Abstract
Aim:
Dracocephalum moldavica L, a traditional Uygur medicine, possesses some key cardiac activities. However, till date, no reports are available on the use of D. moldavica against chronic mountain sickness (CMS), which is a medical condition that affects the residents of high altitude. The present study was designed to explore the treatment efficacy of D. moldavica on CMS.
Materials and Methods:
80 of the 100 Sprague Dawley rats enrolled were bred in simulated high altitude environment and the remaining 20 rats were kept in the plains. Water and alcohol extracts of D. moldavica were prepared. CMS rat model was prepared, and the rat hearts were removed for histopathological analysis. Blood samples were taken for hematological and biochemical analyses. Rat pulmonary artery pressure was determined to study the treatment efficacy.
Results:
In the CMS model group, the levels of interleukin-6 (IL-6), C-reactive protein (CRP), and malondialdehyde (MDA) were found to be significantly higher than the control group; while the concentrations of SOD and GSH-Px decreased. D. moldavica could improve these levels, decrease pulmonary artery pressure, and improve the cardiac pathological state.
Conclusions:
The study results show that IL-6, CRP, MDA, SOD and GSH-Px participate and mediate the formation of CMS and D. moldavica is found to possess noticeable effects on CMS. The present study explored the basics of high altitude sickness and laid the foundation for further progress of Uygur medicines on the treatment of altitude sickness. Further preclinical and clinical studies with more sample size are recommended.
Keywords: Chronic mountain sickness, Dracocephalum moldevica L, myocardial ischemia
INTRODUCTION
Dracocephalum moldavica L is a Labiatae annual herb, which is used as a traditional Uygur medicine for centuries in the name of Baeiranjiboya. D. moldavica possesses important medicinal values against the following diseases: Bronchitis, hypertension, hepatitis, dizziness, biliary tract infections, and other diseases. The water extract of D. moldavica has found to possess an anti-ischemic effect in animal experiments, which significantly prolongs the survival time under hypoxia, acts against isoproterenol-induced increase in myocardial oxygen consumption, improves the hypoxia tolerance, acts against vasopressin-induced myocardial ischemia with acute ST-T changes, reduces heart rate, and prolongs PR interval.[1,2,3,4,5]
D. moldavica is mainly produced in several provinces of Northeast China, Northwest China, and North China; and Southeastern Xinjiang has abundant resources. D. moldavica has a long history as many classical books on Uygur medicines have reported its use. The herb is naturally warm and has aroma, which can exert its effects on brain nerves, cough, asthma, cardiac tissues, diuresis, blood circulation, and resuscitation etc., A portion of minority population in Xinjiang region uses this herb along side tea, and the civil society uses this as a Uygur medicine to treat coronary heart disease, cold nervous headache, colds, and bronchitis etc., It is also used to improve the quality of blood.
Chronic mountain sickness (CMS) is a medical condition in which the residents of high altitude are found to have increased hematocrit and excessive polycythemia. It is also called as “Monge's disease,” after its first description by a Peruvian physician, Carlos Monge in 1925. Reduction of pathophysiological conditions such as hypoxemia and alveolar hypoventilation could act as the ultimate treatment goal for CMS.[6] Pulmonary hypertension is frequently associated, which might lead to congestive heart failure. Hypoxemia due to alveolar hypoventilation may play a central role in the over-stimulation of erythropoiesis leading to increased red cell mass and blood viscosity, systemic and pulmonary hypertension.[7] Till date, no reports are available on the use of D. moldavica against CMS, although the herb has shown efficient cardiac activities.[8,9] Thus, the present study was aimed to explore the effect of D. moldavica on rat CMS model.
MATERIALS AND METHODS
Chemicals and reagents
The rat Interleukin-6 (IL-6) ELISA kit (batch number: 74350026) was purchased from eBioscience (San Diego, USA), and the rat C-reactive protein (CRP, batch number: 3400630) was purchased from Xi Tang Biotechnology Co. Ltd. (Shanghai, China). The key instruments used in the study included the following: Microplate reader, (model: 352; Labsystems Multiskan MS, Finland), washer (model: AC8; Thermo Labsystems, Finland), micro high-speed centrifuge (model: TG16W; Shanghai, China), and water-jacket incubator (model: GNP-9080; Shanghai, China). Individual kits for superoxide dismutase (SOD, total test, batch number: 20130410), malondialdehyde (MDA, batch number: 20130409), and glutathione - catalase (GSH-Px, batch number: 20130401) were purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
Extracts of D. moldavica
For the purpose of this study, the following two extracts of D. moldavica were used: Water and alcohol. For the preparation of extract for D. moldavica alcohol group (DMLAG), 1000 g of D. moldavica 1000 g was added with 3000 ml of methanol, refluxed for 3 h, and filtered. The filtered extract was set aside in the refrigerator. For the preparation of extract for D. moldavica water group (DMLWG), 1000 g of D. moldavica 1000 g was added with 3000 ml of water, refluxed for 3 h, and filtered. The filtered extract was set aside in the refrigerator. 2 g/kg of DMLWG in 0.4 ml of administration, 2 g/kg of DMLAG in 0.4 ml of administration.
Animals and treatment groups
One hundred healthy Sprague Dawley rats (both sex, body weight: 160-200 g) were provided by the Laboratory Animal Center of Xinjiang Medical University, Urumqi, China (license number: SCXK (new) 2011-0004).
Of them, 80 rats were bred in hypobaric chamber which was in the northwest region of medical support peacetime and wartime special environment artificial (located in Urumqi General Hospital of Lanzhou Military Region). The conditions used for these rats included the following: Simulated altitude of 5000 meters, temperature of 18-26°C, humidity of 40-60%, pressure of 54.1 KPa (391.4 mmHg), and oxygen partial pressure of 10.84KPa (80.8 mmHg). The plateau environment after 30 days, these 80 rats wass further randomly divided into four groups (20 in each):
Chronic plateau model group (MG) which had no drug intervention with continued feeding for 15 days in a high altitude environment
Positive control (NE) group which received nifedipine 2.7 mg/kg with continued feeding for 15 days in a high altitude environment
DMLWG which received 0.4ml water extract of D. moldavica by a once daily gavage with continued feeding for 15 days in a high altitude environment
DMLAG which received 0.4ml alcohol extract of D. moldavica by a once daily gavage with continued feeding for 15 days in a high altitude environment.
The remaining 20 rats were used as control and were kept in the plains (CG). They were fed with standard diet and distilled water and kept in clean environment with a temperature of 18-26°C and altitude of 700 meters for 45 days. No drug intervention was given for the rats used in the plains. All rats were fed in consistent conditions, and the following were tested on a daily basis for 45 days: Behavior of rats, spontaneous activity, feeding, drinking, hair, feces, urine, eye, ear, nose, mouth (with or without secretions), and body weight.
Pathological examinations
Ketamine (100 mg/2 ml) + diazapam 1 (10 mg/2 ml) +1 atropine (0.5 mg/ml), evenly mixed then diluted and doubled to 10 ml, then the diluted liquids were intraperitoneally injected by 0.75 ml/100g. After anesthesia and decapitation, the heart was rapidly dissected out and washed immediately with saline and fixed in 10% buffered formalin. The rat heart paraffin block was prepared at 4μm with hematoxyline and eosin. The stained sections were examined under Olympus (Magnus MVX 10) China Ltd. Photomicroscope and photographed. To examine the pathological changes of different treatment groups.
Determination of inflammatory mediators
In accordance to the orbital venous sinus blood sample collection procedures,[10,11] the eyeball blood samples from rats were collected at room temperature, centrifuged at 3000 rpm for 20 min, and the supernatant was separated out. Per the manufacturers’ instructions of various kits, the inflammatory mediators such as CRP, IL-6, MDA, SOD, and GSH-Px were determined.
Detection of pulmonary artery pressure
Equipment and reagents
Northwest special environment artificial experiment module (Urumqi General Hospital of Lanzhou Military Region); BL-420 Biological and functional experimental system and pressure sensors: (Chengdu Taimeng Equipment company); Small animal ventilator (Chengdu Taimeng Equipment company); Rat Operating Table (Chengdu Taimeng Equipment company); operating astral lamp; Medical stainless steel needle (7, Inch 22G); several 15cm of Sterile polyethylene tube; rats trachea cannula; rats artery clamp; injectors (1mm); tissue scissors; ophthalmic scissors; ophthalmic forceps; hemostatic clamp; needle holder; operating knife blade; three limb tube.
Reagents
Nonpyrogenic distilled water; physiological saline; Heparin (5 IU/ml).
Instrument description
Northwest special environment artificial artificial experiment module: The research project was chaired by the Urumqi General Hospital of Lanzhou Military Region. The features of the cabin is in accordance with the design standards that in cabin accurately simulate the altitude of 600 to 10,000 m hypobaric hypoxia status, Ambient temperature +500 C to -500C. The cabin can achieve a single climate simulation and can also achieve complex climate simulation, for example: Achieve altitude environment of low-pressure, cold, strong ultraviolet radiation and high temperature and dry desert environment at the same time. The device is composed of two JEM and a transitional cabin. Including five systems: Cabin structural systems, environmental control system, gas pipeline control systems, automated control systems, TV monitoring system as well as contingency plans for five systems, the total volume of cabin is 117m3 and it can accommodate 20 people at a time. The device can new experimental platform for the study of human acclimatization mechanism and related diseases pathogenesis under the special meteorological environment of plateau, cold, hypoxia, desert and strong ultraviolet; promoting acclimatization and disease control measures as well as the human physiology and disease treatment in the Northwest special environment area.
BL-420 Biological and functional experimental system and pressure sensors
This is an instrument of recording animal blood pressure. Transfer the Animals pressure change signal into electrical signal through pressure signal conversion device (pressure sensor), input it into the computer and amplify, display and record it.
Calibration of the pressure sensor
Calibration of the sensor is an important step in the experiment. It includes nulling the amplifier and calibrating the pressure sensor. First, the zero calibration, if less than zero, you need fine tuning of the sensor; Second, the standard pressure (100 mmHg) calibration. After these two steps you can start the experiment.
ECG measurements
Also use BL-420 Biological and functional experimental system. Use animals standard o connecting standard ECG to lead. The White electrode connects to right forelimb, the red electrode White electrode connected right forelimb, red electrode connects to left hind, and the black electrode connects to right hind. The working principle and pressure measurement are similar.
Small animal ventilator
Its purpose is to prevent the animals’ loss of spontaneous breathing cycle after thoracotomy. Use the Y-tube to connect the animal to the ventilator, breathing frequency is 60 times/min, tidal volume is 6 ml/kg, breathing ratio is 3:2.
Assay method
Endotracheal intubation: Rats were fixed in supine position along the neck midline. The incision and blunt dissection of the subcutaneous skin was performed, and the trachea was exposed along with the parts of the sub-line inverted T incision. The endotracheal intubation was fixed, and the endotracheal tube was directly connected to the ventilator. The ventilator frequency was adjusted to 60 times/min, tidal volume was 6 ml/kg, and breathing ratio was 3:2.
Carotid artery intubation: Rats were fixed in supine position along the neck midline. The incision and blunt dissection of the subcutaneous skin was performed, and the carotid artery exposed, Fixed intubation. Then connect the pressure transducer to record the experimental data by input the pressure changes to biological signal logger.
Pulmonary artery pressure and right ventricular pressure measurement: Ventricular pressure and pulmonary artery pressure were measured using heparinized saline needle at the upper right corner of the right ventricle, and the position of the needle was seen by naked eye. Further end of the needle and catheter made using a pressure transducer was connected to the pressure variation of the biological signal recorder to record the experimental data.[10,11]
Statistical method
Values were expressed as mean ± standard deviation. One-way ANOVA was used for group comparison of means. LSD test was used for post-hoc comparisons of means. All statistical analyses were performed using SPSS for Windows (version 17.0; SPSS Inc. USA), and P < 0.05 were regarded as statistically significant.
RESULTS
Pathological examinations
In the Pathological examination of figure 6 the CG group [Figure 1], both high- and low-power views showed normal tissue structure of heart without any pathological findings. The myocardial cells were stained clearly with dense nucleus and the myocardial fibers were neatly arranged in the same size. The MG group [Figure 2] showed mild hyperemia of subepicardial blood vessels, congested myocardial interstitial blood vessels, and unclear transverse bands under low-power lens; while the high-power view showed partial myocardial swelling, granular degeneration of cytoplasm, eosinophilic cells degeneration of partial myocardial fibers, and occasional infiltration of inflammatory cells. The low-power view showed significant dilatation and congestion of myocardial interstitial blood vessels and unclear transverse bands in the NE group [Figure 3], while the high power view showed mild cellular edema of myocardium and eosinophilic degeneration of small amount of myocardial cells. In the DMLAG group [Figure 4], the low-power view showed nonsubepimyocardial hyperemia with significant congestion of interstitial blood vessels of myocardium, while the high-power view showed an evidence of cellular edema of myocardium and scattered eosinophilic degeneration. In the DMLWG group [Figure 5], neither subepicardial hyperemia nor obvious dilatation or congestion of myocardial interstitial blood vessels was seen under the low-power lens, while the high-power view showed small amount of cellular edema of myocardium and occasional eosinophilic degeneration. DMLWG group compared to the model group had significantly improved cardiac pathology. DMLWG group of pathological conditions close to had normal DMLAG group compared to the model group and has some improvement in cardiac pathology.
Figure 6.
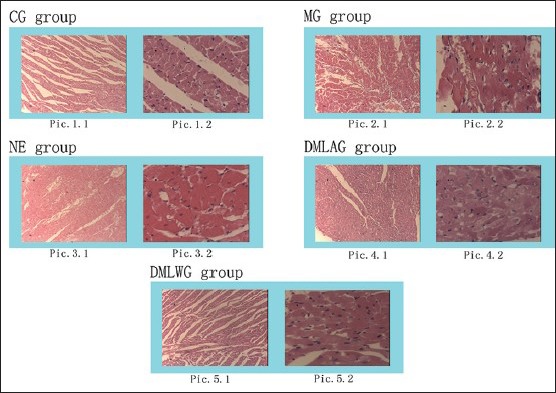
Pathological examinations
Figure 1.
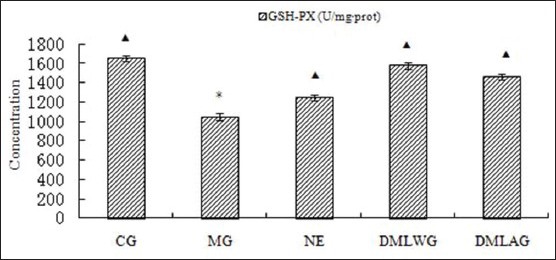
Treatment effects of D. moldavica on GSH-Px
Figure 2.
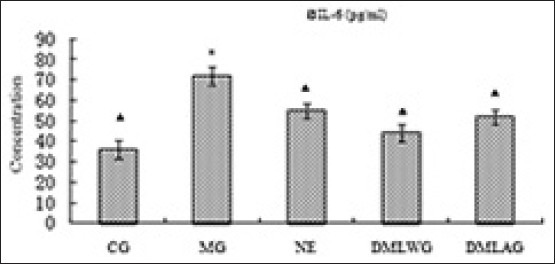
Treatment effects of D. moldavica on IL-6
Figure 3.
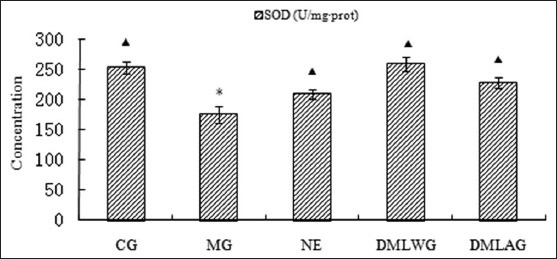
Treatment effects of D. moldavica on SOD
Figure 4.
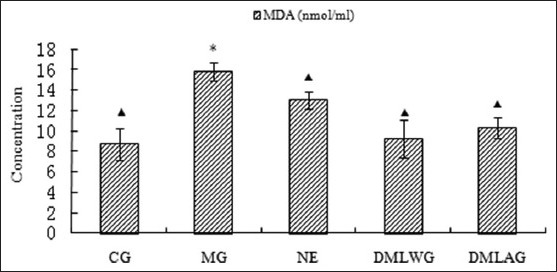
Treatment effects of D. moldavica on MDA
Figure 5.
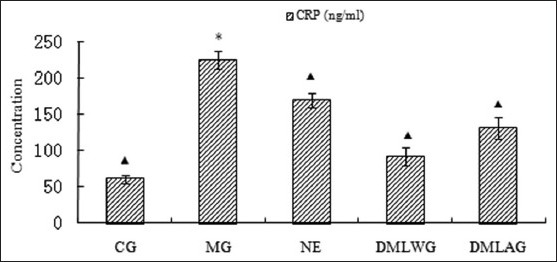
Treatment effects of D. moldavica on CRP
Treatment effects of D. moldavica on IL-6, CRP, MDA, SOD, and GSH-Px
The treatment effects of D. moldavica on IL-6, CRP, MDA, SOD, and GSH-Px are shown in [Figures 1-5]. The concentrations of SOD and GSH-Px were lower in the MG group than in the CG group. The concentrations of IL-6, CRP, and MDA were higher in the MG group than in the CG group. Animals in the DMLWG had significantly higher concentrations of SOD and GSH-Px (P < 0.05) than the MG groups. Animals in the DMLWG had significantly lower concentrations of IL-6, CRP, and MDA (P < 0.05) than the MG groups.
Treatment effects of D. moldavica on pulmonary artery pressure
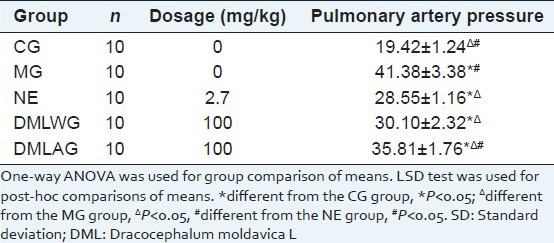
The treatment effects of D. moldavica on pulmonary artery pressure are shown in [Table 1]. Compared with the CG group, MG group had significantly higher pulmonary artery pressure (P < 0.05). Compared with the MG group, NE group, DMLWG, and DMLAG had significantly lower pulmonary artery pressure (P < 0.05). The curative effect was better in DMLWG than that of DMLAG. Also, the reduction of pulmonary artery pressure was better in DMLWG.
Table 1.
Effects of DML treatment on pulmonary artery pressure (x±SD)
DISCUSSION
Dracocephalum moldavica L is widely used in traditional Uyghur medicine for the treatment of complex diseases such as CMS, anti-oxidation and anti-aging. Previous studies have indeed found an anti-oxidation effect of DML in vitro and vivo.[1,3,5] DML also appears to have reduce pulmonary artery pressure and anti-oxidation effects, which may also play a role in its prevention of altitude sickness activity.
We studied several altitude sickness serum markers, including the following relationship. IL-6 is an inflammatory cytokine produced mainly by monocytes/macrophages with wide-induced inflammation and regulation of immune function. The complex network of cytokines promotes the occurrence of atherosclerosis and development.[12] IL-6 is a crucial regulator of vascular injury and myocardial ischemia.[13] It can induce acute phase proteins such as CRPs, promote thrombus formation, and enhance adhesion of self-cells and myocardial cells.[14,15] Increased myocardial cell damage may further induce the vascular endothelium and increase the generation of reactive oxygen species (ROS) clusters. Oxygen free radicals stimulate oxidative stress and endothelial dysfunction cause the increase in the generation of altitude sickness. Increase in IL-6 levels can be done by arachidonic acid metabolism. Increased production of oxygen free radicals could cause cell damage and free radical metabolic imbalance. In the present study, the antioxidant capacity decreased as SOD and GSH-Px activity decreased.[16,17,18,19,20] MDA concentration increased due to the occurrence of oxidative stress.[21,22] Due to the damage to blood vessels and endothelial function, superoxide anion increased. In summary, IL-6, SOD, GSH-Px, MDA, and CRP participate and mediate the formation of CMS. The incidence of cardiovascular events plays an important role.[23,24,25,26,27] The study results indicated that increase in IL-6, MDA, and CRP levels and decrease in SOD and GSH-Px levels were observed in rat serum in a high altitude environment. This shows that the high altitude hypoxia has the effect on rat's cytokines and inflammatory chemokines. DMLWG showed reduced levels of serum IL-6, MDA, and CRP levels, and elevated serum SOD and GSH-Px levels. Thus, the aqueous extract of D. moldavica improved systemic hypoxia in rats kept in the high altitude environment.
In simulated high-altitude environment, the rats in model group had significantly higher pulmonary artery pressure compared with the rats in the plains, confirming the fact that CMS involved simulated altitude sickness. After the administration of D. moldavica, the pulmonary artery pressure significantly reduced close to NE group. This shows that treatment efficacy of this Uygur medicine on altitude sickness. Nifedipine is the first generation calcium antagonists indicated for hypertension and angina. It is widely prescribed for CMS. Its common adverse effects are dizziness, headache, fever, flu, facing red, foot edema, and fluid retention etc., But it is precisely DML without these side effects.
Also, the herb extract showed a protective effect on the heart rats in DMLWG. The present study attempted to explore the basis for the research on clinical development of drugs for high altitude sickness and laid the foundation for further progress of Uygur medicines on the treatment of altitude sickness. Further preclinical and clinical studies with more sample size are recommended.
ACKNOWLEDGMENTS
This research work was supported by the national basic research program of china (2012CB517802). This research was supported by the special culture of Xinjiang minority talents of science and technology plan project(201423125).
Footnotes
Source of Support: This research work was supported by the Xinjiang Uygur Autonomous Region cardiovascular research laboratory project (XJDX0903-2013-01)
Conflict of Interest: None declared.
REFERENCES
- 1.Dastmalchi K, Damien HJ, Koşar M, Hiltunen R. Chemical composition and in vitro antioxidant evaluation of a water-soluble Moldavian balm (Dracocephalum moldavica L.) extract. LWT-Food Sci Technol. 2007;40:239–48. [Google Scholar]
- 2.Holm Y, Hiltunen R, Nykanen I. Capillary gas chromatographic-mass spectrometric determination of the flavour composition of dragonhead (Dracocephalum moldavica L.) extract. Flavour Fragr J. 1988;3:109–12. [Google Scholar]
- 3.Dastmalchi K, Damien HJ, Laakso I, Hiltunen R. Chemical composition and antioxidative activity of Moldavian balm (Dracocephalum moldavica L.) extracts. LWT-Food Sci Technol. 2007;40:1655–63. [Google Scholar]
- 4.Povilaityte V, Cuvelier ME, Berset C. Antioxidant properties of Moldavian dragonhead (Dracocephalum moldavica L.) J Food Lipids. 2001;8:45–64. [Google Scholar]
- 5.Guo CM, Wu RL, Feng S, Wang JD. Extraction and determination of Dracocephalum Moldavica polysaccharides and its effects on reducing the free radical oxygen. Food Fermen Ind. 2005;3:129–32. [Google Scholar]
- 6.Penaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes healthy highlanders and chronic mountain sickness. Circulation. 2007;115:1132–46. doi: 10.1161/CIRCULATIONAHA.106.624544. [DOI] [PubMed] [Google Scholar]
- 7.Richalet JP, Rivera-Ch M, Maignan M, Pham I, Privat C, Velarde FL. Pulmonary hypertension and monge's disease. PVRI Rev. 2009;12:114–9. [Google Scholar]
- 8.Monge CC, Arregui A, Leon-Velarde F. Pathophysiology and epidemiology of chronic mountain sickness. Int J Sports Med. 1992;13(Suppl 1):79–81. doi: 10.1055/s-2007-1024603. [DOI] [PubMed] [Google Scholar]
- 9.Ou LC, Salceda S, Schuster SJ, Dunnack LM, Brink-Johnsen T, Chen J, et al. Polycythemic responses to hypoxia: Molecular and genetic mechanisms of chronic mountain sickness. J Appl Physiol. 1998;84:1242–51. doi: 10.1152/jappl.1998.84.4.1242. [DOI] [PubMed] [Google Scholar]
- 10.Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1:87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parasuraman S, Raveendran R. Measurement of invasive blood pressure in rats. J Pharmacol Pharmacother. 2012;3:172–7. doi: 10.4103/0976-500X.95521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–7. [PubMed] [Google Scholar]
- 13.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: Induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–8. [PubMed] [Google Scholar]
- 14.Zee RY, Ridker PM. Polymorphism in the human C-reactive protein (CRP) gene, plasma concentrations of CRP, and the risk of future arterial thrombosis. Atherosclerosis. 2002;162:217–9. doi: 10.1016/s0021-9150(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 15.Yudkin JS, Stehouwer CD, Emeis JJ, Coppock SW. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction a potential role for cytokines originating from adipose tissue. Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 16.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 17.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–30. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 18.Lebovitz RM, Zhang H, Vogel H, Cartwright JR, Dionne L, Lu N, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93:9782–7. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974;104:580–7. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 20.Look MP, Rockstroh JK, Rao GS, Kreuzer KA, Barton S, Lemosh H, et al. Serum selenium, plasma glutathione (GSH) and erythrocyte glutathione peroxidase (GSH-Px)-levels in asymptomatic versus symptomatic human immunodeficiency virus-1 (HIV-1)-infection. Eur J Clin Nutr. 1997;51:266–72. doi: 10.1038/sj.ejcn.1600401. [DOI] [PubMed] [Google Scholar]
- 21.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1989;186:421–31. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 22.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 23.Engler RL, Dahlgren MD, Morris DD, Peterson MA, Schmid-Schonbein GW. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986;251:314–23. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- 24.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: An overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez RM. Effect of the hexane extract of Piper auritum on insulin release from β-cell and oxidative stress in streptozotocin-induced diabetic rat. Pharmacogn Mag. 2012;8:308–13. doi: 10.4103/0973-1296.103661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koti BC, Nagathan S, Vishwanathswamy A, Gadad PC, Thippeswamy A. Cardioprotective effect of vedic guard against doxorubicin-induced cardiotoxicity in rats: A biochemical, electrocardiographic, and histopathological study. Pharmacogn Mag. 2013;9:176–81. doi: 10.4103/0973-1296.111287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aikemu A, Yusup A, Umar A, Berké B, Moore N, Upur H. The impact of the Uighur medicine abnormal savda munziq on antitumor and antioxidant activity in a S180 and Ehrlich ascites carcinoma mouse tumor model. Pharmacogn Mag. 2012;8:141–8. doi: 10.4103/0973-1296.96568. [DOI] [PMC free article] [PubMed] [Google Scholar]


