Abstract
Background:
Achyranthes coynei Sant. (Family: Amaranthaceae) is a rare endemic medicinal plant used by local traditional practitioners to treat various diseases. The plant has been reported for promising antibacterial and antioxidant activities. However, the plant is not explored for its phytocompounds, especially triterpenoids.
Objective:
To study the accumulation and trends in distribution of triterpenoids: betulinic, oleanolic and ursolic acids (BA, OA and UA) in leaf, stem, root and inflorescence of A. coynei.
Materials and Methods:
Extraction was achieved using refluxing and reversed phase-ultra flow liquid chromatographic (RP-UFLC) technique was employed for determination. Separation of triterpenoids was achieved on a Hibar 250-4.6 mm, 5 μ, Lichrospher 100, C18e column using methanol and water (90:10) as mobile phase (pH adjusted to 5.0 using GAA) in an isocratic mode.
Results:
Oleanolic acid was higher in leaf (0.172 ± 0.009%) followed by stem (0.035 ± 0.002%) and root (0.028 ± 0.001%). Ursolic acid was accounted to be highest in the inflorescence (0.099 ± 0.005%). The contents of BA and UA were lower than OA in leaf and stem while it was remained undetected in roots of A. coynei.
Conclusion:
The triterpenoids: BA, OA and UA were detected, quantified and reported for the first time from A. coynei. In the present study leaves were found to be the major source of BA and OA, whereas inflorescence was for UA.
Keywords: Achyranthes coynei, betulinic acid, oleanolic acid, RP-UFLC, triterpenoids, ursolic acid
INTRODUCTION
Achyranthes coynei Sant. (Family: Amaranthaceae) is a profusely branching perennial shrub growing up to height of 2.0-4.5 m. It is a rare and endemic plant reported from Maharashtra and Karnataka states of India.[1,2] Achyranthes coynei is used by local traditional practitioners to treat various diseases.[1] The plant is reported to have antioxidant[3] and antibacterial[4] activities. Antibacterial properties of such traditionally used medicinal preparations are attributed mainly to group of triterpenoids.[5]
Triterpenoids are modified terpenes resembling steroids in their chemical structures [Figure 1].[6] Triterpenoids are the major group in A. aspera and oleanolic acid (OA) is reported to be the major compound.[6,7,8,9,10,11] Recently betulinic acid (BA) along with OA has been reported from A. aspera.[12] Reports also suggest BA, OA and ursolic acid (UA) individually or in combination to possess wide array of biological activities viz. anticancer, anti HIV, hepatoprotective and many other.[13]
Figure 1.
Structures of (a) Betulinic acid; (b) Oleanolic acid; (c) Ursolic acid
BA, OA and UA are three hydroxyl pentacyclic triterpenoic acids (HPTAs) found in number of plants as glycones of saponins and free acids.[14] There are no reports on phytochemical evaluation on A. coynei as per best of our knowledge. Thus the present study deals with determination of BA, OA and UA in various parts of A. coynei using RP-UFLC analysis.
MATERIALS AND METHODS
Plant materials and chemicals
Plant material of A. coynei was obtained from a single population from Madanabhavi, Belgaum district, Karnataka, India and a specimen was authenticated and deposited at Herbarium, Regional Medical Research Centre, Belgaum (Voucher Number: RMRC 785). All the solvents viz. methanol, water and glacial acetic acid (GAA) were of HPLC grade (Fischer Scientific, Mumbai, India). HPLC grade BA (≥98% pure) OA (≥97% pure) and UA (≥98% pure) were procured from Sigma-Aldrich, USA.
Extraction
The plant materials (leaf, stem, root and inflorescence), obtained from wild were dried at room temperature and finely powdered. Method given by Rajpal[15] was employed for extraction of triterpenoids, with minor modifications. A volume of 25 mL 50% (v/v) aqueous methanol was added to 5 g of powder and heated for 2-3 min for complete dissolution. A 75 mL of distilled water and 10 mL of concentrated H2SO4 was added with shaking. The mixture was refluxed for 5-6 hours over water bath at 95 ± 2°C. Cooled contents were filtered and transferred to separating funnel. 25 mL of chloroform was added and allowed for layers separation. The chloroform layer was separated and the procedure was repeated twice. The 50 mL chloroform layer was washed with distilled water to get acid free chloroform. The acid free chloroform layer was dried to obtain residue. The residue was dissolved in methanol and the same was used for RP-UFLC analysis.
Quantification of BA, OA and UA using RP-UFLC analysis
Previously described RP-UFLC method of Pai et al.,[12] was employed during the present investigation. Shimadzu chromatographic system (Model no. LC-20AD) consisting of a quaternary pump, manual injector, degasser (DGU-20A5), and dual λ UV absorbance diode array detector SPD-M20A was used for RP–UFLC analysis. The built-in LC-Solution software system was used for data processing. Methanol and water (90:10 v/v) mobile phase was used for chromatographic separation with pH 5.0 (adjusted using GAA) in an isocratic mode through Hibar 250-4.6 mm, 5 μ, Lichrospher 100, C18e column. The flow rate was 1 mL/min−1 for separation. Injection volume of standard and sample was 20 μL and the detection wavelength was set at 210 nm. The analysis time was 15 min for both standard and sample. Signal/noise ratios of 3.3 and 10 were applied for estimating the LOD and LOQ, respectively. The system suitability test was assessed by three replicate injections of the standard solutions at a particular concentration. The peak areas were used to evaluate repeatability of the method and their peaks were analyzed for resolution.
Statistical analysis
Values are represented as mean readings of three injections ± SD. Experimental data were subjected to analysis of variance (ANOVA) and significant (P < 0.05) means were determined using Duncan's multiple-range test to distinguish differences between content means at 5% level using SPSS for Windows version 16.0.
RESULTS AND DISCUSSION
The extracts of different parts of A. coynei were evaluated for percent yields (leaf: 0.322%, stem: 0.546%, root: 0.730% and inflorescence: 0.600%). These values were considered further to calculate percent (%) BA, OA and UA from respective parts of A. coynei.
Quantification of BA, OA and UA using RP-UFLC analysis
Six different concentrations (0.05, 1, 10, 20, 40 and 80 μg/mL) of standard BA, OA and UA were detected at 210 nm wavelength using RP-UFLC technique [Figure 2a and b]. The chromatogram profiles with retention time 11.492 ± 0.026 (BA), 12.462 ± 0.045 (OA) and 13.234 ± 0.035 (UA) min were obtained [Figure 2a]. The peaks obtained for the standards injected were sharp indicating no mixture of compounds near to its retention time (RT) and pure. The early peaks during detection were attributed to impurities in solvent system, although they do not have any compatibility issues in separation and quantification. The linearity curves for standards were obtained with coefficient of determination (R2) not more than 0.998 [Figure 2b and Table 1].
Figure 2.
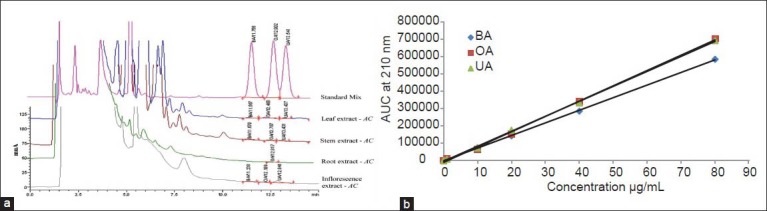
RP-UFLC profile of (a) Standard mixture of BA, OA, UA (80 μg/mL) and leaf, stem root, inflorescence extracts of A. coynei; (b) Six point calibration curve for standard BA, OA and UA (0.05, 1, 10, 20, 40, 80 μg/mL)
Table 1.
RP-UFLC analysis attributes and results obtained for BA, OA and UA
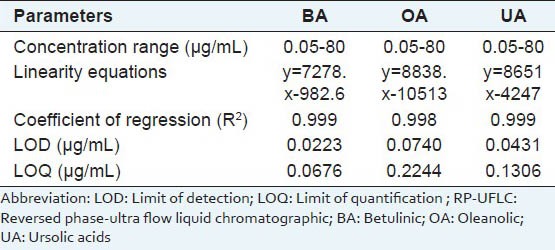
Above chromatographic conditions were used to analyze linearity and sensitivity of the method used. Three independent calibration equations were obtained for each analytes (BA, OA and UA) by correlating the detector signals with respective concentrations prepared. Details regarding concentration ranges, linearity equation, coefficient of regression, LOD and LOQ are presented in Table 1. These results indicated good linearity and significant validity for the calibration values used. Similarly, three injections each of three different concentrations of the analytes (1, 10, 20 μg/mL) had significant inter- and intra-day precision. Lower RSD (<0.72%) value of retention time indicated acceptable reproducibility of the method. The theoretical plate number (N) was found to be 9111.151 (BA), 9400.486 (OA) and 9547.568 (UA) for the column used during the study (250 × 4.6 mm i.d., particle size 5 μm), demonstrating acceptable column efficiency. All these results assure the competence of the current UFLC method for analysis of BA, OA and UA.
The chromatogram profiles of the samples were obtained at same conditions as that of standards [Figure 2a]. The results were expressed in percent dried powder of leaf, stem, root, and inflorescence [Figure 3]. Betulinic acid ranged from 0.028-0.125%, OA from 0.028-0.172% and UA from 0.024-0.099% in dried plant material. The RP-UFLC analysis revealed that, leaves sourced highest in content of BA (0.125 ± 0.006%) and OA (0.172 ± 0.009%) while UA was higher in inflorescences (0.099 ± 0.005%). The results were in accordance to that of A. aspera, wherein leaves had higher triterpenoids than other parts.[12] However, the contents of BA and OA were much higher in A. aspera than in A. coynei observed in present study.[12]
Figure 3.
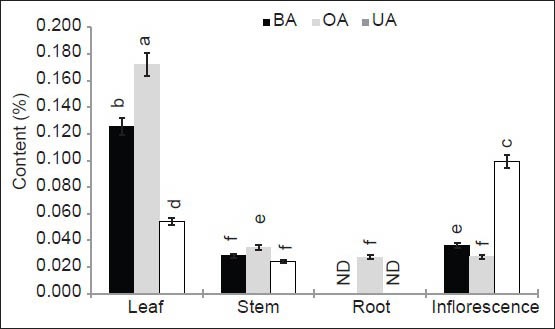
Histogram showing BA, OA, UA content (%) in leaf, stem, root and inflorescence extract of A. coynei Within each group, values with different letters indicate significant difference at P < 0.05 using Duncan's multiple-range test
Stem of A. coynei contain 0.035 ± 0.002% of OA followed by BA (0.028 ± 0.001%) and UA (0.024 ± 0.001%). Unlike other parts in the study accumulation of BA (0.036 ± 0.002%) was higher than OA (0.028 ± 0.001%) in the inflorescence. A trend in distribution of triterpenoids was observed in the magnitude from OA > BA > UA in all parts except inflorescence. However, BA and UA were not detected in roots of A. coynei. Furthermore, it would be interesting to note that there is a great variation (0.006-2.190%) in content of OA in different parts from the plants of the genus Achyranthes, wherein various methods of quantification were employed by different workers.[6,7,8,9,10,11,12,16,17]
CONCLUSION
The accumulation levels of triterpenoids (BA, OA and UA) varied in leaf, stem, root and inflorescence of A. coynei and significant variations in their trends of distribution were also observed. The content of triterpenoids was higher in leaves followed by inflorescence and stem. BA and UA remain undetected in root extracts of A. coynei. The present work is the first report of determining these triterpenoids using RP-UFLC analysis in the plant. These promising results need further attention and detailed study to discover its medicinal potential.
ACKNOWLEDGMENTS
Authors acknowledge Officer-in-charge, RMRC, Belgaum, Dr. R. K. Joshi, Phytochemistry Division, RMRC, Belgaum, for kind support and Mr. Ashok Naik, Biostatistics Department, RMRC, Belgaum for help in statistical analysis. SRP is indebted to SERB, DST, New Delhi for financial support during the work. VU is thankful to Indian Council of Medical Research, New Delhi for providing ICMR-SRF grant for the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pai SR, Upadhya V, Hegde HV, Kholkute SD. Achyranthes coynei Santapau (Amranthaceae)- An addition of endemic taxon to Flora of Karnataka, India. J Threat Taxa. 2011;3:1875–9. [Google Scholar]
- 2.Sharma BD, Kulkarni BG. Achyranthes coynei Santapau, Amaranthaceae. In: Nayar MP, Sastry AR, editors. Red Data Book of Indian Plants. Vol. 2. Calcutta: Botanical Survey of India; 1987. pp. 8–9. [Google Scholar]
- 3.Upadhya V, Pai SR, Ankad G, Pramod HJ, Hegde HV. Phenolic contents and antioxidant properties from aerial parts of Achyranthes coynei Sant. Indian J Pharm Sci. 2013;75:483–6. doi: 10.4103/0250-474X.119812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ankad G, Upadhya V, Pai SR, Hegde HV, Roy S. In vitro antimicrobial activity of Achyranthes coynei Sant. Asian Pac J Trop Dis. 2013;3:930–5. [Google Scholar]
- 5.Fontanay S, Grare M, Mayer J, Finance C, Duval RE. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J Ethnopharmacol. 2008;120:272–6. doi: 10.1016/j.jep.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Tandon N, editor. Vol. 9. New Delhi: Indian Council of Medical Research; 2011. Quality Standards of Indian Medicinal Plants; pp. 18–31. [Google Scholar]
- 7.Nehete JY, Deshmukh VN, Shewale VV, Narkhede MR, Aurangabadkar VM. Quantitation of oleanolic acid in Achyranthes aspera L. roots and leaves extracts by high performance thin layer chromatography. Int J Pharm Res Dev Online. 2009:7. [Google Scholar]
- 8.Mehta FA, Patel BG, Pandya SS, Ahir KB. Densitometric HPTLC method for analysis of oleanolic acid in Achyranthes aspera L. J Planar Chromatogr. 2010:23. [Google Scholar]
- 9.Aeri V, Khan MI, Alam S. A validated HPLC method for the quantification of oleanolic acid in the roots of Achyranthes aspera Linn. and marketed formulation. Int J Pharm Sci. 2010;2:74–8. [Google Scholar]
- 10.Li-Lan O, Xin Y, Chun Z, Ye Z. Pharmacognostical identification of Achyranthes aspera L. roots. J Med Plant. 2012;3:5–8. [Google Scholar]
- 11.Suhagia BN, Rathod IS, Shah SA, Sindhu S. Validated chromatographic method for the determination of oleanolic acid in Achyranthes aspera Linn. Int J Phytother. 2014;4:16–21. [Google Scholar]
- 12.Pai SR, Upadhya V, Hegde HV, Joshi RK, Kholkute SD. New report of triterpenoid betulinic acid along with oleanolic acid from Achyranthes aspera by reversed-phase–ultra flow liquid chromatographic analysis and confirmation using high-performance thin-layer chromatographic and fourier transform–infrared spectroscopic techniques. J Planar Chromatogr. 2014;27:38–41. [Google Scholar]
- 13.Acton QA. Georgia: Scholarly Editions Atlanta; 2012. Pentacyclic triterpenes-advances in research and application; pp. 1–10. [Google Scholar]
- 14.Tian S, Shi Y, Yu Q, Upur H. Determination of oleanolic acid and ursolic acid contents in Ziziphora clinopodioides Lam. by HPLC method. Pharmacogn Mag. 2010;6:116–9. doi: 10.4103/0973-1296.62898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajpal V. Vol. 2. New Delhi: Eastern Publishers; 2005. Standardization of botanicals; pp. 232–4. [Google Scholar]
- 16.Wu MH, Li XL, Wang M, Liu JY, Zhang MY. Determination of oleanolic acid in Achyranthes bidentata Blume. and its preparations by super critical fluid chromatography. Yao Xue Xue Bao. 1992;27:690–4. [PubMed] [Google Scholar]
- 17.Li X, Hu S. Determination of oleanolic acid in the root of Achyranthes bidentata Bl. from different places of production by TLC-scanning. Zhongguo Zhong Yao Za Zhi. 1995;20:459–60. [PubMed] [Google Scholar]


