Abstract
Background:
Traditional Chinese medicine (TCM) formula has been used for over 1000 years and most of them contain complicate chemical constituents. Chromatographic fingerprinting has been widely accepted as a crucial method for qualitative and quantitative analyses for TCM. Zhi Zhu Wan (ZZW), a classical Chinese medical formula, has been commonly used for the treatment of gastrointestinal disease, which pose a serious challenge to its quality control.
Materials and Methods:
In this work, a sensitive and reliable method of high-performance liquid chromatography coupled with photodiode array detector (HPLC-PDA) was developed to control the quality of ZZW for chemical fingerprint analysis and quantitative analysis of four major bioactive constituents, including hesperidin, naringin, neohesperidin, and atractylenolide I. The chromatographic separation was performed on a Waters Symmetry C18 column (4.6 mm × 250 mm, 5 μm particle size), with an aqueous 0.095% phosphate acid and acetonitrile mobile phase gradient.
Results:
Optimization of other experimental conditions was validated with satisfactory accuracy, precision, repeatability, and recovery. In quantitative analysis, the four components showed good regression (R > 0.9994) within test ranges, and the recovery method ranged from 99.32% to 100.630%. HPLC fingerprints of the ZZW samples were compared by performing similarity analysis.
Conclusion:
The results indicated that the newly developed HPLC-PDA fingerprint method would be suitable for quality control of ZZW.
Keywords: Chromatographic fingerprinting, high-performance liquid chromatography coupled with photodiode array detector, quality control, traditional Chinese medicine, Zhizhu Wan
INTRODUCTION
Traditional Chinese medicine (TCM) has been used for disease prevention and therapy in China for a long time and is becoming increasingly popular over the world.[1] Usually, TCM is used as whole plant and/or combination of several herbs, and multiple constituents are responsible for the therapeutic effects. Quality control is vital for ensuring the safety and efficacy of TCM.[2] To date, the valid method for quantitatively evaluating the quality of TCM is poor. TCM is complex mixtures and contain usually hundreds of chemically different constituents, which make the quality control of crude drugs and their medical preparations extremely difficult.[3] Therefore, better analytical strategies to assure their efficacy, safety, and consistency are in great demand. Chromatographic fingerprinting (CF) technique of TCM has proved to be a comprehensive strategy for assessing the intact quality and exploring the complexity of herbal medicines.[4,5] Also, it is a quality-relevant data high-throughput and integral tool to explore chemically the complexity of TCM.
Zhi Zhu Wan (ZZW), a classical Chinese medical formula, consists of Atractylodes Rhizome and Fructus Citrus Immaturus, and it is used clinically for the treatment of gastrointestinal diseases.[6] It has been officially listed in Chinese Pharmacopoeia (State Pharmacopoeia 2010) as two different crude herbs. Atractylodis Rhizoma is defined as the rhizome of Atractylodes macrocephala Koidz (A. Koidz). The active compounds in this rhizome are atractylone, atractylenolid I, and atractylenolid III. The other combination crude drug in ZZW is Fructus Citrus Immaturus, which is derived from both Citrus aurantium L. and Citrus sinensis Osbeck. Fructis Citrus Immaturus originated from Citrus aurantium L. is used to prepare blue-labeled ZZW (BZZW), whereas Fructis Citrus Immaturus from Citrus sinensis Osbeck is used to prepare red-labeled ZZW.[7,8] In this prescription, A. Koidz is the most important crude drug and can relieve the symptoms of abdominal distention, dyspepsia, nausea, vomiting, etc., in gastrointestinal diseases. It has been the principal drug in many Chinese medicinal prescriptions and has been used to treat symptoms of the gastrointestinal tract, such as anorexia, for 2000 years.[9] Citrus aurantium L. or Citrus sinensis Osbeck is the most important crude drug. It has been reported that citrus plants possess a wide range of pharmacological properties, including moderate diuretic, antioxidant, anti-inflammatory, free radical scavenging, and lipid peroxidation inhibitory effects.[10] However, the quality control of ZZW preparation has not been reported thoroughly.
Chemical fingerprint and quantitative analysis have become one of the most frequently applied approaches in the quality control of TCM.[11,12,13,14,15] Studies about combining chromatographic fingerprint and multi-ingredient quantification by high-performance liquid chromatography coupled with photodiode array detector (HPLC-PDA) for the quality control of ZZW preparation have not been reported. Thus, a simple, accurate, and practical HPLC-PDA method was developed for the simultaneous determination of multi-bioactive components in ZZW (BZZW and RZZW) preparation. The chemical fingerprints of ZZW were established and investigated by similarity analysis (SA). The combination of chromatographic fingerprint analysis and the simultaneous determination of the bioactive components offers a more comprehensive strategy for the quality evaluation of ZZW.
Experimental section
Materials, reagents, and chemicals
Methanol, HPLC grade, was purchased from Dikma Technology Corporation (Richmond Hill, USA). Deionized water was purified on a Milli-Q system (Millipore, Bedford, USA). Formic acid and phosphoric acid, analytical grade, were obtained from Beijing Reagent Company (Beijing, China). All other organic solvents were of analytical grade. BZZW and RZZW were purchased from Experimental Pharmaceutical Company of China Academy of Chinese Medical Science (Beijing, China). Hesperetin and naringenin (purify, 98%) were purchased from MP Biomedicals, Inc. (Sichuan, P. R. China). Neohesperidin was purchased from Sigma Corporation. Atractylenolide I was provided by Hokkaido University, Japan. Synephrine, hesperidin, and naringin were purchased from Inspection agency of Pharmaceutical and Biological Products (Beijing, China).
Preparation of standard solution
Standard samples were accurately weighed and dissolved in methanol to produce a solution containing 179.2 μg/mL hesperidin, 56.0 μg/mL naringin, 268.8 μg/mL neohesperidin, and 9.2 μg/mL atractylenolide I, which was used as the reference solution.
Preparation of sample solution
Each sample (1.0 g powder) was extracted with 50 mL of methanol by three times under ultrasonic. The final solution was filtered through a 0.45-μm membrane prior to use. An aliquot of 10 μL of each sample solution was injected into the HPLC system for analysis.
Instrumentation and chromatographic condition
The 2695 liquid chromatography system (Waters, USA) comprised a Waters 600 controller, two Waters 600 pumps, a 2695 auto-injector, a Waters 2695 column oven, and a Waters 2996 diode array detector. The HPLC column consisted of a Waters Symmetry C18 column (4.6 mm × 250 mm, 5 μm) connected to Nova-Pak C18 Guard-PakTM guard column (2 mm × 4 mm, 5 μm). The gradient elution was employed using solvent A (water with 0.095% phosphate acid, v/v) and solvent B (acetonitrile) at 30°C for 10 min. Gradient elute procedure: 0-15 min, A 100-90%; 15-60 min, A 90-75%; 60-110 min, A 75-20%; 110-130 min, A 20-100%. The flow rate was set at 1.0 mL/min. The detection wavelengths were set at 200-400 nm. A volume of 10 μL of sample was subjected to HPLC for analysis.
RESULTS AND DISCUSSION
Optimization of HPLC conditions
Different HPLC parameters were examined and compared, including various columns, mobile phases, detection wavelengths, and gradient elution conditions to obtain as much chemical information as possible and to determine the best separation mechanism in chromatograms. Three kinds of reversed-phase columns, namely, VP-ODS column (4.6 mm × 150 mm, 5 μm), Kromasil C18 column (4.6 mm × 200 mm, 5 μm), and Waters Symmetry C18 column were investigated and compared [Figure 1]. The Waters Symmetry C18 column had good peak separation and sharp peaks. The effect of mobile phase composition (methanol-water and acetonitrile-water with different modifiers including acetic acid, formic acid, and phosphoric acid) on chromatographic separation was investigated [Figure 2]. Aqueous 0.095% phosphate acid in the mobile phase provided a better resolution and separation of the bioactivity components and resulted in high precision sensitivity and selectivity. Based on the maximum absorption and full-scan experiment of the marker components in the UV spectra of the two-dimensional chromatograms obtained by PDA detection, the detection wavelengths at 225, 256, 283, 300, 310, and 350 nm were selected to compare the peak number and peak resolution of all marker compounds. Finally, the wavelength was set at 300 nm [Figure 3].
Figure 1.
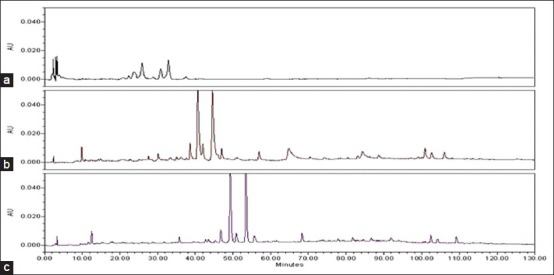
The HPLC chromatograms of Zhi Zhu Wan under different column conditions. (a) VP-ODS column (4.6 mm × 150 mm, 5 μm); (b) Kromasil C18 column (4.6 mm × 200 mm, 5 μm); (c) Waters Symmetry C18 column
Figure 2.
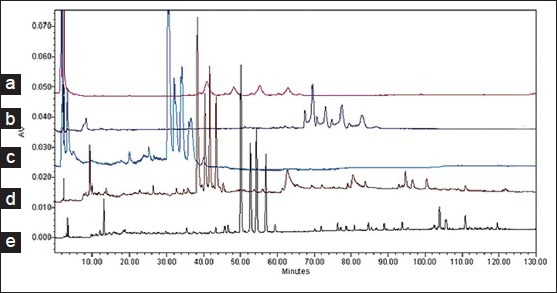
HPLC chromatograms of Zhi Zhu Wan in different elution conditions. (a) Acetonitrile-0.5% acetic acid aqueous solution; (b) Methanol-aqueous solution; (c) Methanol-1% aqueous acetic acid; (d) Acetonitrile-0.5% aqueous acetic acid; (e) Acetonitrile-0.095% aqueous phosphoric acid
Figure 3.
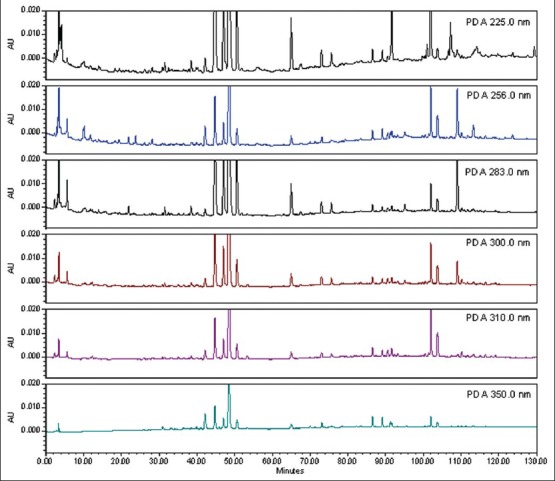
HPLC chromatograms of ZZW in different wavelength detection conditions
Optimization of extraction methods
Satisfactory extraction efficiency was obtained by comparing water-refluxing, ultrasonic, and Soxhlet extraction methods. Ultrasonic extraction was simpler and more effective than the other methods. Therefore, this method was used in further experiments. In this study, different concentrations of ethanol solutions, sample-solvent ratios, and extraction times were used for the ZZW extraction procedure. As a result, the best extraction condition was established as follows: The samples (1.0 g powder) were extracted by ultrasonic extraction using 50 mL of methanol as the extraction solvent, and the duration was 30 min.
Screening and determination of the main constituents in vitro
For quality control of the administered samples, HPLC fingerprints of ZZW from two different types were obtained [Figure 4]. The number of peaks was measured and compared to reference compounds. 18 and 16 bioactive constituents in RZZW and BZZW were simultaneously detected using the HPLC-UV method.
Figure 4.
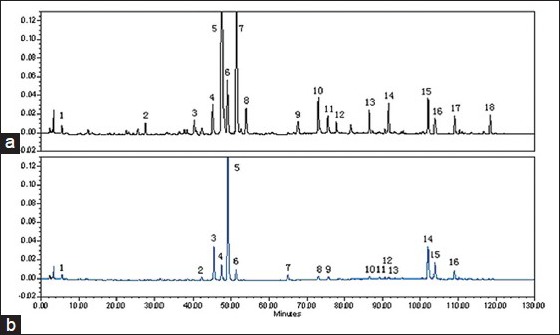
The determination of the main constituents in RZZW (a) and BZZW (b). A: 1, synephrine; 3, narirutin; 4, hesperidin; 5, naringin; 6, neohesperidin; 8, naringenin; 9, hesperetin; 10, nobiletin; 12, tangeritin; 15, atractylenolide I. B: 1, synephrine; 4.narirutin; 5, Naringin; 6, hesperidin; 7, neohesperidin; 10, hesperetin; 11, naringenin; 13, nobiletin; 14, tangeritin; 15, atractylenolide I
Method validation of quantitative analysis
The method was validated in terms of linearity, precision, reproducibility, stability, and recovery test.
Calibration curves
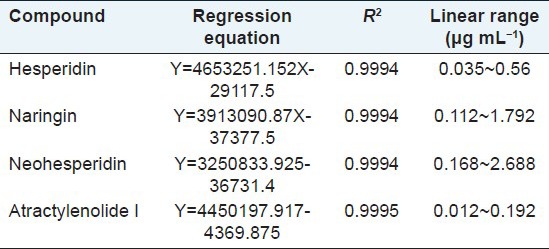
Methanol stock solutions containing four analytes were diluted to appropriate concentrations for calibration curve construction. The analyte solutions at six different concentrations were injected in triplicate, and the calibration curves were established by plotting the peak area (y) versus the concentration (x) of each component. The detailed information regarding the calibration curves and linear ranges of the four compounds are listed in Table 1.
Table 1.
Regression data for the four bioactive constituents
Precision, reproducibility, stability, and recovery
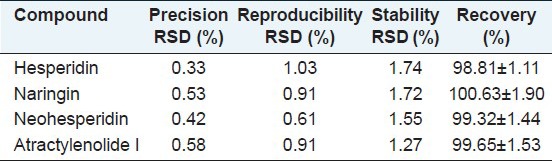
As shown in Table 2, the precision based on the peak area measurements of the four bioactive components were higher than 0.58% (RSD, n = 5). The reproducibility (RSD, n = 5) of the proposed method based on five replicate injections was in the range of 0.61%-0.93%. The stability (RSD, n = 5) of the measurements over 3 days for the four compounds was 1.27%-1.74%. The recovery test was performed by the standard addition method. Low, medium, and large high amounts of the standards were added to the known sample. The mean recovery was calculated according to the following formula: Recovery (%) = (amount found - original amount)/amount spiked × 100%, and RSD (%) = (SD/mean) × 100%. The mean recovery of the four bioactive compounds was 99.65%-100.63%, and the RSD value was 1.11%-1.90%.
Table 2.
Precision, reproducibility, stability, and recovery of the four bioactive constituents (n=6)
Sample analysis
HPLC chromatograms of ZZW samples and reference substance are shown in Figure 5. The newly established analytical method was subsequently applied to determine simultaneously the four bioactive components in samples of ZZW from manufacturers in China. All samples were analyzed using the optimized extraction method in optimized HPLC conditions. Each sample was analyzed in four to determine the mean content (mg·g-1), and the results are tabulated in Figure 6.
Figure 5.
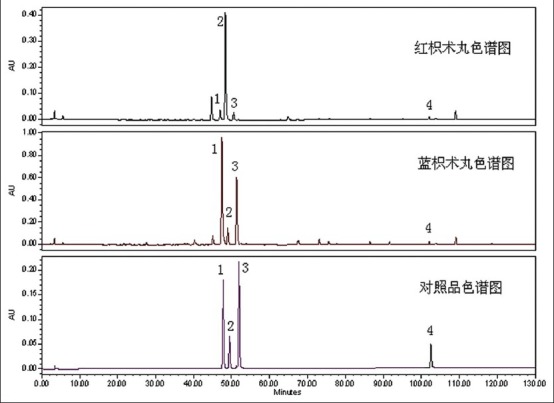
HPLC chromatograms of ZZW samples and reference substance. 1, naringin; 2, hesperidin; 3, neohesperidin; 4, atractylenolide I
Figure 6.
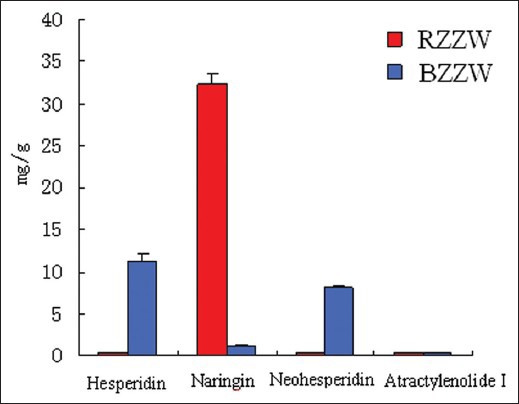
The mean content of naringin, hesperidin, neohesperidin, and atractylenolide I in ZZW samples
HPLC fingerprint of ZZW
Altogether, 10 batches of samples were analyzed, and all chromatograms were introduced into the Computer-Aided Similarity Evaluation System for Chromatographic Fingerprint of TCM (China Committee of Pharmacopeia). Peaks existing in all sample chromatograms were assigned as the “common peak,” and 18 common peaks were observed between 5 and 120 min in all 10 batches [Figure 7]. Peak 5 (Naringin), which was one of the most important active constituents of ZZW, was chosen as the internal reference peak to calculate the relative retention time (RRT) and relative peak area (RPA) of the other peaks. Figure 4 shows that the investigated naringin, hesperidin, neohesperidin, atractylenolide I, and other compounds in ZZW were separated and determined using the developed HPLC-PDA method. The differences of the bioactive components from BZZW and RZZW were recognized, and the components could be rapidly and efficiently differentiated by the chromatographic method.
Figure 7.
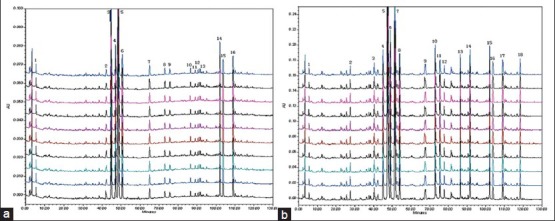
HPLC fingerprint of 10 batches of ZZW samples. (a) 1, synephrine; 3, narirutin; 4, hesperidin; 5, naringin; 6, neohesperidin; 8, naringenin; 9, hesperetin; 10, nobiletin; 12, tangeritin; 15, atractylenolide I. (b) 1, synephrine; 4.narirutin; 5, naringin; 6, hesperidin; 7, neohesperidin; 10, hesperetin; 11, naringenin; 13, nobiletin; 14, tangeritin; 15, atractylenolide I
Fingerprint SA
The SFDA suggested that all herbal chromatograms should be evaluated in terms of similarity by calculating the correlation coefficient and/or angle cosine value of the original data.[16,17,18] Therefore, SA was conducted based on the mean peak area of standard fingerprints using correlation coefficient method and the cosine of the angle method to calculate the similarity of fingerprints. The same samples had similar constituents with the slight difference resulting from the BZZW and RZZW. The similarity of two methods was more than 0.99.
CONCLUSION
Quality control is one of the bottleneck problem limiting the applications and development of TCM. In recent years, the research on TCMs has already made a great progress. A simple, sensitive, and reliable HPLC method has been developed for simultaneous determination of major constituents in ZZW, a multiherbal formula. Ten batches of sample were used to establish the fingerprint and quantitative analyses. The combination of quantitative and chromatographic fingerprint analysis can be used for the quality assessment of ZZW. The method was validated for repeatability, precision, stability, accuracy, and selectivity. For the first time, the feasibility and advantages of employing chromatographic fingerprint were investigated and demonstrated for the evaluation of ZZW with the professional analytical software recommended by SFDA. By comparing the retention times, and UV data with reference standards, four characteristic peaks in the chromatograms were successfully confirmed as corresponding to naringin, hesperidin, neohesperidin, and atractylenolide I. The established method was successfully applied to quantify the marker compounds in ZZW, which provided a useful basis of overall evaluation of the quality of ZZW. This readily available, low-cost, and reliable HPLC-PAD method improved the quality control of TCM preparations consisting of complex compounds with different structures. The inherent advantages of HPLC, such as its simplicity, make itself feasible for the quality control of TCM preparations.
ACKNOWLEDGMENTS
This work was supported by grants from the Key Program of Natural Science Foundation of State (grant No. 90709019, 81173500, 81302905, 81102556, 81202639), Key Science and Technology Program of Heilongjiang Province, China (grant No. GC06C501, GA08C303, GA06C30101), National Key Technology Research and Development Program of the Ministry of Science and Technology of China (grant No. 2011BAI03B03, 2011BAI03B06, 2011BAI03B08), Foundation of Heilongjiang University of Chinese Medicine (grant No. 201209), and National Key Subject of Drug Innovation (grant No. 2009ZX09502-005).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Zhang A, Sun H, Yuan Y, Sun W, Jiao G, Wang X. An in vivo analysis of the therapeutic and synergistic properties of Chinese medicinal formula Yin-Chen-Hao-Tang based on its active constituents. Fitoterapia. 2011;82:1160–8. doi: 10.1016/j.fitote.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Yang Z, Luo H, Wang H, Hou H. Preparative isolation of bufalin and cinobufagin from Chinese traditional medicine ChanSu. J Chromatogr Sci. 2008;46:81–5. doi: 10.1093/chromsci/46.1.81. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, David B, Tu P, Barbin Y. Recent analytical approaches in quality control of traditional Chinese medicines--a review. Anal Chim Acta. 2010;657:9–18. doi: 10.1016/j.aca.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Zhang A, Sun H. Future perspectives of Chinese medical formulae: Chinmedomics as an effector. OMICS. 2012;16:414–21. doi: 10.1089/omi.2011.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin Q, Wang P, Zhang A, Sun H, Wu X, Wang X. Ultra-performance LC-ESI/quadrupole-TOF MS for rapid analysis of chemical constituents of Shaoyao-Gancao decoction. J Sep Sci. 2013;36:1238–46. doi: 10.1002/jssc.201201198. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Jing Z, Tang X, Wang X, Zhang S, Yu Y, et al. To compare the efficacy of two kinds of Zhizhu pills in the treatment of functional dyspepsia of spleen-deficiency and qi-stagnation syndrome: A randomized group sequential comparative trial. BMC Gastroenterol. 2011;11:81. doi: 10.1186/1471-230X-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun H, Dong T, Zhang A, Yang J, Yan G, Sakurai T, et al. Pharmacokinetics of Hesperetin and Naringenin in the Zhi Zhu Wan, a Traditional Chinese Medicinal Formulae, and its Pharmacodynamics Study. Phytother Res. 2013;27:1345–51. doi: 10.1002/ptr.4867. [DOI] [PubMed] [Google Scholar]
- 8.Ghen JY, Qiu JR, Pan F. Clinical observation on treatment of gastro-esophageal reflux with modified zhizhu pill. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24:25–7. [PubMed] [Google Scholar]
- 9.Jiang H, Shi J, Li Y. Screening for compounds with aromatase inhibiting activities from Atractylodes macrocephala Koidz. Molecules. 2011;16:3146–51. doi: 10.3390/molecules16043146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao H, Chen X, Sun H, Sakurai T, Zhou J, Sun W, et al. Pharmacokinetics-based elucidation on disparity in clinical effectiveness between varieties of Zhi Zhu Wan, a Traditional Chinese Medical formula. J Ethnopharmacol. 2010;128:606–10. doi: 10.1016/j.jep.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Yan G, Zhang A, Li Y, Wang Y, Sun H, et al. Rapid discovery and global characterization of chemical constituents and rats metabolites of Phellodendri amurensis cortex by ultra-performance liquid chromatography-electrospray ionization/quadrupole-time-of-flight mass spectrometry coupled with pattern recognition approach. Analyst. 2013;138:3303–12. doi: 10.1039/c3an36902a. [DOI] [PubMed] [Google Scholar]
- 12.Zhang A, Sun H, Wang X. Potentiating therapeutic effects by enhancing synergism based on active constituents from traditional medicine. Phytother Res. 2014;28:526–33. doi: 10.1002/ptr.5032. [DOI] [PubMed] [Google Scholar]
- 13.Liang Y, Xie P, Chau F. Chromatographic fingerprinting and related chemometric techniques for quality control of traditional Chinese medicines. J Sep Sci. 2010;33:410–21. doi: 10.1002/jssc.200900653. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Liu Y, Yin L, Wang X, Peng J. Simple and reliable methods for the determination of sixteen marker components for quality control of Daochi pill by HPLC coupled with diode array detection. Phytochem Anal. 2009;20:385–94. doi: 10.1002/pca.1138. [DOI] [PubMed] [Google Scholar]
- 15.Yan GL, Zhang AH, Sun H, Han Y, Shi H, Zhou Y, et al. An effective method for determining the ingredients of Shuanghuanglian formula in blood samples using high-resolution LC-MS coupled with background subtraction and a multiple data processing approach. J Sep Sci. 2013;36:3191–9. doi: 10.1002/jssc.201300529. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Lu T, Mao C, Wu H, Zhang X, Wang J, et al. Simultaneous determination of 10 components in traditional Chinese medicine Dachaihu Granule by reversed-phase-high-performance liquid chromatographic-diode array detector. Pharmacogn Mag. 2013;9:33–8. doi: 10.4103/0973-1296.108136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong W, Wang P, Meng X, Sun H, Zhang A, Wang W, et al. Ultra-performance liquid chromatography-high-definition mass spectrometry analysis of constituents in the root of radix stemonae and those absorbed in blood after oral administration of the extract of the crude drug. Phytochem Anal. 2012;23:657–67. doi: 10.1002/pca.2370. [DOI] [PubMed] [Google Scholar]
- 18.Yang A, Wang X, Xiong C, Chen J, Ruan J. Quality control of tuirejieduling granules using high-performance liquid chromatography fingerprint method and simultaneous determination of four main active ingredients. Pharmacogn Mag. 2013;9:162–6. doi: 10.4103/0973-1296.111284. [DOI] [PMC free article] [PubMed] [Google Scholar]


