Abstract
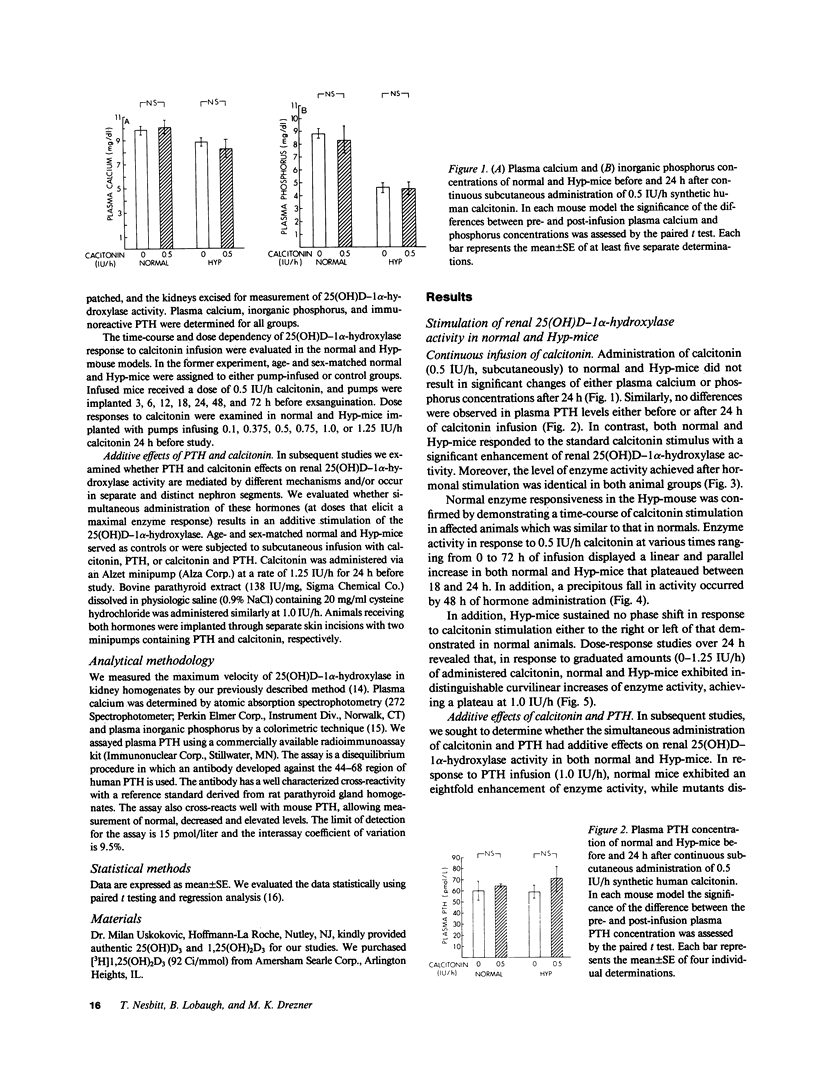
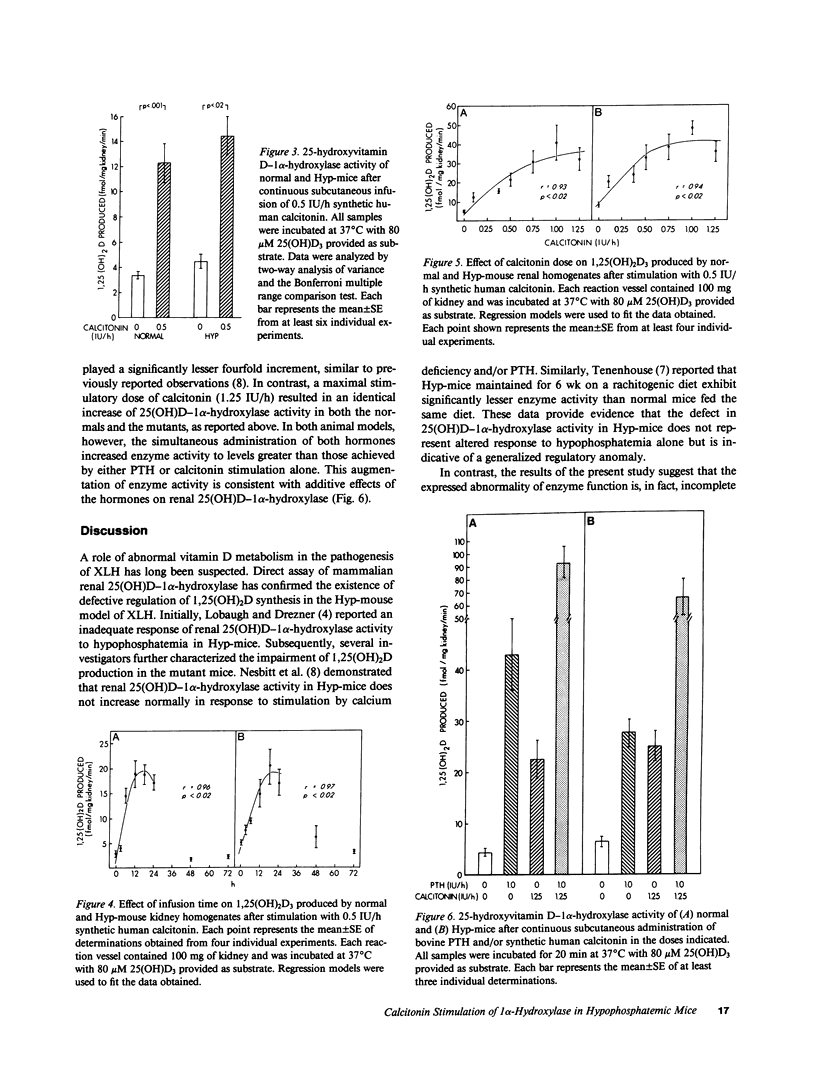
Hypophosphatemia (Hyp) mice have defective regulation of 25(OH)D-1 alpha-hydroxylase activity in response to hypophosphatemia, hypocalcemia, and parathyroid hormone (PTH) administration. However, recent observations support the existence of anatomically distinct, independently regulated renal 1 alpha-hydroxylase systems in mammalian proximal convoluted and straight tubules. To more completely define the extent of the 1 alpha-hydroxylase regulatory defect in Hyp-mice, we compared enzyme maximum velocity in normal and mutants after infusion of calcitonin. Upon stimulation, renal 1 alpha-hydroxylase activity increased to similar levels in normal and Hyp-mouse renal homogenates. Moreover, time-course and dose-dependence studies revealed similar patterns of response in the animal models. Subsequently, we examined whether PTH and calcitonin stimulatory effects on enzyme activity are mediated through different mechanisms. In both animal models administration of PTH and calcitonin increased enzyme activity to levels greater than those obtained after maximal stimulation by either hormone alone, consistent with additive effects. These observations indicate that a calcitonin-sensitive component of 1 alpha-hydroxylase is not compromised in the X-linked hypophosphatemic syndrome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiba T., Endou H., Koseki C., Sakai F., Horiuchi N., Suda T. Localization of 25-hydroxyvitamin D3-1 alpha-hydroxylase activity in the mammalian kidney. Biochem Biophys Res Commun. 1980 May 14;94(1):313–318. doi: 10.1016/s0006-291x(80)80222-1. [DOI] [PubMed] [Google Scholar]
- Brunette M. G., Chabardes D., Imbert-Teboul M., Clique A., Montégut M., Morel F. Hormone-sensitive adenylate cyclase along the nephron of genetically hypophosphatemic mice. Kidney Int. 1979 Apr;15(4):357–369. doi: 10.1038/ki.1979.47. [DOI] [PubMed] [Google Scholar]
- Cowgill L. D., Goldfarb S., Lau K., Slatopolsky E., Agus Z. S. Evidence for an intrinsic renal tubular defect in mice with genetic hypophosphatemic rickets. J Clin Invest. 1979 Jun;63(6):1203–1210. doi: 10.1172/JCI109415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- Delvin E. E., Glorieux F. H. Serum 1,25-dihydroxyvitamin D concentration in hypophosphatemic vitamin D-resistant rickets. Calcif Tissue Int. 1981;33(2):173–175. doi: 10.1007/BF02409431. [DOI] [PubMed] [Google Scholar]
- Dennis V. W., Brazy P. C. Intracellular processes that affect renal phosphate transport. Adv Exp Med Biol. 1984;178:21–24. doi: 10.1007/978-1-4684-4808-5_2. [DOI] [PubMed] [Google Scholar]
- Fukase M., Avioli L. V., Birge S. J., Chase L. R. Abnormal regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase activity by calcium and calcitonin in renal cortex from hypophosphatemic (Hyp) mice. Endocrinology. 1984 Apr;114(4):1203–1207. doi: 10.1210/endo-114-4-1203. [DOI] [PubMed] [Google Scholar]
- Galante L., Colston K. W., MacAuley S. J., MacIntyre I. Effect of calcitonin on vitamin D metabolism. Nature. 1972 Aug 4;238(5362):271–273. doi: 10.1038/238271a0. [DOI] [PubMed] [Google Scholar]
- Giasson S. D., Brunette M. G., Danan G., Vigneault N., Carriere S. Micropuncture study of renal phosphorus transport in hypophosphatemic vitamin D resistant rickets mice. Pflugers Arch. 1977 Oct 19;371(1-2):33–38. doi: 10.1007/BF00580769. [DOI] [PubMed] [Google Scholar]
- Horiuchi N., Suda T., Takahashi H., Shimazawa E., Ogata E. In vivo evidence for the intermediary role of 3',5'-cyclic AMP in parathyroid hormone-induced stimulation of 1alpha,25-dihydroxyvitamin D3 synthesis in rats. Endocrinology. 1977 Sep;101(3):969–974. doi: 10.1210/endo-101-3-969. [DOI] [PubMed] [Google Scholar]
- Horiuchi N., Takahashi H., Matsumoto T., Takahashi N., Shimazawa E., Suda T., Ogata E. Salmon calcitonin-induced stimulation of 1 alpha,25-dihydroxycholecalciferol synthesis in rats involving a mechanism independent of adenosine 3':5'-cyclic monophosphate. Biochem J. 1979 Nov 15;184(2):269–275. doi: 10.1042/bj1840269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima H., Kurokawa K. Unique hormonal regulation of vitamin D metabolism in the mammalian kidney. Miner Electrolyte Metab. 1983;9(4-6):227–235. [PubMed] [Google Scholar]
- Kawashima H., Torikai S., Kurokawa K. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1 alpha-hydroxylase in proximal straight tubule of rat kidney. Nature. 1981 May 28;291(5813):327–329. doi: 10.1038/291327a0. [DOI] [PubMed] [Google Scholar]
- Kawashima H., Torikai S., Kurokawa K. Localization of 25-hydroxyvitamin D3 1 alpha-hydroxylase and 24-hydroxylase along the rat nephron. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1199–1203. doi: 10.1073/pnas.78.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins R. G., MacAuley S. J., Rapoport A., Martin T. J., Tulloch B. R., Byfield P. G., Matthews E. W., MacIntyre I. Effects of nucleotides, hormones, ions, and 1,25-dihydroxycholecalciferon on 1,25-dihydroxycholecalciferol production in isolated chick renal tubules. Clin Sci Mol Med. 1974 May;46(5):569–582. doi: 10.1042/cs0460569. [DOI] [PubMed] [Google Scholar]
- Lobaugh B., Drezner M. K. Abnormal regulation of renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the X-linked hypophosphatemic mouse. J Clin Invest. 1983 Feb;71(2):400–403. doi: 10.1172/JCI110783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobaugh B., Drezner M. K. Measurement of 25-hydroxyvitamin D-1 alpha-hydroxylase activity in mammalian kidney. Anal Biochem. 1983 Mar;129(2):416–424. doi: 10.1016/0003-2697(83)90571-7. [DOI] [PubMed] [Google Scholar]
- Lorenc R., Tanaka Y., DeLuca H. F., Jones G. Lack of effect of calcitonin on the regulation of vitamin D metabolism in the rat. Endocrinology. 1977 Feb;100(2):468–472. doi: 10.1210/endo-100-2-468. [DOI] [PubMed] [Google Scholar]
- Lyles K. W., Clark A. G., Drezner M. K. Serum 1,25-dihydroxyvitamin D levels in subjects with X-linked hypophosphatemic rickets and osteomalacia. Calcif Tissue Int. 1982 Mar;34(2):125–130. doi: 10.1007/BF02411222. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Jr, Gray R. W., Kiebzak G. M., Mish P. M. Altered vitamin D, cyclic nucleotide and trace mineral metabolism in the X-linked hypophosphatemic mouse. Adv Exp Med Biol. 1980;128:351–359. doi: 10.1007/978-1-4615-9167-2_40. [DOI] [PubMed] [Google Scholar]
- Nesbitt T., Drezner M. K., Lobaugh B. Abnormal parathyroid hormone stimulation of 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the hypophosphatemic mouse. Evidence for a generalized defect of vitamin D metabolism. J Clin Invest. 1986 Jan;77(1):181–187. doi: 10.1172/JCI112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Wong M., Bikle D., Goodman D. B. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972 Sep;51(9):2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenhouse H. S. Abnormal renal mitochondrial 25-hydroxyvitamin D3-1-hydroxylase activity in the vitamin D and calcium deficient X-linked Hyp mouse. Endocrinology. 1983 Aug;113(2):816–818. doi: 10.1210/endo-113-2-816. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S. Investigation of the mechanism for abnormal renal 25-hydroxyvitamin D3-1-hydroxylase activity in the X-linked Hyp mouse. Endocrinology. 1984 Aug;115(2):634–639. doi: 10.1210/endo-115-2-634. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R., McInnes R. R., Glorieux F. H. Renal handling of phosphate in vivo and in vitro by the X-linked hypophosphatemic male mouse: evidence for a defect in the brush border membrane. Kidney Int. 1978 Sep;14(3):236–244. doi: 10.1038/ki.1978.115. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R. Renal adaptation to phosphate deprivation in the Hyp mouse with X-linked hypophosphatemia. Can J Biochem. 1979 Jun;57(6):938–944. doi: 10.1139/o79-114. [DOI] [PubMed] [Google Scholar]



