Abstract
Background:
Cervi Cornu Pantotrichum has been a well known traditional Chinese medicine, which is young horn of Cervus Nippon Temminck (Hualurong: HLR). At present, the methods used for the quality control of Cervi Cornu Pantotrichum show low specificity.
Objective:
To describe a holistic method based on chemical characteristics and splenocyte-proliferating activities to evaluate the quality of HLR.
Materials and Methods:
The nucleosides and bases from HLR were identified by high performance liquid chromatography electrospray ionization mass spectrometry (HPLC-ESI-MS), and six of them were chosen to be used for simultaneous HPLC quantification according to the results of proliferation of mouse splenocytes in vitro.
Results:
In this study, eight nucleosides and bases have been identified. In addition, uracil, hypoxanthine, uridine, inosine, guanosine, and adenosine were chosen to be used for simultaneous HPLC quantification. Simultaneous quantification of these six substances was performed on ten groups of HLR under the condition of a TIANHE Kromasil C18 column (5 μm, 4.6 mm × 250 mm i.d.) and a gradient elution of water and acetonitrile. Of the ten groups, HLR displayed the highest total nucleoside contents (TNC, sum of adenosine and uracil, 0.412 mg/g) with the strongest splenocyte-proliferating activities.
Conclusion:
These results suggest that TNC (such as particularly highly contained adenosine and uracil) in HLR has a certain correlation with the activity of splenocyte-proliferating, and it may be used as a quality control for HLR. This comprehensive method could be applied to other traditional Chinese medicines to ameliorate their quality control.
Keywords: Cervus Nippon Temminck, electrospray ionization mass spectrometry, high performance liquid chromatography, immunomodulatory, nucleosides and bases
INTRODUCTION
Lurong (LR) or Cervi Cornu Pantotrichum has been a well-known traditional Chinese medicine and health food widely used in China for thousands years and also listed in the Chinese Pharmacopoeia,[1] which is a young horn of Cervus Nippon Temminck (Hualurong: HLR).
HLR is commonly used in China for various activities, such as antioxidation, anti-aging, anti-tumor, immunomodulatory and other biological activities, especially for improving sexual function.[2,3,4,5,6,7,8,9,10] Traditional medical reports show that HLR is made of many components such as amino acids, fatty acids, lipids, polypeptides, polysaccharides, polyamines, inorganic elements, biological bases, and other chemical compositions.[11] Recently, the analysis methods have been established for separately determining biological bases (i.e., uracil, hypoxanthine and uridine) in antler velvet.[12] However, there has been no report regarding the quantification of biological bases and splenocyte-proliferating activity in HLR. A simultaneous quantification is required because several nucleosides and bases will individually promote spleen cell proliferation. Therefore, this study was aimed to evaluate the splenocyte-proliferating activity of HLR and to analyze the content of the active principles in HLR.
MATERIALS AND METHODS
Chemical reagents, materials, and instruments
Acetonitrile used was of chromatographically grade and procured from Merck (Germany). The water used was treated with a Milli-Q water purification system (Millipore, Molsheim, France). Standards of uracil, hypoxanthine, uridine, inosine, guanosine, adenosine, 2’-deoxyguanosine, and guanine were procured from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) [Figure 1]. Concanavalin A (ConA) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were procured from Sigma (St. Louis, MO, USA). RPMI1640 medium, penicillin–streptomycin and fetal bovine serum (FBS) were obtained from Hyclone (Utah, USA). Other reagents and solvents used were of analytical grade.
Figure 1.
The structures of 8 standard compounds
A total of 10 samples of HLR were collected from the cultivation areas in Jilin and Liaoning provinces of China, which four collected from Shuangyang (ShuangYang01, ShuangYang02, ShuangYang03, ShuangYang04), two collected from Dongfeng (DongFeng01, DongFeng02), two collected from Xifeng (XiFeng01, XiFeng02), and two collected from Aodong (AoDong01, AoDong02). All samples were verified by Prof. Ming-Lu Deng (Changchun University of Chinese Medicine, Changchun, China).
Agilent Technology 1100 Series high performance liquid chromatography (HPLC) system equipped with a quaternary pump, degasser, thermostatic auto-sampler, and photodiode array detector (DAD) was used for analysis. The MS instrument used to perform the studies was a 6320 ion trap LC/MS from Agilent.
Sample preparation
A 1-g sample of the fine-ground powder was accurately weighted and extracted with 20 mL of boiling H2O for 60 min and centrifuged at 12000 rpm for 15 min, and the supernatant was collected. The extraction was lyophilized, and the lyophilized powder was extracted with 60% methanol by ultrasonication for 15 min at room temperature. The mixture was then centrifuged at 12000 rpm for 15 min, and the supernatant was collected. The supernatant was dried in the rotary evaporator at 160 rpm at 30°C, which was named total nucleoside contents (TNC) for using on proliferation of mouse splenocytes in vitro. The powder of TNC was accurately weighed and dissolved in 60% methanol (1 mg/mL) for LC/MS analysis and determination. It was filtered through a membrane filter (0.45 μm pore size) prior to injection and analyzed three times.
Standard preparation
The reference standards of the eight nucleosides and bases were accurately weighed and dissolved in 60% methanol; six of them (uracil, hypoxanthine, uridine, inosine, guanosine, and adenosine) were then diluted to appropriate concentration ranges for the establishment of calibration curves. All stock and working standard solutions were stored in brown bottles at 4°C until used for analysis.
HPLC-DAD-electrospray ionization-mass spectrometry analysis of TNC
HPLC procedures. The analysis was performed on an Agilent 1100 liquid chromatography system, equipped with a diode array detector working in the range of 190-400 nm, a quaternary solvent delivery system, a column temperature controller, and an autosampler. The chromatographic data was recorded and processed with Agilent Chromatographic Work Station software.
Chromatographic separations were carried out on a C18 analytical column (TIANHE Kromasil C18, 4.6 mm × 250 mm, 5 μm) supplied by Dalian Elite Analytical Instruments, Dalian, China. The mobile phase consisted of water (A) –acetonitrile (B); A: B was as follows: 0 min, 99:1; 5 min, 98:2; 10 min, 98:2; 20 min, 96:4; 40 min, 96:4; 42 min, 45:55; 55 min, 40:60; and 60 min, 0:100. The flow rate and column temperature were set constantly at 0.3 mL/min and 30°C, respectively. The detection wavelength was fixed at 260 nm. All solvents were filtered through a 0.45-μm pore size filter and then degassed by sonication in an ultrasonic bath before use.
ESI-MS parameter. The HPLC analytical conditions for liquid chromatography-mass spectrometry (LC-MS) were same as those used for the HPLC-DAD analysis, except that one-third of the elutant was introduced into the MS system by using split technique. Agilent 1100 HPLC/MSD Trap mass spectrometer (Agilent, Wilmington, Germany) equipped with an electrospray ionization source was used in positive ion mode. An HPLC system coupled with DAD was controlled by an HPLC-MSD ChemStation software system. Auto MS2 mode of Mass spectrometer was chosen to analyze the sample. The following operation parameters were used: Capillary voltage: 4000V; nebulizer pressure: 35 psi; drying gas: 9.0 L/min; gas temperature: 350°C; fragmentor voltage: 200V; skimmer voltage: 60V. LC–ESI-MS accurate mass spectra were recorded across the range from 50 to 400 m/z. The data recorded was processed with the applied HPLC-MSD ChemStation software system.
Proliferation of mouse splenocytes in vitro
An assay method described by Kan F et al.[13] was used to measure the splenocyte-proliferating activity of TNC and eight nucleosides and bases of HLR. Aliquots (100 μL) of the Splenocyte suspension and 100 μL of test samples were added to each well of a 96-well plate. PBS and ConA (5 μg/mL) served as the negative and positive controls, respectively. After incubation at 37°C in a 5% CO2 atmosphere for 72 h, 20 μL MTT were added to each well, and the incubation continued for further 4 h. Extinction values at 570 nm were measured using an enzyme-linked immunosorbent assay (ELISA) reader (Model 680, Takara, Japan). The proliferation rate was calculated according to the following formula: Splenocyte proliferation rate (%) = [A570 (sample) − A570(negative control) ]/A570 (negative control) ] × 100%.
Simultaneous determination of six nucleosides and bases in TNC of HLR
Chromatographic conditions. The HPLC conditions of determination coincide with those used for HPLC analysis in LC-MS.
LC method validation. The HPLC analysis method used in this study has been validated through several tests. Linearity was evaluated by the value of R2 (correlation coefficient) in the calibration curve of serial concentrations. Sensitivities were determined by the value of limit of detection (LOD) and limit of quantification (LOQ). They were calculated with corresponding standard solution on the basis of signal-to-noise ratio (S/N) of 3 and 10, respectively. The stability of the analysis method was assessed by measurements of the intra- and inter-day variability. To evaluate the accuracy, the recovery test was performed, spiking each standard compound to the sample solution.
RESULTS AND DISCUSSION
Optimization of HPLC conditions
DAD was used in HPLC analysis, and full scan runs were made initially to select the optimum wavelength that provided the best result in chromatographic analysis. Chromatogram at 260 nm showed the most abundant components information than at other wavelengths, in which Figure 2 demonstrates the separation obtained for a typical sample of the six nucleosides and bases; the six insets are DAD UV scan of uracil (3), hypoxanthine (5), uridine (6), inosine (7), guanosine (8), and adenosine (10) peak (190-400 nm). An optimum formula consisting of solvent A and solvent B was selected. Solvent A was H2O and solvent B was acetonitrile. This composition was selected because it showed a better separation and the solvents were environment-friendly. A gradient elution method was employed for better chromatographic separation on a wide range of polarity in a shorter time.
Figure 2.
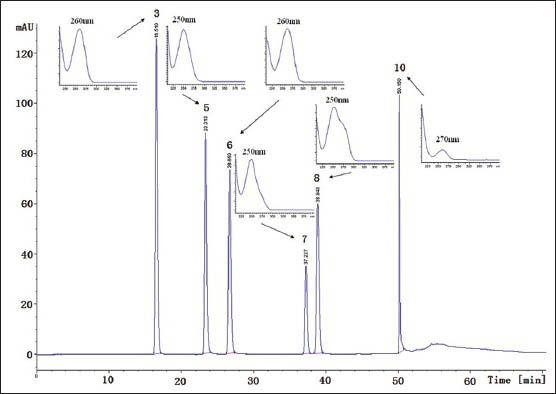
HPLC chromatogram of nucleosides and bases in HLR at 260 nm wavelength. The six insets are DAD UV scan of uracil (3), hypoxanthine (5), uridine (6), inosine (7), guanosine (8) and adenosine (10) peak (190-400 nm)
HPLC-ESI-MS analysis
HPLC-ESI-MS was employed to analyze the components separated by HPLC. In ESI-MS experiment, molecular mass of the components can be obtained. Comparing MS results with the uracil, hypoxanthine, uridine, inosine, guanosine, adenosine, 2’-deoxyguanosine and guanine standard, and those in the literature,[14,15] we have deduced eight structures of nucleosides and bases in the HPLC peaks. Since positive ionization ESI mode was used, all of the m/z data are [M+H]+. The retention times and mass spectra of products exactly matched with the corresponding standard compounds, which are shown in Figure 3 and Table 1.
Figure 3.
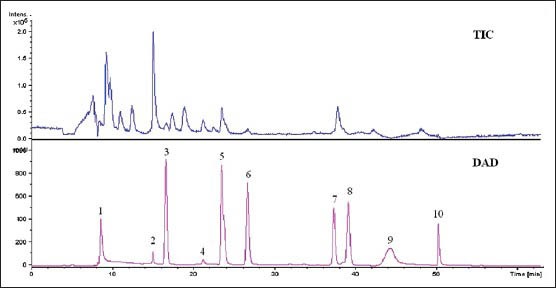
The HPLC/DAD chromatogram and total ion chromatogram in positive ion mode of HLR
Table 1.
HPLC-ESI-MS data of nucleosides and bases in HLR
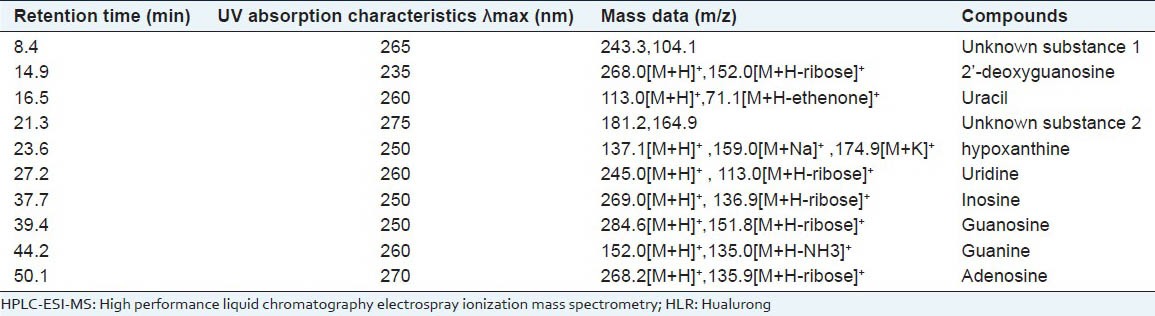
Effect of eight nucleosides and bases and the TNC of HLR on mouse splenocyte proliferation in vitro
Reports about immunomodulatory of protein and peptides in LR were very much in recent years. However, no specific studies have been carried out regarding water-soluble components of small molecular weight in HLR and their immunomodulatory activities. In the present study, the proliferation effect of eight nucleosides and bases and the TNC of HLR on mouse splenocytes was examined by MTT assay.
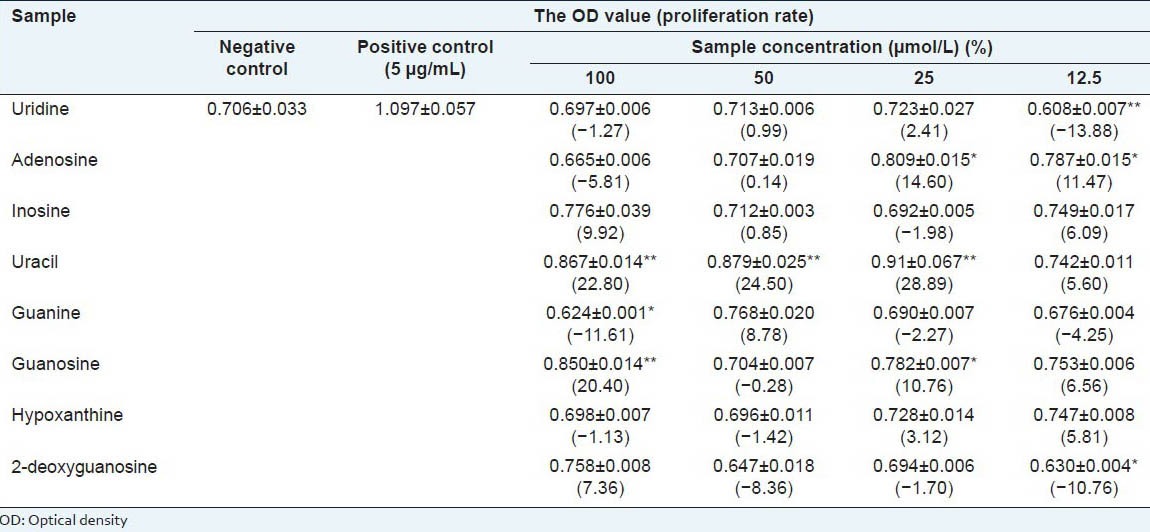
Effect of eight compounds. Exposure of mouse splenocytes to different concentrations (12.5, 25, 50, and 100 μmol/L) of eight compounds in HLR increased cell proliferation in each case [Table 2]. Proliferation rates in samples treated with uracil was dose dependently increased, the proliferation rates at 50 μmol/L and 100μmol/L of uracil treatment was significantly higher than that of negative control. Interestingly, the proliferation rates from adenosine treatment present significantly higher at lower dose such as 12.5 μmol/L and 25 μmol/L compared to negative control, the proliferation effect of adenosine was diminished following the increase of dose. In the case of other six compounds, the effect was marginal. Further study is still required to investigate the immunomodulatory effect of uracil and adenosine.
Table 2.
Effect of eight nucleosides and bases on the proliferation of mouse splenocytes (x±s. n=3)
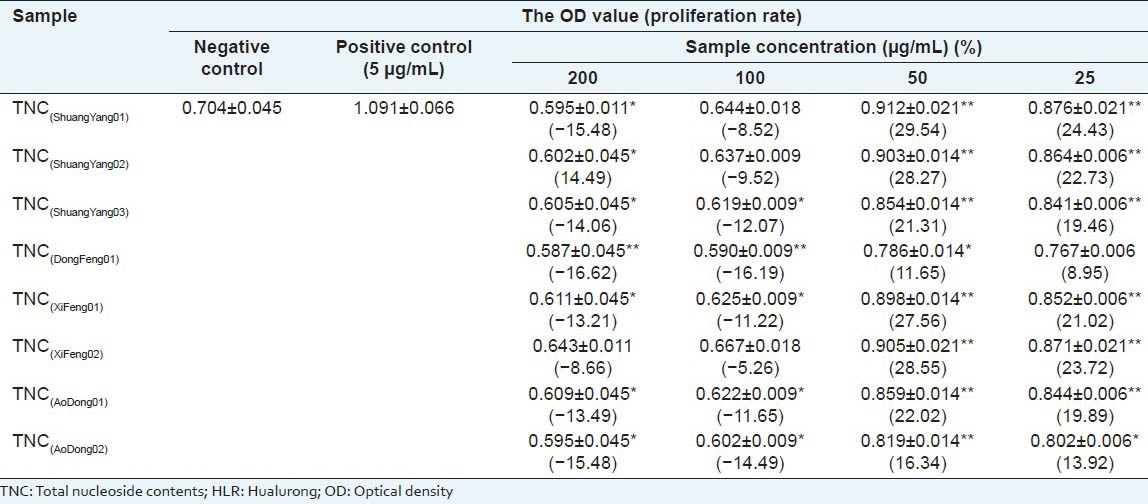
Effect of the TNC of HLR. Exposure of mouse splenocytes to TNC (25, 50, 100 and 200 μg/mL) of HLR increased cell proliferation in each case [Table 3]. Proliferation rates were much higher after the treatment of 25 μg/mL and 50 μg/mL of TNC compared to negative control. In case of higher concentrations, TNC resulted in decrease in the splenocyte viability. It indicated that TNC in higher concentrations (100 and 200 μg/mL) might have some cytotoxic effect on mouse splenocytes.
Table 3.
Effect of the TNC of HLR on the proliferation of mouse splenocytes (x±s. n=3)
LC method validation
The optimized HPLC method was validated by examining the linearity, sensitivity, stability, and accuracy.
Linearity, LOD, and LOQ. The working solutions were all prepared as described above to construct calibration curves. Each calibration curve contained five different concentrations and was performed in triplicate. An aliquot (10 μL) of each standard working solution was subjected to LC analysis. The linearity for each compound was established by plotting the peak area (y) versus concentration (x) of each analyte. Every R2 value was more than 0.9995, verifying the linearities of the calibration equations.
Stock solution containing six reference compounds was diluted to a series of appropriate concentrations, and an aliquot (20 μL) of the diluted solutions was injected into LC for analysis. LOD and LOQ for each analyte were calculated with corresponding standard solution on the basis of S/N of 3 and 10, respectively. Data for LOD and LOQ has been shown in Table 4.
Table 4.
Calibration curves, LOD, LOQ and recoveries of the analytes (n=5)
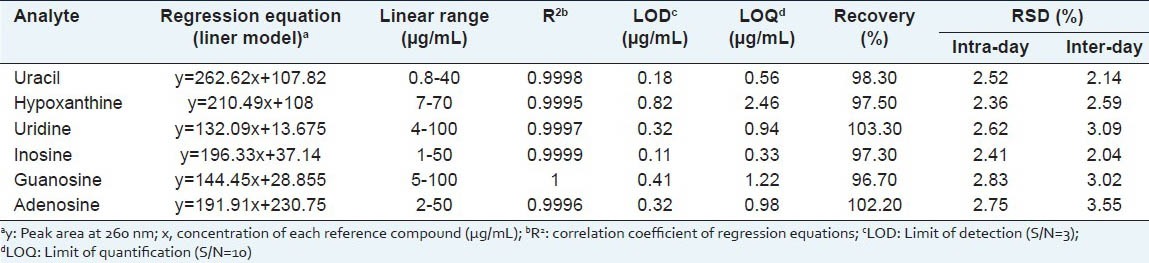
Stability and accuracy. Intra-day and inter-day variations were utilized to determine the precision of the developed assay. The intra-day variation was determined by analyzing the six replicate samples (1.0 g, Shuangyang, Jilin province, ShuangYang-01) within 1 day and inter-day variation was determined on three consecutive days. To confirm the repeatability, five different working solutions prepared from the same sample (1.0 g, Shuangyang, Jilin province, ShuangYang-01) were analyzed. Variations were expressed as relative standard deviations. The overall intra-day variations were < 2.83% and inter-day variations were <3.55% for the analytes [Table 4].
The accuracy of the method was tested in terms of recovery percentage. The recovery test was determined by standard addition method. Six nucleosides and bases in a mixed standard solution were spiked into the samples (0.5 g, Shuangyang, Jilin province, ShuangYang-01), and then extracted, processed and quantified in accordance with the established procedures, and finally the recovery rates were calculated. The average recovery with RSD values is presented in Table 4.
The results were considered to be satisfactory for subsequent analysis of all samples and the method proposed in our paper was accurate for the quantitative determination of the six nucleosides and bases in HLR.
Quantitative Determination of HLR
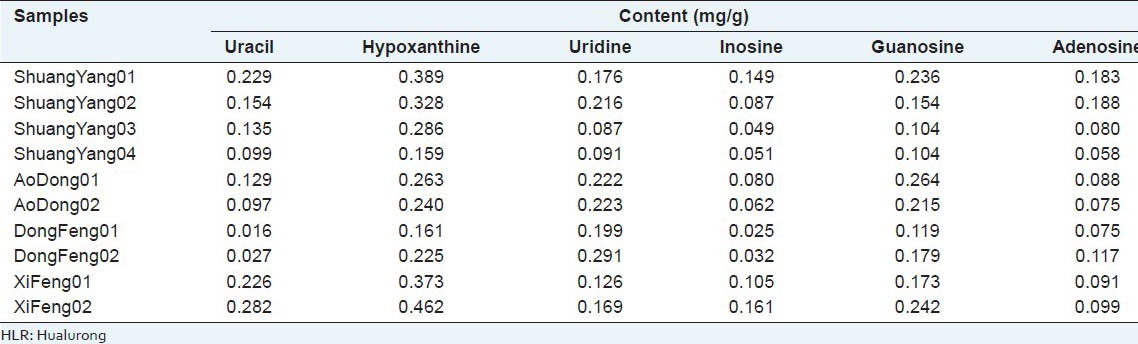
The developed analytical method was successfully applied to the simultaneous determination of uracil, hypoxanthine, uridine, inosine, guanosine, and adenosine in ten samples of HLR, which were obtained from various cities in Jilin province. Each sample was determined in triplicate. Peaks in the chromatograms were identified by comparing the retention times and online UV spectra with those of the standards. HPLC-DAD profiles are illustrated in Figure 2. Table 5 shows the content of the six nucleosides and bases in ten samples of HLR. It was found that the content of each analyte varied greatly among the different samples. In the majority of analytes, hypoxanthine was the main component whose content varied from 0.159 to 0.462 mg/g. Similar variation could also be found for the other nucleosides and bases. The variation in content of constituents could certainly lead to the variation of therapeutic effects. Hence, each procedure involved should be standardized.
Table 5.
Content of each analyte in ten HLR samples and fingerprint similarity (n=3)
CONCLUSION
In this study, a series of nucleosides and bases have been identified by HPLC-ESI-MS and adenosine, uracil, and TNC of HLR demonstrated immunomodulatory potential via stimulation of splenocyte proliferation. It is suggested that nucleosides and bases should act as the active principles of in HLR for its splenocyte-proliferating activity. Meanwhile, a validated rapid HPLC method for simultaneous rapid quantification of six nucleosides and bases in HLR samples for quality control was developed. This comprehensive strategy, providing reliable and adequate scientific evidence, could be applied to other traditional Chinese medicines to ameliorate their quality control.
ACKNOWLEDGMENTS
This research work was supported by the National Natural Science Foundation of China grants (81373936), the Science and Technology funds of Jilin province Department of Education (JiJiaoKeHeZi[2014] No. 71) and the Science and Technology funds of Health Department of Jilin Province (2012Z044). The authors are grateful to Linlin Zhang, Xueting Jia and Prof. Xiuchang Li, Development Center of Traditional Chinese Medicine and Bioengineering, Changchun University of Chinese Medicine, for statistical expertise, data collection and logistic support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chinese Pharmacopoeia Commission. Vol. 1. Beijing: China Medical Science Press; 2010. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- 2.Ke LJ, Lin DY, Huang XN, Huo YS, Rao PF, Ye XY. Comparison of protein composition and activities of pilose antler processed by different methods. Zhong Yao Cai. 2008;31:11–4. [PubMed] [Google Scholar]
- 3.Shi B, Li G, Wang P, Yin W, Sun GD, Wu QB, et al. Effect of antler extract on corticosteroid-induced avascular necrosis of the femoral head in rats. J Ethnopharmacol. 2010;127:124–9. doi: 10.1016/j.jep.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Shin KH, Yun C, Hye S, Lim SS, Won DH, Kim JK. Immuno-stimulating, anti-stress and anti-thrombotic effects of unossified velvet antlers. Nat Prod Sci. 1999;5:54–9. [Google Scholar]
- 5.Tian YZ, He JX, Wang FM, Wang GX, Wu Y, Ma QS. Morphometry of the effect of Pilose Antler on the testicle of rat. J Qinghai Med Coll. 1997;18:154–5. [Google Scholar]
- 6.Xu JP, Sun YX, Zhang HX, Song YR, Hou DY. The determination of the activity of superoxide dismutase in toast-drying and freeze-drying pilose antler. J Jilin Univ (Science Edition) 1991;4:103–4. [Google Scholar]
- 7.Zhang YH, Huang XW, Sun JH, OU XY, Zhou M, Deng YF. Protective effect of alcholic extractive of Cervi Cornu against myocardial damage of acute myocardial Infarction model rats and Influence on plasma ET. Chin J Inf Tradit Chin Med. 2007;14:40–1. [Google Scholar]
- 8.Zhou R, Li SF. In vitro antioxidant analysis and characterisation of antler velvet extract. Food Chem. 2009;114:1321–7. [Google Scholar]
- 9.Zhou R, Li SF, Zhang DC. Combination of supercritical fluid extraction with ultrasonic extraction for obtaining sex hormones and IGF-1 from antler velvet. Chin J Chem Eng. 2009;17:373–80. [Google Scholar]
- 10.Zhou R, Wang JY, Li SF, Liu Y. Supercritical fluid extraction of monoamine oxidase inhibitor from antler velvet. Sep Purif Technol. 2009;65:275–81. [Google Scholar]
- 11.Bo SR, Li QJ, Wang CY, Yuan XL, Wang QK. Research progress on chemical composition and pharmacological effects of cervi cornu pantotrichum. J Econ Anim. 2010;4:243–8. [Google Scholar]
- 12.Zhou R, Wang F, Yu HS, Chang M, Hao J, Li SF. Study on the extraction process of three biological bases from antler velvet. J Anhui Agric Sci. 2011;39:21805–8. [Google Scholar]
- 13.Kan F, Sun J, Tian L, Li C, Xu MX, Fang Y, et al. Isolation and proliferation effect of polysaccharide extracted from grub on the immunocytes of mice in vitro. J Nanjing Agric Univ. 2009;32:161–4. [Google Scholar]
- 14.Fan H, Li SP, Xiang JJ, Lai CM, Yang FQ, Gao JL, et al. Qualitative and quantitative determination of nucleosides, bases and their analogues in natural and cultured Cordyceps by pressurized liquid extraction and high performance liquid chromatography–electrospray ionization tandem mass spectrometry (HPLC–ESI–MS/MS) Anal Chim Acta. 2006;567:218–28. [Google Scholar]
- 15.Liu R, Ji J, Wang L, Chen S, Guo S, Wu H. Characterisation of nucleosides and nucleobases in Mactra veneriformis by high performance liquid chromatography coupled with diode array detector-mass spectrometry (HPLC–DAD–MS) Food Chem. 2012;135:548–54. doi: 10.1016/j.foodchem.2012.05.019. [DOI] [PubMed] [Google Scholar]


