Abstract
Background:
‘Ge-Gen-Qin-Lian’ Decoction derived from ‘Shang-Han-Lun’ compiled by Zhang Zhongjing. It is widely used in the treatment of acute gastroenteritis, bacillary dysentery, virus diarrhea. This paper describes a sensitive and specific assay for the determination of the 11-marker compounds using ultra performance liquid chromatography (UPLC).
Objective:
To develop an UPLC method for simultaneous determination of 11 bioactive compounds in ‘Ge-Gen-Qin-Lian’ preparations.
Materials and Methods:
The chromatography analysis was performed on an Agilent Proshell 120 EC-C18 column (4.6 × 50 mm, 2.7 μm) at 30°C with a gradient elution of methanol, 0.5% formic acid and 0.5% ammonium acetate at a flow rate 1.0 ml/min and UV detected at 270 nm.
Results:
All calibration curves showed good linear regression (r ≥ 0.9993) within tested ranges. Limits of detection (LOD) and limits of quantification (LOQ) fell in the range between 0.0691-1.04 μg/ml and 0.23–3.43 μg/ml, respectively. The mean recovery of each herbal medicine ranged from 96.60 to 102.11%.
Conclusion:
The method was validated for repeatability, precision, stability, accuracy, and selectivity. The validated method was successfully applied to simultaneous analysis of these active components in ‘Ge-Gen-Qin-Lian’ decoction.
Keywords: Determination, eleven components, Ge-Gen-Qin-Lian decoction, quality evaluation, UPLC
INTRODUCTION
Traditional Chinese medicines (TCMs) have a long history in medical practice and health care in China. Owing to the change in global trend, TCM have become popular worldwide for health promotion and adjuvant therapy. Unlike a single chemical entity determining the therapeutic use of chemical drugs, TCM preparations are usually composed of several herbal medicines, each herbal medicine is, itself, a complex mixture containing lot of chemical components. It poses a serious challenge to establish comprehensive and pragmatic quality control methods to guarantee the safety and efficacy of TCM. Currently, selecting a single or a few analytical compounds from only a certain herbal medicine, which determine either its efficacy or quality is contrary to TCM principles. It cannot afford to provide sufficient quantitative information, for other medicinal compositions in a complex TCM preparation cannot and accurately reflect the total quality of TCM products.[1,2,3] Therefore, the methods of simultaneous measurement of multi-components from different medicinal herbs have attracted great attention due to the major advantage of providing valuable qualitative and quantitative information.
’Ge-Gen-Qin-Lian’ Decoction consists of Radix puerariae, Radix scutellariae, Rhizoma coptidis and Radix glycyrrhizae, derived from ‘Shang-Han-Lun’ compiled by Zhang Zhongjing, a distinguished pharmacist in the Han Dynasty of China. It has been shown to have beneficial effects on acute gastroenteritis, bacillary dysentery, virus diarrhea, sensation of heat in the chest and epigastrium in clinic.[4]
Pharmacology studies have revealed that the main bioactive components of Radix puerariae, Radix scutellariae, Rhizoma coptidis and Radix glycyrrhizae are isoflavonoids, flavonoids and alkaloids, respectively. The pharmacological reports were focused on isoflavonoids (puerarin, daidzin), flavonoids (baicalin, baicalein, wogonoside, wogonin and liquiritin), and alkaloids (berberine, palmatine, jatrorrhizine and coptisine), and these compounds were proved to be the key pharmacological constituents of Ge-Gen-Qin-Lian decoction.[5,6,7,8,9,10,11]
A number of high-performance liquid chromatography (HPLC) methods[12,13,14] have been developed for component determination in crude drugs and various ‘Ge-Gen-Qin-Lian’ preparations, with the main focuses on puerarin, baicalin, berberine and glycyrrhizic acid, which are assumed to have high contents in the crude drugs of ‘Ge-Gen-Qin-Lian’ decoction. Among these methods, HPLC has been considered a powerful tool. However, there are many components in ‘Ge-Gen-Qin-Lian’ preparations, and the conventional column used by previous methods could interfere with the co-eluted peaks due to its lower resolution. Owing to the utilization of sub-2.7 μm particles as stationary phase, ultra performance liquid chromatography (UPLC) showed many advantages over the conventional HPLC including increased peak capacity, improved resolution, shorter retention time, less solvent consumption and higher sensitivity.[15]
This study stated the first application of the UPLC method in the determination of eleven contents (puerarin, daidzin from Radix puerariae, coptisine, jatrorrhizine, berberine, palmatine from Rhizoma Coptidis, baicalin, wogonoside, baicalein and wogonin from Radix scutellariae, liquiritin from Radix glycyrrhizae) of preparation of ‘Ge-Gen-Qin-Lian’ decoction. Compared with the conventional analytical methods, the established methods can exhibit more accuracy, higher resolution, shorter analysis time, and reduced solvent consumption. Sample preparation, chromatographic conditions, and method validation were objectives of the research reported. The presented method was also applicable to analyze ‘Ge-Gen-Qin-Lian’ preparations of different pharmaceutical forms, such as dispensing granule, tablet and pill.
MATERIALS AND METHODS
Materials and reagents
Radix Puerariae, Radix Scutellariae, Rhizoma Coptidis and Radix Glycyrrhizae were purchased from Hongqiao Medicinal Materials Electuary Co., Ltd (Shanghai, PR China) and verified by Prof. Zhi-Li Zhao (Shanghai University of Traditional Chinese Medicine).
Puerarin, daidzin, liquilitin, coptisine, jatrorrhizine, berberine, palmatine, baicalin were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Wogonoside, baicalein and wogonin were purchased from Shanghai u-sea biotech Co.Ltd (China), the purity of these reference compounds were determined to be more than 98% by UPLC analysis. Methanol, formic acid and ammonium acetate were of chromatographic-grade, which were purchased from Merck (Darmstadt, Germany). Water for chromatographic analysis was purified with a Milli-Q water system (Millipore, MA, USA). Other reagents were of analytical grade. All solvents and samples were filtered through a 0.45 μm nylon filter.
Instrument and chromatographic conditions
Chromatography was performed on a Waters ACQUITY Ultra Performance LC™ system (Milford, MA, USA) equipped with a binary solvent delivery pump, an autosampler, a thermostated column compartment, a photodiode array λ detector and Masslyn × 4.1 workstation. The chromatographic analysis was carried out on an Agilent Proshell 120 EC-C18 (4.6 × 50 mm, 2.7 μm) column. The mobile phase A was methanol, mobile phase B consisted of 0.5% formic acid and 0.5% ammonium acetate in water. A gradient program was used as follows: 0-6 min, 22%A → 32%A, 6-9 min, 32%A, 9-10 min, 32%A → 45%A, 10-20 min, 45%A → 60%A. Flow rate and detection wavelength were set at 1.0 ml/min and 270 nm, respectively. Column temperature was maintained at 30°C.
Preparation of sample solutions and negative control samples
A certain amount of the crude drugs (Radix puerariae 15.0 g, Radix scutellariae 9.0 g, Rhizome coptidis 9.0 g and Radix glycyrrhizae 6.0 g) equivalent to a daily dose of ‘Ge-Gen-Qin-Lian’ decoction was weighed. Radix puerariae was boiled for 20 min prior to mixing the other crude drugs, and then the mixture was decocted in boiling water twice, totaling 1.5 h. The supernatants were filtered and mixed when they were still hot, and was let to evaporate to an appropriate volume, and diluted. Finally, this standard decoction was extracted by an ultrasonic bath for 20 min, diluted with 80% methanol to a concentration of 8 mg/ml, and filtered with a 0.45 μm milliporous membrane before use. The negative control samples of ‘Ge-Gen-Qin-Lian’Decoction were prepared by deriving one herb from the prescriptions. The herbs were accurately weighed according to specified ratio of the prescription of ‘Ge-Gen-Qin-Lian’decoction and was prepared as similar to the procedure of the sample preparation protocol.
Preparation of standard solutions and calibration curve
The standard solutions of puerarin (187.2 μg/ml), daidzin (116.4 μg/ml), liquilitin (50.40 μg/ml), coptisine (56.40 μg/ml), jatrorrhizine (34.40 μg/ml), berberine (390.0 μg/ml), palmatine (82.00 μg/ml), baicalin (420.0 μg/ml), wogonoside (100.0 μg/ml), baicalein (10.08 μg/ml) and wogonin (21.20 μg/ml) were prepared in water/methanol (50/50, v/v). Working solutions of low concentration were prepared by appropriate dilution of the stock solution. The stock and working solutions were stored at 4°C.
RESULTS AND DISCUSSION
Chromatographic separation
To optimize the extraction conditions for achievement of quantitative extraction, variables involved in the procedure such as solvent, sample-solvent ratio and extraction time were optimized. Pure and aqueous methanol solutions are often used as the extraction solvents. In the present study, 80% methanol and 50% methanol were examined as the extracting solvent. Most of the (iso) flavonoides, alkaloids and saponins can be extracted completely with 80% methanol as the diluted solvent. Furthermore, the extraction efficiency for all the analytes was evaluated by repeating extracting samples three times by ultrasonication, all above 98%, which suggested that one-step ultrasonic extraction could meet the extraction requirement. Additionally, the method of ultrasonication is comparatively simpler.
A suitable chromatographic column, mobile phase, elution mode and detection wavelength are important for good separation. Different types of chromatographic columns such as XB-C18 column (4.6 × 250 mm, 5 μm), ZORBAX SBC18 (4.6 × 250 mm, 5 μm) Lichrosorb C18 (4.6 × 150 mm, 5 μm), ACQUITY UPLC BEH C18 (2.1 × 50 mm, 1.7 μm), Proshell 120 EC-C18 (4.6 × 50 mm, 2.7 μm) were tested. Different mobile phases consisting of acetonitrile-water and methanol-water with some modifiers including phosphoric acid, acetic acid, and formic acid solutions adjusted by ammonium acetate at different pH values were investigated under different gradient elution modes. The UV spectra of the analytes in methanol between 190-400 nm were acquired by use of the photodiode array detector. Considering the sensitivity and simplicity of sample analysis, 270 nm was selected for analyzing these contents. At this wavelength, the contents of samples have enough sensitivity to be detected. The typical chromatographic profiles of the standard solution, sample solution and negative control samples solution were shown in Figures 1 and 2.
Figure 1.
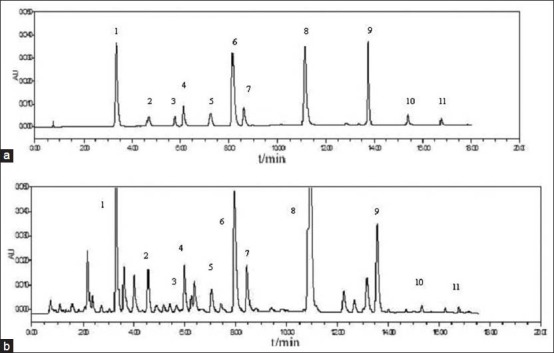
Chromatograms obtained from a mixed standard solution (a) and sample solution (b)
Figure 2.
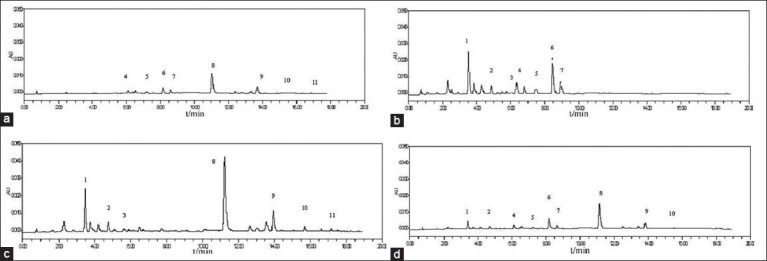
Chromatograms of the negative control samples
Calibration curves, LOD, and LOQ
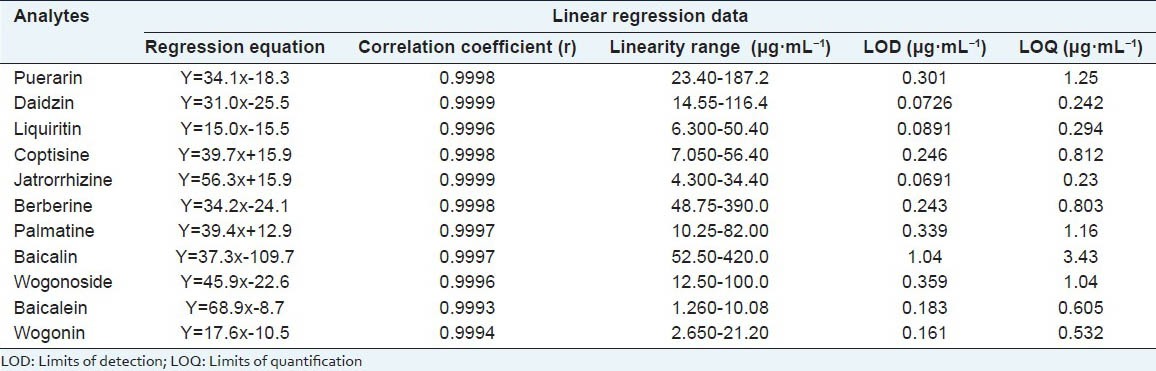
The linearity calibration curves were constructed by five concentration assays of each reference compound in triplicate. An aliquot (10 μl) of each standard solution was subjected to analysis. Integrated chromatographic peak areas (Y) were plotted against the corresponding concentrations (X μg/ml) of the 11constituents in the standard solutions to obtain calibration curves based on linear regression analysis. The regression curves had good linearity (r > 0.9993) in the investigated ranges. LOD and LOQ expressed by 3- and 10-fold of the ratio of the signal to-noise (S/N) were also acquired. Detailed information regarding calibration curves, linear ranges, LOD and LOQ are listed in Table 1.
Table 1.
Linear regression data, LOD, LOQ of analytes
Precision, repeatability and stability
The precision of the developed method was evaluated by performing intra-assay and inter- ssay by replicate (n = 5) injection of a mixed standard solution. The intra-assay precisions were evaluated with the standard solutions at 4-h intervals during the same day, and the inter-assay precisions were determined in 5 consecutive days. Inter- and intra-assay precisions for all the investigated components expressed as relative standard deviation (R.S.D.) were between 0.31% and 1.4%. Add. The results are shown in Table 2.
Table 2.
Precision, repeatability and stability of analytes
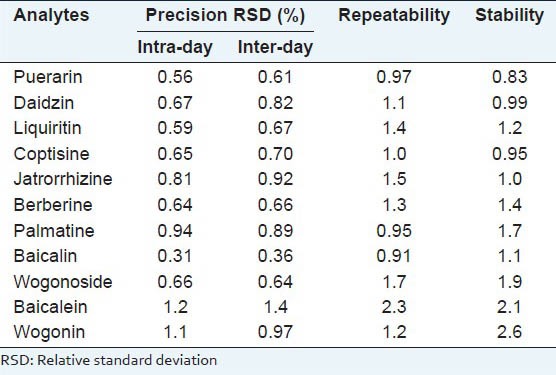
Six independent sample solutions of ‘Ge-Gen-Qin-Lian’decoction were prepared, analyzed and evaluated for repeatability. The RSD values varied from 0.91 to 2.3%. Stability was also tested at room temperature, and samples were analyzed in triplicate every 8 h and was continued for 48 h. The sample solution can be regarded as stable because the RSD values of peak areas varied from 0.83 to 2.6%. The results are shown in Table 2.
Accuracy
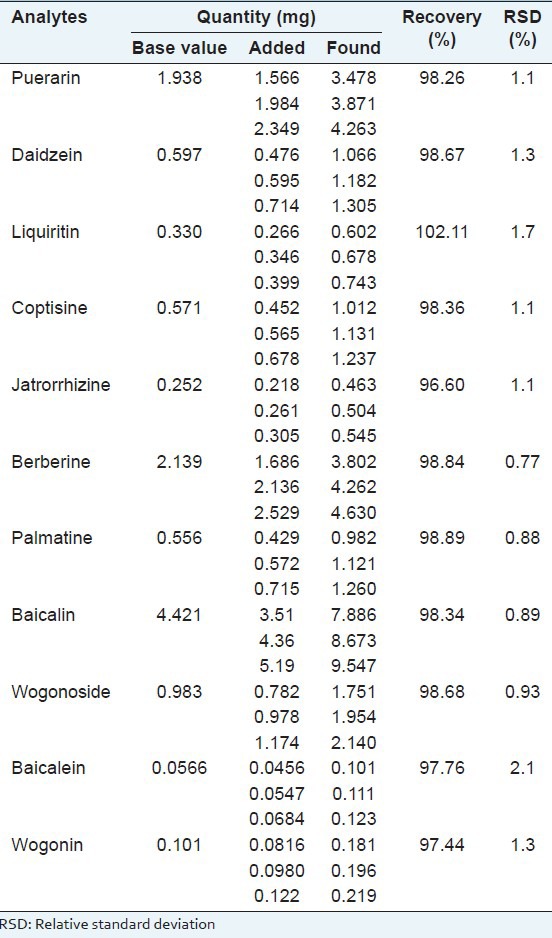
In the recovery test, the proposed method was applied to the samples spiked with the mixed standard solution at high, middle and low concentration levels, and the mixtures were extracted and analyzed in triplicate using the proposed method. Average recoveries of the investigated targets ranged from 96.60% to 102.11%, and the RSD values were all <3% as shown in Table 3. It was clear that the method enables highly accurate simultaneous analysis of the 11 analytes. The results are shown in Table 3.
Table 3.
The result of the recovery test (n=3)
Sample analysis
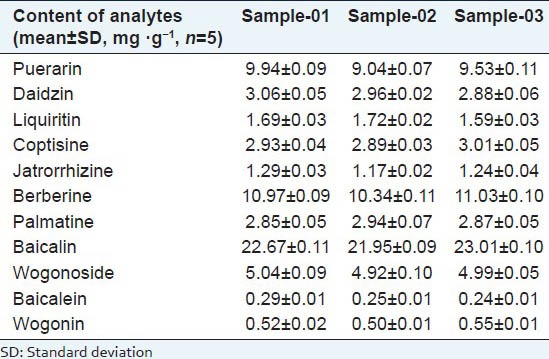
The validated UPLC method was applied to analyze active markers of ‘Ge-Gen-Qin-Lian’decoction. Five injections of each sample were performed to obtain the mean value and the RSD value. Contents of the 11 components in the samples are listed in Table 4. The results indicate that all the 11-marker compounds were detectable in each sample. The variation of the amounts among the three batches of decoction was found based on the quality of the herbal materials (what its cultivar was, where it was planted, and when it was harvested) and the efficiency of the extraction procedure. To avoid such variations, the samples can be collected immediately, and then extracted with the selected solvents and analyzed instantly.
Table 4.
Amounts of the active components in three different samples of ‘Ge-Gen-Qin-Lian’ decoction
CONCLUSION
The marker components of Chinese herbal preparations could be influenced by different sources of their crude drugs and by the efficiency of the extraction procedure; therefore the sensitivity and the accuracy of an analytical method are of great significance in identification, determination and quality evaluation of multiple constituents in Chinese herbal preparations. This is the first report for simultaneous determination of the 11-marker compounds by UPLC. UPLC is a new technique used in the chromatographic separation and determination in all samples. The high resolution obtained in extremely short analysis time makes UPLC a very attractive tool for the pharmaceutical analysis. The developed method is valuable for improving the quality control of the medicinal products, and can be applied in the quality control of other related pharmaceutical preparations containing Radix puerariae, Radix scutellariae, Rhizoma coptidis and Radix glycyrrhizae.
ACKNOWLEDGMENTS
This study was supported by Natural Science Foundation of Shangshai (12ZR1431500), Shanghai Municipal Education commission (11ZZ110, 11YZ69), Program for Shanghai Innovative Research Team in University (2009).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yin L, Lu B, Qi Y, Xu L, Han X, Xu Y, et al. Simultaneous determination of 11 active components in two well-known traditional Chinese medicines by HPLC coupled with diode array detection for quality control. J Pharm Biomed Anal. 2009;49:1101–8. doi: 10.1016/j.jpba.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Wang D, Wu J, Yu B, Zhu D. Identification of multiple constituents in the traditional Chinese medicine formula GuiZhiFuLing-Wan by HPLC-DAD-MS/MS. J Pharm Biomed Anal. 2009;49:267–75. doi: 10.1016/j.jpba.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Feng C, Cai YL, Ruan JL. Simultaneous determination of 10 active components in traditional Chinese medicine “YIGONG” capsule by RP-HPLC-DAD. J Pharm Biomed Anal. 2008;47:442–7. doi: 10.1016/j.jpba.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Wu JY, Yu LZ, Luo JB, Shao HX. Comparative study on bacteriostasis among compositions of Gegen Qinlian Decoction against Escherichia coli. J Pharmacol Clin Chin Mater Med. 2003;19:3–5. [Google Scholar]
- 5.Xiong FL, Sun HX, Gan L, Yang XL, Xu HB. Puerarin protects rat pancreatic islets from damage by hydrogen peroxide. Eur J Pharmacol. 2006;529:1–7. doi: 10.1016/j.ejphar.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Hosoda K, Furuta T, Yokokawa A, Ogura K, Hiratsuka A, Ishii K. Plasma profiling of intact isoflavone metabolites by high-performance liquid chromatography and mass spectrometric identification of flavone glycosides daidzin and genistin in human plasma after administration of kinako. Drug Metab Dispos. 2008;36:1485–95. doi: 10.1124/dmd.108.021006. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Li G, Zou Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm Drug Dispos. 2011;32:427–45. doi: 10.1002/bdd.771. [DOI] [PubMed] [Google Scholar]
- 8.Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 9.Wu DZ, Yuan JY, Shi HL, Hu ZB. Palmatine, a protoberberine alkaloid, inhibits both Ca (2+)- and cAMP-activated Cl(-) secretion in isolated rat distal colon. Br J Pharmacol. 2008;153:1203–13. doi: 10.1038/sj.bjp.0707684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Cao A, Zhou J, Hu Z, Wu D. Effect of jatrorrhizine on delayed gastrointestinal transit in rat postoperative ileus. J Pharm Pharmacol. 2012;64:413–9. doi: 10.1111/j.2042-7158.2011.01407.x. [DOI] [PubMed] [Google Scholar]
- 11.Dong X, Zhao SP, Liu Y, Fu GX, Li KM, Li P. Protective effect of Liquiritin on cardiocyte injury of neonate rat induced by aconitine. China J Tradit Chin Med Pharm. 2009;24:163–6. [Google Scholar]
- 12.Qu HB, Ma YH, Yu K, Cheng YY. Development of an HPLC Method for the Quality Evaluation of ‘Ge-Gen-Qin-Lian’ Tablets Derived from Traditional Chinese Medicine. Chromatographia. 2007;65:713–8. [Google Scholar]
- 13.Chen LH, Wang Q. Influence of various compatibilities on the content of baicalin and berberine in Gegen Qinlian Decoction. J Northwest Pharma. 2005;20:147–9. [Google Scholar]
- 14.Qi LW, Yu QT, Li P, Li SL, Wang YX, Sheng LH, et al. Quality evaluation ofRadix Astragali through a simultaneous determination of six major active isoflavonoids and four main saponins by high-performance liquid chromatography coupled with diode array and evaporative light scattering detectors. J Chromatogr A. 2006;1134:162–9. doi: 10.1016/j.chroma.2006.08.085. [DOI] [PubMed] [Google Scholar]
- 15.Yu K, Little D, Plumb R, Smith B. High-throughput quantification for a drug mixture in rat plasma-a comparison of Ultra Performance liquid chromatography/tandem mass spectrometry with high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:544–52. doi: 10.1002/rcm.2336. [DOI] [PubMed] [Google Scholar]


