Liaw C, Oldfield BJ, Tschop MH, Bagnol D. Effect of central administration of QRFP(26) peptide on energy balance and characterization of a second QRFP receptor in rat. Brain Res. 2006;1119:133–149. doi: 10.1016/j.brainres.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 45.Bruzzone F, Lectez B, Alexandre D, Jegou S, Mounien L, Tollemer H, Chatenet D, Leprince J, Vallarino M, Vaudry H, Chartrel N. Distribution of 26RFa binding sites and GPR103 mRNA in the central nervous system of the rat. J Comp Neurol. 2007;503:573–591. doi: 10.1002/cne.21400. [DOI] [PubMed] [Google Scholar]
- 46.Yeo GS, Heisler LK. Unraveling the brain regulation of appetite: lessons from genetics. Nature Neurosci. 2012;15:1343–1349. doi: 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]
- 47.Gouarderes C, Mazarguil H, Mollereau C, Chartrel N, Leprince J, Vaudry H, Zajac JM. Functional differences between NPFF1 and NPFF2 receptor coupling: high intrinsic activities of RFamide-related peptides on stimulation of 35S GTPS binding. Neuropharmacology. 2007;52:376–386. doi: 10.1016/j.neuropharm.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 48.Gouarderes C, Puget A, Zajac JM. Detailed distribution of Neuropeptide FF receptors (NPFF1 and NPFF2) in the rat, mouse, octodon, rabbit, guinea pig and marmoset monkey brains: a comparative audoradiographic study. Synapse. 2004;51:249–269. doi: 10.1002/syn.10305. [DOI] [PubMed] [Google Scholar]
- 49.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 50.Heinrichs SC, Menzaghi F, Pich EM, Hauger RL, Koob GF. Corticotropin-releasing factor in the paraventriuclar nucleus modulates feeding induced by neuropeptide Y. Brain Res. 1993;611:18–24. doi: 10.1016/0006-8993(93)91771-j. [DOI] [PubMed] [Google Scholar]
- 51.Konturek PC, Konturek JW, Czesnikiewicz-Guzik M, Brzozowski T, Sito E, Konturek SJ. Neuro-hormonal control of food intake: basic mechanisms and clinical implications. J Physiol Pharmacol. 2005;56(Suppl 6):5–25. [PubMed] [Google Scholar]
- 52.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 53.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 54.Tiesjema B, la Fleur SE, Luijendijk MC, Brans MA, Lin EJ, During MJ, Adan RA. Viral mediated neuropeptide Y expression in the rat paraventricular nucleus results in obesity. Obesity (Silver Spring) 2007;15:2424–2435. doi: 10.1038/oby.2007.288. [DOI] [PubMed] [Google Scholar]
- 55.Woods SC, Figlewicz DP, Madden LJ, Porte D, Sipols AJ, Seeley RJ. NPY and food intake: Discrepancies in the model. Regul Pept. 1998;75–76:403–408. doi: 10.1016/s0167-0115(98)00095-0. [DOI] [PubMed] [Google Scholar]
- 56.Barnes MJ, Lapanowski K, Conley A, Rafols JA, Jen KL, Dunbar JC. High fat feeding is associated with increased blood pressure, sympathetic nerve activity and hypothalamic mu opioid receptors. Brain Res Bull. 2003;61:511–519. doi: 10.1016/s0361-9230(03)00188-6. [DOI] [PubMed] [Google Scholar]
- 57.Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ. Opioid receptor involvement in the effect of AgRP− (83–132) on food intake and food selection. Am J Physiol Regul Integr Comp Physiol. 2001;280:R814–R821. doi: 10.1152/ajpregu.2001.280.3.R814. [DOI] [PubMed] [Google Scholar]
- 58.Koegler FH, York DA, Bray GA. The effects on feeding of galanin and M40 when injected into the nucleus of the solitary tract, the lateral parabrachial nucleus, and the third ventricle. Physiol Behav. 1999;67:259–267. doi: 10.1016/s0031-9384(99)00075-x. [DOI] [PubMed] [Google Scholar]
- 59.Leibowitz SF, Wortley KE. Hypothalamic control of engery balance: different peptides, different functions. Peptides. 2004;25:473–504. doi: 10.1016/j.peptides.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Zhang M, Kelley AE. Enhanced intake of high fat food following striatal mu-opiod stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 61.Beck B, Richy S. Suppression of QRFP 43 in the hypothalamic ventro-medial nucleus of Long-Evans rats fed a high-fat diet. Biochem Biophys Res Commun. 2009;383:78–82. doi: 10.1016/j.bbrc.2009.03.132. [DOI] [PubMed] [Google Scholar]
- 62.Galusca B, Jeandel L, Germain N, Alexandre D, Leprince J, Anouar Y, Estour B, Chartrel N. Orexigenic neuroptpdie 26Rfa: new evidence for an adaptive progile of appetite regulation in anorexia nervosa. J Clin Endocrinol Metab. 2012;97:2012–2018. doi: 10.1210/jc.2011-3396. [DOI] [PubMed] [Google Scholar]
- 63.Mulumba M, Jossart C, Granata R, Gallo D, Escher E, Ghigo E, Servant MJ, Marleau S, Ong H. GPR103b functions in theperipheral regulation of adipogenesis. Mol Endocrinol. 2010;24:1615–1625. doi: 10.1210/me.2010-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Marec O, Neveu C, Lefranc B, Dubessy C, Boutin JA, Do-Rego JC, Costentin J, Tonon MC, Tena-Sempere M, Vaudry H, Leprince J. Structure-activity relationships of a series of analogues of teh RFamide-related peptide 26RFa. J Med Chem. 2011;54:4806–4814. doi: 10.1021/jm200418c. [DOI] [PubMed] [Google Scholar]
- 65.Neveu C, Lefranc B, Tasseau O, Do-Rego JC, Bourmaud A, Chan P, Bauchat P, Le Marec O, Chuquet J, Guilhaudis L, Boutin J, Segalas-Milazzo I, Costentin J, Vaudry H, Baudy-Floc’fh M, Vaudry D, Leprince J. Rational design of a low molecular weight, stable, potent, and long-lasting GPR103 aza-B3-pseudopeptide agonist. J Med Chem. 2012;55:7516–7524. doi: 10.1021/jm300507d. [DOI] [PubMed] [Google Scholar]
- 66.Barson JR, Morganstern I, Leibowitz S, Leibowitz SF. Neurobiology of consummatory behavior: mechanisms underlying overeating and drug use. ILAR J. 2012;53:35–58. doi: 10.1093/ilar.53.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee AK, Mojtahed-Jaberi M, Kyrikou T, Astarloa EAO, Arno M, Marshall NJ, Brain SD, O’Dell SD. Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition. 2010;26:411–422. doi: 10.1016/j.nut.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leibowitz SF. Regulation and efects of hypothalamic galanin: relation to dietary fat, alcohol ingestion, circulating lipids and energy homeostasis . Neuropeptides. 2005;39:327–332. doi: 10.1016/j.npep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 69.Deshmukh RR, Sharma PL. Stimulation of accumbens shell cannabinoid CV(1) receptors by noladin ether, a putative endocannabinoid, modulates food intake and dietary selection in rats. Pharmacol Res. 2012;66:276–282. doi: 10.1016/j.phrs.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Higuchi S, Ohji M, Araki M, Furuta R, Katsuki M, Yamaguchi R, Akitake Y, Matsuyama K, Irie K, Mishima K, Mishima K, Iwasaki K, Fujiwara M. Increment of hypothalamic 2-arachidonoylgylcerol induces the preference for a high fat diet via activation of cannabinoid 1 receptors. Behav Brain Res. 2011;216:477–480. doi: 10.1016/j.bbr.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 71.Katsuura Y, Heckmann JA, Taha SA. Mu-opioid receptor stimulation in the nucleus accumbens elevates fatty tastant intake by incresing palatability and suppressing satiety signals. Am J Physiol Regulatory Integrative Comp Physiol. 2011;301:R244–R254. doi: 10.1152/ajpregu.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]