Abstract
Background:
It is known that when crude Pinelliae rhizome and Pinelliae rhizoma preparatum are combined with Aconiti Radix Cocta respectively, the toxicity of the combination varies. However, the component's transformation between different compatibility have remained unclear.
Objective:
In this paper, a novel approach using rapid resolution liquid chromatography-quadrupole time-of-flight mass spectrometry (RRLC-Q-TOF-MS) coupled with multivariate statistical analysis was established for exploring the influence of processing adjuvants (PAs) on the compatibility of Aconiti Radix Cocta and Pinelliae rhizome.
Materials and Methods:
In order to obtain information about the representative markers between different groups, an exhaustive study of different protocols based on adding or removing different PAs step by step was carried out and the influence of PAs on compatibility was investigated.
Results:
It was found that lime can facilitate diester diterpenoid alkaloids with high toxicity in Aconiti Radix Cocta to be converted into low-toxic or non-toxic derivatives. Glycyrrhizae Radix et Rhizoma had no remarkable effect on the process.
Conclusion:
The established method in this study will be of great significance to process research mechanism and study on traditional Chinese Medicine compatibility and clinical application.
Keywords: Aconiti Radix Cocta, Pinelliae rhizome, processing adjuvants, RRLC-Q-TOF-MS
INTRODUCTION
Paozhi is a common practice that usually occurs before most traditional Chinese herbs were prescribed.[1,2] The typical processing methods include steaming, boiling, adding adjuvants and so on. During Processing, PAs are additional aiding materials with specific property, flavor and function. Different adjuvants have different properties and functions. They can co-ordinate medicines, strengthen and change drug efficacy, reduce or eliminate drug toxicity and side-effect, influence the physico-chemical properties of basic remedy and change the medicinal position or drug effect.[3,4] Therefore, characterizing the variation patterns in composition during processing, as well as the compatibility function, is of great importance in clinical application.
Pinelliae rhizome (Banxia, [BX]) is the dry root tuber of the Araceae plant of Pinellia ternata (Thunb.) Breit [Figure 1a]. Pinelliae rhizoma preparatum [Figure 1b], known as Fabanxia (FBX), is a processed product obtained by steeping crude Pinelliae rhizome (Shengbanxia, [SBX]) with Glycyrrhizae Radix et Rhizoma (GR) and lime and then drying them together. Chuanwu (CW) is dry mother root of Aconitum carmichaelii Debx. CW is highly toxic and its processed product, Aconiti Radix Cocta [Zhichuanwu, ZCW, Figure 1c], is commonly used in clinical application. The main ingredients of ZCW are five major groups of alkaloids, namely, diester diterpene alkaloids (DDAs), monoester diterpene alkaloids (MDAs), amine diterpenoid alkaloids (ADAs), non-ester alkaloids (NEAs) and lipo-alkaloids (LOAs).[5] DDAs and MDAs were considered as the major toxic aconite alkaloid in ZCW.[6,7,8] BX and ZCW are commonly used as Chinese medicine in clinical application. However, several classic works on traditional Chinese medical science recorded that BX and ZCW should not be used together or should be used cautiously, whereas some scholars also believe that the two are compatible. There are more than 20 commonly used proprietary herbal products for stroke, cancer, bone disease and rheumatism from both historical literature and modern clinical reports, containing BX and ZCW as the main ingredients, such as “Chi wan fang,” “Wutoubanxiasan,” “Zhenfangbaiwanzi,” etc.[9,10,11] However, the components transformation in the compatibility process of BX and ZCW is still unclear.
Figure 1.

(a) SBX; (b) FBX; (c) ZCW; (d) BX1; (e) BX2
Some reports showed that compared with the extracting solution of ZCW, the extracting solution from boiling SBX and ZCW contained slightly more, less or the same amount of highly toxic DDAs, whereas the extracting solution from FBX and ZCW contained relatively less highly toxic DDAs.[12,13] The major difference between FBX and SBX is that PAs (GR and Lime) have been used in FBX. Thus, whether BX and ZCW can be used together may depend on the raw BX, processing method and adjuvant used.
In this paper, we used RRLC-Q-TOF-MS analysis platform to investigate the role of PAs in reducing the toxicity of the compatibility process of BX and ZCW, tried to discover the influence of GR and lime on the chemical transformation in content and/or in amount in the processing procedure and compatibility and revealed features of concerted application of BX and ZCW.
MATERIALS AND METHODS
Chemicals, materials and instruments
Agilent 1200 RRLC-Q-TOF-MS (RRLC-Q-TOF-MS, US Agilent Company), qualitative analysis B.04.00 data analysis software (US Agilent Company), Sartorius BS224S electronic balance (Beijing Sartorius Instrument and System Engineering Co., Ltd.) and TGL-16C centrifuge (Shanghai Anting Scientific Instrument Factory) were used in this study. Acetonitrile (ACN, HPLC grade) was purchased from Merck (Darmstadt, Germany). Other chemical reagents were all of analytical grade.
ZCW was obtained from Sichuan Jiangyou Zhongba Aconiti Science and Technology Development Co. Ltd., the SBX and GR were obtained from the Anguo Medical Herbs Market. They were authenticated as Aconiti Radix Cocta, Pinelliae rhizome and GR by Dr. Zhang Lu. The voucher specimens (No. 20120701-20120703) were deposited in the Laboratory of the Chinese Herbal Medicine School of Tianjin University of TCM, China.
Sample preparation
According to Chinese Pharmacopoeia (2010), SBX is added GR and lime when processing to FBX. Therefore, GR and lime were used as the processing markers in sample preparation. Different BX samples were prepared by adding different PAs. If only lime was missing while other adjuvants and procedures were the same, then the mixture obtained is SBX-GR processed product [BX1, Figure 1d], whereas if only GR was missing and the other adjuvants and procedures were the same, then the mixture obtained is SBX-lime processed product [BX2, Figure 1e].
SBX (1 g), FBX (1 g), BX1 (1 g) and BX2 (1 g) were respectively mixed with 1 g of ZCW. 10 times of distilled water were added and the mixtures were soaked for 30 min, then boiled for 0.5 h. Afterward, the mixtures were centrifuged at 10,000 g for 10 min, then 8 times of distilled water were added to the residue, boiled for another 0.5 h and centrifuged at 10,000 g for 10 min. The filtered liquors were combined, which were mixed solutions and diluted mixed solutions in 100 mL measuring flasks and marked as ZCW-SBX, ZCW-BX1, ZCW-BX2 and ZCW-FBX. Meanwhile, 1 g of ZCW without adding any kind of BX, was extracted according to the procedure above, the solution was marked as the control solution of ZCW (ZCW-C), to show the integrity of chemical component information of ZCW in the same RRLC-Q-TOF-MS spectrum.
Conditions of data acquisition
The sample separation was performed with an Agilent 1200 rapid resolution liquid phase system equipped with a Q-TOF mass spectrometer (RRLC-Q-TOF-MS, US Agilent Company) and with data processing Qualitative Analysis B.04.00 software. Using high-purity nitrogen as assist spray ionization and desolventizing gas, the flow rate of drying gas was 10 mL/min and the temperature was set at 350°C; the atmospheric pressure of the atomizer was 310 kPa; flow rate of N2 was 600 L/h; cone gas flow (N2), 50 L/h. Electrospray ionization capillary voltage was set at +4.0 kV for positive ion mode. The mass scan was over the range of 50-1000 m/z. A ZORBAX SB-Aq C18 column (2.1 × 100 mm, 1.8 µm) (Agilent, USA) was used. The mobile phase was a gradient prepared from 0.1% formic acid aqueous solution (component (A) and 0.1% formic acid ACN (component (B). Gradient profile consisted of 0-2 min: B 1%; 2-5 min: B 1-6%; 5-15 min: B 6-10%; 15-20 min: B 10-20%; 20-31 min: B 20-30%; 31-36 min: B 30-50%; 36-38 min: B 50-70%; 38-39 min: B 70-99%; 39-40 min: B 99%; 40-45 min B 99-1%; 45-50 min: B 1%. Total run time, including conditioning of the column prior to the initial conditions, was 50 min. The injection volume was 5 µL. The flow rate was kept constant at 0.3 mL/min and analyzes were conducted at 40 ± 1°C.
Pattern recognition analysis
After the extraction, peak alignment, peak match and peak intensity correction of ion pair from collected spectrogram by Mass Profiler Professional (USA, Agilent), the data was imported to SIMCA-P 12.0 (Sweden, Umetrics AB) for principal component analysis (PCA), partial least squares-discriminant analysis (PLS-DA) and orthogonal to PLS-DA. Mass data were introduced into SPSS 13.0 (Chicago, IL, USA) for statistical analysis. Statistical differences were considered to be significant if the test P < 0.05.
RESULTS AND DISCUSSION
Multivariate statistical analysis and chemical markers exploring
Given that ZCW compatibility of BX's main components were alkaloids, positive ion mode was used to monitor and optimize RRLC-Q-TOF-MS conditions.[14] Figure 2 showed the BPC picture of ZCW before and after concerted application with different BX in positive ion mode. About 1500 mass signals were collected for further multivariate data analysis and study on ZCW and BX's compatibility mechanism. In order to compare the differences between different groups, the compounds were classified with PCA and PLS-DA method to find out the differentiated markers. After Pareto scaling with mean centering, the data were displayed as score plot [Figure 3a]. PCA score plot showed sample aggregation and discreteness.[15] The score plot shows that the experimental samples clearly clustered into different groups, indicating that processing procedures caused changes in the composition and/or content of components between various BX samples and ZCW. ZCW-FBX and ZCW-BX2 gathered on one side of the score plot region, but they were far from ZCW; ZCW-SBX and ZCW-BX1 gathered on the other side which was closer to ZCW. In order to find the potential chemical markers for the discrimination between ZCW's combination with SBX and FBX, extended statistical analysis was performed to generate a loading plot [Figure 3b]. In the loading plot, every spot represented a variable including its intensity and its corresponding tR-m/z pair. Chemical markers were selected according to the distance from the origin on the loading plot. The S-plot was used to identify the chemical markers according to the orders of their contributions to the separation of clustering [Figure 3c]. Variable importance parameters (VIP) value was a coefficient that reflected variable importance to the model, wherein, the larger the value, the more important the variable was; a variable with VIP >1 was considered as an important chemical marker to the model.[16,17,18,19] The potential chemical markers were selected according to the former VIP value mentioned and loading plot of PLS-DA model. To ensure the accuracy of final result, the established PLS-DA model must be firstly evaluated. R2Y (cum) and Q2 (cum) parameters usually indicated the fitness and prediction of the model. The model with low ratio of the two parameters was not accepted, as the model has over fitted.[20,21,22] We checked our model established in this study and all the models were proper and all the analysis were right. According to the criterion of data processing established above, 18 components were identified and selected as chemical markers to distinguish significant differences among different groups.
Figure 2.
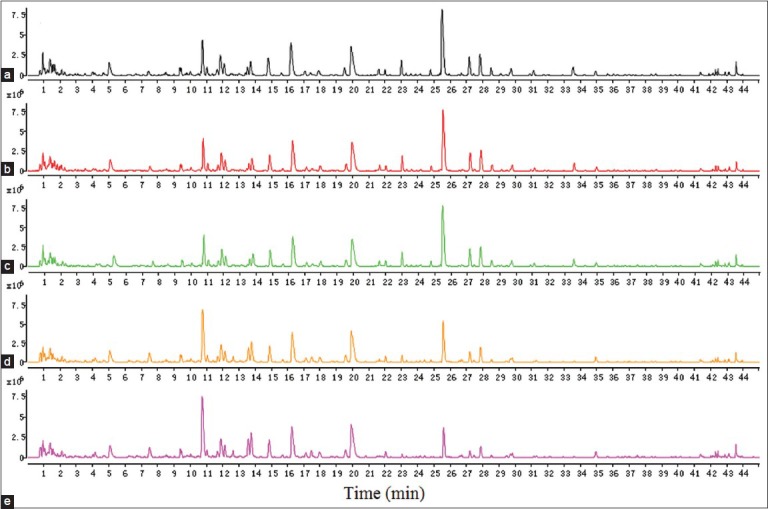
The representative chromatograms of Zhichuanwu (ZCW) decoction (a) and co-decoction of ZCW with SBX (b), BX1 (c), BX2 (d) and FBX (e)
Figure 3.
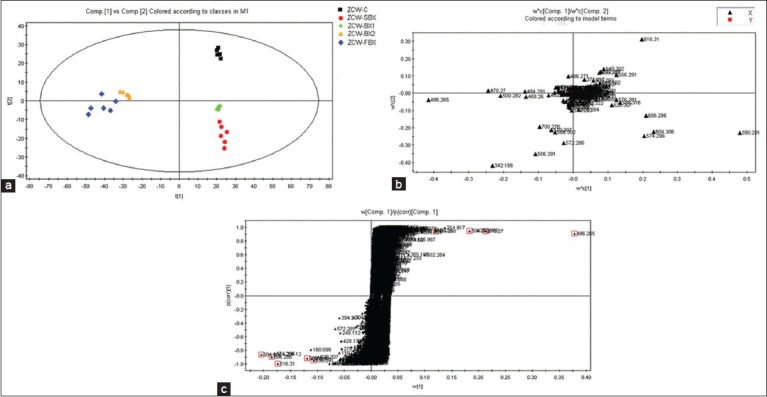
(a) Principal component analysis score plots of Zhichuanwu (ZCW) and its compatibility of different products of BX. (b) Loading plots corresponding partial least squares-discriminant analysis for pattern recognition. (c) S-plot of ZCW-C and ZCW-FBX
Identification of chemical markers
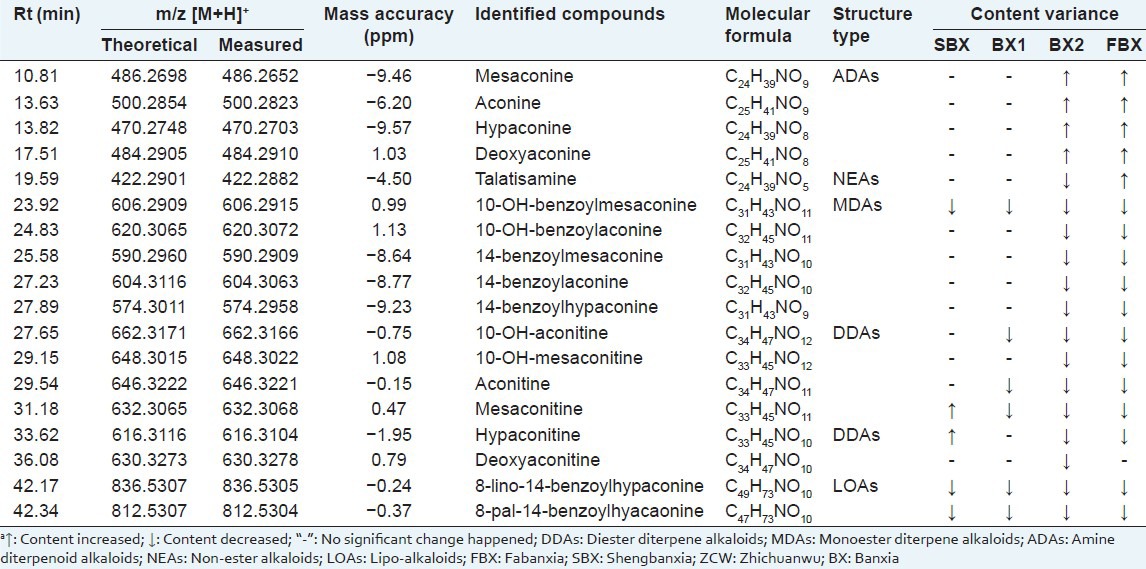
Under the present chromatographic and spectrometric conditions, chemical markers were identified by comparing accurately measured mass value with the theoretical exact mass. The identities of 18 most changed components were identified or tentatively assigned by comparison with the reference compounds or matching the molecular weight with those of the published compounds of ZCW. Taking mesaconitine as an example VIP value, 1.5; mass-to-charge ratio of ion [M + H]+, 632.3068; MS fragments, 572[M + H–C2H4O2]+, 540[M + H–C3H8O3]+, 512[M + H–C4H8O4]+, 105[M + H–C26H41NO10]+. This data showed the characteristics of mesaconitine and its elemental composition may be C33H45NO11. After treatment in line with the procedures above, 5 chemical markers were found in synergy of ZCW-C and ZCW-SBX, which were hypaconitine, mesaconitine, 10-OH-benzoylmesaconine, 8-lino-14-benzoyl-hypaconine and 8-pal-14-benzoylhyacaonine [Table 1]; 6 chemical markers in synergy of ZCW-C and ZCW-BX1: 10-OH-aconitine, aconitine, mesaconitine, 10-OH-benzoyl-mesaconine, 8-lino-14-benzoylhypaconine and 8-pal-14-benzoylhyacaonine [Table 1]; and 18 chemical markers in synergy of ZCW-C and ZCW-BX2: Mesaconine, aconine, hypaconine, deoxyaconine, talatisamine, 10-OH-benzoylmesaconine, 10-OH-benzoylaconine, 14-benzoylmesaconine, 14-benzoylaconine, 14-benzoylhypaconine, 10-OH-aconitine, 10-OH-mesaconitine, aconitine, mesaconitine, hypaconitine, deoxyaconitine, 8-lino-14-benzoylhypaconine and 8-pal-14-benzoylhyacaonine. [Table 1], 17 chemical markers in synergy of ZCW-C and ZCW-FBX: Mesaconine, aconine, hypaconine, deoxyaconine, talatisamine, 10-OH-benzoyl-mesaconine, 10-OH-benzoylaconine, 14-benzoylmesaconine, 14-benzoylaconine, 14-benzoylhypaconine, 10-OH-aconitine, 10-OH-mesaconitine, aconitine, mesaconitine, hypaconitine, 8-lino-14-benzoylhypaconine and 8-pal-14-benzoyl-hyacaonine [Table 1]. These chemical markers belonged to DDAs, MDAs, ADAs, NEAs and LOAs, all of them were from ZCW. Under the same RRLC-Q-TOF/MS condition, there were no markers detected from BX sample, thus, combination may have a greater impact on alkaloid compounds in ZCW.
Table 1.
Potential chemical markers were found between ZCW-C and ZCW-SBX, ZCW-BX1, ZCW-BX2, ZCW-FBXa
Intensity change of chemical markers
Comparing chemical markers of ZCW before and after its synergy with various BX samples, the DDAs in ZCW, such as hypaconitine and mesaconitine, increased in ZCW-SBX group and decreased in ZCW-BX1 group [Figure 4]. The decrease was especially significantly in ZCW-BX2 group and ZCW-FBX group. Whilst other DDAs in ZCW, such as 10-OH-mesaconitine, aconitine and deoxyaconitine, dropped in ZCW-BX2 group and ZCW-FBX group, no obvious decrease was observed in ZCW-SBX group and ZCW-BX1 group; and the ZCW-BX2 group was similar to ZCW-FBX group in terms of DDAs decrease. As shown in Figure 4, the MDAs, such as 10-OH-benzoylmesaconine, also exhibited some decrease in four combination groups. The ZCW-FBX and ZCW-BX2 groups had similar content of MDAs, such as 10-OH-benzoylaconine, 14-benzoylmesaconine, 14-benzoylaconine and 14-benzoylhypaconine, but much lower than that of ZCW-C group. In respect of ADAs, ZCW-FBX and ZCW-BX2 groups had much higher content of mesaconine, aconine, hypaconine and deoxyaconine than the other three groups, whereas those of ZCW-SBX and ZCW-BX1 were similar to ZCW-C group. In terms of NEAs, such as talatisamine, their content in ZCW-C was similar to ZCW-SBX and ZCW-BX1, but lower than ZCW-FBX and ZCW-BX2. LOAs, such as 8-lino-14-benzoylhypaconine and 8-pal-14-benzoylhyacaonine, also decreased significantly in four combination groups.
Figure 4.
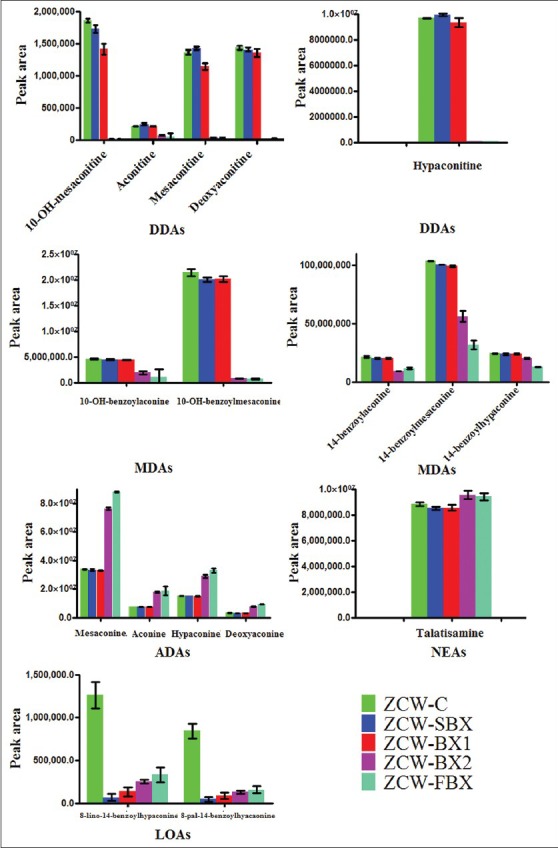
The relative intensity of chemical markers between different groups
Based on former mentioned results, it can be concluded that the compatibility of ZCW and FBX may have an important function on reducing the content of aconite alkaloid in ZCW, which may be mainly due to the different PAs used step by step when processing SBX to FBX. The results demonstrated that FBX can facilitate DDAs reduction or the transformation to non-toxic ADAs. As both SBX and FBX were decocted together with ZCW, the difference on content change of aconite alkaloid after ZCW combination with SBX and FBX was not due to the combination extraction. The different effect of two combinations may be related to the distinction between SBX and FBX. As former discussed, FBX was a processed product obtained by steeping SBX. Moreover, the content change of aconite alkaloid in ZCW-FBX and ZCW-BX2 showed the same trend and ZCW-SBX and ZCW-BX1 had similar behavior. This finding indicated that lime could effectively lower aconite alkaloid content of ZCW-FBX group, whereas the function of GR was not notable.
At present, there was no consensus on the reason why combination of ZCW and FBX can facilitate DDAs reduction or the transformation to non-toxic ADAs. A number of researchers thought it was due to GR and literature about organic acid in GR can reduce toxicity of aconitum in medical materials.[23,24] However, the amount of GR must be at least half of the amount of ZCW.[23] Only 15 kg of GR was needed per 100 kg of SBX when processing to FBX, the amount was far from enough to reduce ZCW toxicity. Others believed that lime had the function of dis-intoxication and some component of FBX may undergo denaturation precipitation or adsorption in alkaline solution to reduce toxicity.[25] These two assumptions were based on experience and lack of systematic experimental study. Our research showed that lime may have an important role in the DDAs reduction of ZCW and FBX combination. Moreover, sample solutions of ZCW-SBX and ZCW-BX1 were stickier, probably because the extraction solution contained macromolecular components such as starch from BX, whereas sample solutions of ZCW-FBX and ZCW-BX2 were very clear because lime caused denaturation of starch, polyoses and other macromolecular components.
CONCLUSION
This study adopted RRLC-Q-TOF-MS technology to analyze the Pas’ influence on compatibility of ZCW's combination with SBX and FBX. Multivariate pattern recognition technology was used to study the overall changes in chemical composition before and after combination. The results indicated that PAs affected the plant chemical composition and combination mechanism. For example, lime can facilitate the change of high toxic DDAs into low-toxic or non-toxic components, whereas GR did not have an important role in this process. This study can help to fully understand the differences of chemical composition of ZCW in synergy with BX, as well as provide useful information for potential chemical markers variation. In this study, the established method will be of great significance to processing mechanism research and study of TCM compatibility and clinical application.
ACKNOWLEDGEMENT
The authors would like to thank National Basic Research Program of China (973 Program) (2011CB505300, 2011CB505302) for their financial support.
Footnotes
Source of Support: The National Basic Research Program of China (973 Program) (2011CB505300, 2011CB505302)
Conflict of Interest: None declared.
REFERENCES
- 1.Görög S. Drug safety, drug quality, drug analysis. J Pharm Biomed Anal. 2008;48:247–53. doi: 10.1016/j.jpba.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Chen X, Huang Q, Fang D, Li G, Zhang G. Toxicity of raw and processed roots of Polygonum multiflorum. Fitoterapia. 2012;83:469–75. doi: 10.1016/j.fitote.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Sun H, Ni B, Zhang A, Wang M, Dong H, Wang X. Metabolomics study on Fuzi and its processed products using ultra-performance liquid-chromatography/electrospray-ionization synapt high-definition mass spectrometry coupled with pattern recognition analysis. Analyst. 2012;137:170–85. doi: 10.1039/c1an15833c. [DOI] [PubMed] [Google Scholar]
- 4.Geng L, Sun H, Yuan Y, Liu Z, Cui Y, Bi K, et al. Discrimination of raw and vinegar-processed Genkwa Flos using metabolomics coupled with multivariate data analysis: A discrimination study with metabolomics coupled with PCA. Fitoterapia. 2013;84:286–94. doi: 10.1016/j.fitote.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Wang Y, Su L, Li L, Zhang Y. Exploring potential chemical markers by metabolomics method for studying the processing mechanism of traditional Chinese medicine using RPLC-Q-TOF/MS: A case study of radix aconiti. Chem Cent J. 2013;7:36. doi: 10.1186/1752-153X-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niitsu H, Fujita Y, Fujita S, Kumagai R, Takamiya M, Aoki Y, et al. Distribution of Aconitum alkaloids in autopsy cases of aconite poisoning. Forensic Sci Int. 2013;227:111–7. doi: 10.1016/j.forsciint.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Chan TY. Contributory factors in herb-induced fatal aconite poisoning. Forensic Sci Int. 2012;223:40–3. doi: 10.1016/j.forsciint.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q, Zhuo L, Liu L, Zhu S, Sunnassee A, Liang M, et al. Seven cases of fatal aconite poisoning: Forensic experience in China. Forensic Sci Int. 2011;212:e5–9. doi: 10.1016/j.forsciint.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Li XH, Wang WY, Zhang Y. Experimental study for calming panting of cold wheezing pill. J Hubei Med Staff Coll. 2004;17:9–11. [Google Scholar]
- 10.Zhang SJ, Gong K, He KQ, Wang Q. Clinical safty observation of wutoubanxiasan. J Sichuan Tradit Chin Med. 2009;27:55–6. [Google Scholar]
- 11.Yan HJ, Liu GL, Gao WH. The application of Radix aconiti combination with Rhizoma pinelliae. J Shandong Univ TCM. 1999;23:152–5. [Google Scholar]
- 12.Weng XG, Nie SQ, Huang LQ. Determination of content changes of hypaconitine in preparations of aconite matching other herbs in “pinellia tuber, snakegourd fruit, fritillaria, Japanese ampelopsis root and common bletilla tuber counteract aconite” by HPLC. Chin Pharm J. 2004;39:57–9. [Google Scholar]
- 13.Wang C, Wang YG, Liang QD, Rang WQ, Gao Y. Analysis of chemical composition in the combination of monkshood and pinellia by UPLC/Q-TOFMS with multivariate statistical analysis. Yao Xue Xue Bao. 2010;45:1301–6. [PubMed] [Google Scholar]
- 14.Gao P, Lu C, Zhang F, Sang P, Yang D, Li X, et al. Integrated GC-MS and LC-MS plasma metabonomics analysis of ankylosing spondylitis. Analyst. 2008;133:1214–20. doi: 10.1039/b807369d. [DOI] [PubMed] [Google Scholar]
- 15.Yin P, Zhao X, Li Q, Wang J, Li J, Xu G. Metabonomics study of intestinal fistulas based on ultraperformance liquid chromatography coupled with Q-TOF mass spectrometry (UPLC/Q-TOF MS) J Proteome Res. 2006;5:2135–43. doi: 10.1021/pr060256p. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Enciso M, Tenenhaus M. Prediction of clinical outcome with microarray data: A partial least squares discriminant analysis (PLS-DA) approach. Hum Genet. 2003;112:581–92. doi: 10.1007/s00439-003-0921-9. [DOI] [PubMed] [Google Scholar]
- 17.Liang X, Chen X, Liang Q, Zhang H, Hu P, Wang Y, et al. Metabonomic study of Chinese medicine Shuanglong formula as an effective treatment for myocardial infarction in rats. J Proteome Res. 2011;10:790–9. doi: 10.1021/pr1009299. [DOI] [PubMed] [Google Scholar]
- 18.Lin L, Huang Z, Gao Y, Chen Y, Hang W, Xing J, et al. LC-MS-based serum metabolic profiling for genitourinary cancer classification and cancer type-specific biomarker discovery. Proteomics. 2012;12:2238–46. doi: 10.1002/pmic.201200016. [DOI] [PubMed] [Google Scholar]
- 19.Zhang A, Sun H, Dou S, Sun W, Wu X, Wang P, et al. Metabolomics study on the hepatoprotective effect of scoparone using ultra-performance liquid chromatography/electrospray ionization quadruple time-of-flight mass spectrometry. Analyst. 2013;138:353–61. doi: 10.1039/c2an36382h. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Shan YH, Yan X, Zhao SM, Yi FY, Zhao XJ, et al. A metabonomics approach based on liquid chromatography-mass spectrometry: From metabolic profiling to potential biomarker. Sci China. 2009;39:1268–76. [Google Scholar]
- 21.Poliquin PO, Chen J, Cloutier M, Trudeau LÉ, Jolicoeur M. Metabolomics and in-silico analysis reveal critical energy deregulations in animal models of Parkinson's disease. PLoS One. 2013;8:e69146. doi: 10.1371/journal.pone.0069146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang AH, Sun H, Han Y, Yan GL, Yuan Y, Song GC, et al. Ultraperformance liquid chromatography-mass spectrometry based comprehensive metabolomics combined with pattern recognition and network analysis methods for characterization of metabolites and metabolic pathways from biological data sets. Anal Chem. 2013;85:7606–12. doi: 10.1021/ac401793d. [DOI] [PubMed] [Google Scholar]
- 23.Wang FF, Song ZH, Zhang LL, Zhou SP, Feng F. Effect of Aconiti radix Preparata combined with Glycyrrhizae radix in different ratios on monoester alkaloids. Chin Tradit Herb Drug. 2012;43:1101–4. [Google Scholar]
- 24.Zhang YY, Yang JH. Effect of the compatibility of aconite and glycyrrhiza on the contents of aconitine, glycyrrhizin and liquirtin. Chin Pharm J. 2009;44:11–4. [Google Scholar]
- 25.Zhong LY, Wu H, Zhang L, Zhu T. Overview of toxic components and processing mechanism of Rhizoma pinelliae. Shanghai J Tradit Chin Med. 2007;41:72–4. [Google Scholar]


