Abstract
Background:
Ziziphi Spinosae Semen (ZSS), the seed of Ziziphus jujuba var. spinosa (Bunge) Hu ex H. F. Chow., is a traditional herb for insomnia and anxiety in eastern Asia. However, few researches have been concerned with its effect on ameliorating memory and learning performance.
Objective:
To investigate the constituents of ZSS water soluble extract and its ameliorating learning and memory in mice.
Materials and Methods:
A new high performance liquid chromatography coupled with tandem mass spectrometry (HPLC-ESI-MS/MS) method was developed and validated to determine the main constituents in the extract. The effect of ZSS water soluble extract on memory and learning performance was investigated in mice by Y-maze and passive avoidance test.
Results:
The extract could significantly decrease the number of errors (NOE), and increase the transfer latency time (TLT) and electrical stimuli time (EST). In addition, spinosin, jujuboside A (JuA) and jujuboside B (JuB) were simultaneously identified and quantified in the extract. Their contents in the extract were as followed: Spinosin (223.51mg/g), JuA (63.76mg/g) and JuB (26.29mg/g).
Conclusion:
The extract played a promising role in ameliorating memory in mice with alcohol induced memory retrieval disorders, and might help to improve learning capacity to some extent. Spinosin, JuA and JuB were the predominant constituents, which might be mainly responsible for the definite activity.
Keywords: HPLC-ESI-MS/MS, Jujuboside A, learning and memory, spinosin, water-soluble extract, Ziziphi Spinosae Semen
INTRODUCTION
Ziziphi Spinosae Semen (ZSS), a traditional Chinese herb or nutraceutical, is the dried ripe seed of Ziziphus jujuba var. spinosa (Bunge) Hu ex H. F. Chow. It has been used to treat insomnia and anxiety for over two thousand years in China.[1,2] Due to its definite clinical activities, masses of pharmacological researches have been performed on ZSS, which demonstrate it with various bioactive effects, such as hypnotic effect,[3] sedative effect,[4] antidepressant-like effect,[5] and so on.[6,7,8]
Commonly, mild cognitive disorder is always featured with the dysfunction of memory, which is also the essential incipient symptom of senile dementia. It has been demonstrated that mild cognitive disorder exhibits close relevance with Alzheimer disease.[9,10] In order to treat these disorders (including dementia, amnesia and Alzheimer's disease), it is meaningful to exploit natural nutraceutical with the effect of ameliorating learning and memory. Recently, it is found that ZSS oil exhibits certain improving effects of learning and memory in mice,[11] which manifested the great potential of ZSS as a dietary supplement for treatment of these disorders.
In the previous reports, it has been showed that the various bioactivities of ZSS are mainly attributed to its polar components, i.e. flavonoids, saponins and polysaccharides.[12] Among them, spinosin,[13] jujuboside A (JuA)[14,15,16,17,18] and jujuboside B (JuB)[19] are the most representative ingredients, that exert significant activity in sedation and hypnosis through a variety of mechanisms and targets, especially 5-HT metabolism, GABAergic receptors and hippocampal neurons. It is well known that these mechanisms and targets are also involved in the processes of learning and memory.[20,21,22] As a result, these polar components in ZSS might also have a predictable benefit for learning and memory. However, to the best of our knowledge, there have been no reports on the relevant research.
In this study, the effects of ZSS water-soluble extract on the learning and memory in dysmnesia mice induced by alcohol were investigated with Y maze and passive avoidance tests. In addition, the main individual ingredients of spinosin, JuA and JuB in the extract were identified and quantified with a new rapid and sensitive HPLC-ESI-MS/MS method. Collectively, the aim of the present research was to make a good recognition of ZSS water-soluble compounds effect on learning and memory, so as to elucidate its bioactivity mechanism and exploit a variety of products.
MATERIALS AND METHODS
Plant materials and chemicals
ZSS was gathered from Jixian County (Tianjin, China), and authorized to be ripe seeds of Ziziphus jujuba var. spinosa (Bunge) Hu ex H. F. Chow by Dr. Junbo Xie. Spinosin (Batch No. 120519, 99.2%), JuA (Batch No. 110312, 99.0%) and JuB (Batch No. 110927, 99.1%) were purchased from Chengdu Scholar Bio-tech CO., Ltd. (Chengdu, China). Piacetam (Batch No. 10071027) was from Jinshi Pharmaceutical CO., Ltd. (Tianjin, China). HPLC grade acetonitrile and deionized water were all purchased from J.T.Baker (USA). 96% formic acid was from Tedia CO., Ltd. (USA). All other chemicals were of analytical grade.
Preparation of water-soluble extract from ZSS
Water-soluble extract from ZSS (WSE) was prepared according to the modified process of the reported method.[23] 3.0kg of ZSS were dried and ground into powder (40 mesh particle size). After being degreased with petroleum ether (60-90°C), the residue was refluxed with 70% ethanol (1:10 m/v) for 6h (three times). The extract was collected and evaporated with a PE-5205 rotary evaporator (Shanghai China). The residue obtained was dissolved in water and then purified with AB-8 macroporous adsorption resin column chromatography (Tianjin, China). The elution was performed with water, 20 ethanol, 50 ethanol and 70% ethanol successively (5 times of column volume). The selected eluent (50% ethanol) was concentrated under reduced pressure and then freeze-dried. The ultimate yield was 16.10g.
Preparation of standard and sample solutions for HPLC-ESI-MS/MS analysis
The standard stock solutions were prepared with accurately weighed spinosin, JuA and JuB (10mg each) separately into three 10 ml volumetric flasks. Acetonitrile of 35% was used to dissolve them and made up to the volume. Then, precise amount (1.0 ml) of the each stock solution was put into the same volumetric flask (10 ml). After dilution with 35% acetonitrile to the volume, the compound standard stock solution with nominal concentrations of 0.1 mg/ml for the three components (spinosin, JuA and JuB) was obtained.
The extract sample (WSE) solution was prepared by dissolving 25mg of WSE in a 100ml volumetric flask with 35% acetonitrile, and then diluted to obtain 0.25 mg/ml of sample solution. The solution was diluted further to obtain the concentration of 10 μg/ml. Before being injected into the HPLC system, all solutions were filtered through 0.45μm membrane filters. Before analysis, all the solutions were diluted into the proper concentration.
The conditions of HPLC-ESI-MS/MS analysis
The HPLC analysis were performed on Agilent 1200 Series HPLC system (Agilent, USA) which consisted of a binary pump (G1312B), a column heater (G1316B) and an autosampler SL Plus (G1367D). The chromatographic separation was performed on an Agilent Poroshell 120 EC C18 column (50 mm × 2.1 mm, 2.7μm) at 30°C. HPLC analysis was carried out by gradient elution with a mobile phase of acetonitrile–aqueous phase (0.1% formic acid) at a flow rate of 0.3ml/min. The percentage of acetonitrile in the mobile phase was programmed as follows: 30% (0 min) - 30% (6 min) - 80% (11 min). Then, the column was re-equilibrated for 3 min before the next analysis. The injection volume was 40 μl.
Agilent Technologies 6410 Triple Quad LC-MS/MS system equipped with electrospray ionization (ESI) was performed for the MS/MS detection. Ionization was achieved by using electrospray in the negative-ion mode with the spray voltage of 4000V. High purity N2 was used as nebulizer gas at the pressure of 35p.s.i with source temperature of 100°C. Desolvation gas (N2) was heated to 350°C and delivered at a flow rate of 10 L/min. For collision-induced dissociation (CID), high purity N2 was used at the pressure of 0.15MPa. Quantification was performed by multiple reactions monitoring (MRM). The peak widths of precursor and product ions were maintained at 0.7amu at half-height in the MRM mode. All the MS/MS fragmentation and collision energy conditions have been studied. Under the optimized HPLC-ESI-MS/MS conditions, the three biomarkers were separated and detected efficiently in only 7 min [Figure 1].
Figure 1.
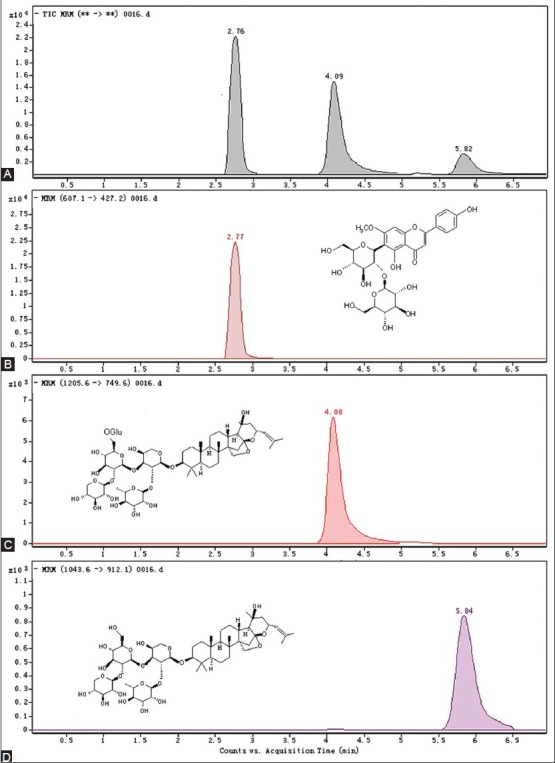
The typical chromatograms and chemical structures of the three components. A: the mixed standards; B: Spinosin; C: JuA; D: JuB
Validation of the assay method
Sensitivity and Linearity: The stock solution containing three reference compounds was diluted to a serial of appropriate concentrations with 35% acetonitrile. The calibration curve was constructed by plotting the peak areas versus the concentration for each analyte (six various concentrations). The limits of detection (LOD) and limits of quantification (LOQ) for each analyte were determined under the present chromatographic conditions at a signal-to-noise ratio (S/N) of about 3 and 10, respectively.
Precision, Reproducibility and Recovery: The intra- and inter-day variations were determined to investigate the precision of the developed method. Certain concentration (250 ng/ml) of standard solution was tested with three times on the same day and on three consecutive days, respectively. Variations were expressed by the relative standard deviation (R.S.D.). Five different WSE sample solutions (the same batch) were analyzed to investigate the reproducibility, which was also expressed by R.S.D. Recoveries were examined by spiking accurate amounts of reference compounds (low, middle and high level; n = 3 each) with sample and then analyzed as previously described. The recovery was determined by calculating the ratio of measured increment of bioactive markers and the actual added amount into the sample.
Animals and treatments
The animals used in the research were all 6 to 8 weeks old Chinese Kunming mice (20-25g) from Tianjin Experimental Animal Center. All the experimental procedures were performed under the National Guidelines on the proper care and use of animals in laboratory research, and approved by the local ethical committee of Tianjin University of Commerce.
In the present investigation, the mice were divided randomly divided into 12 groups (12 mice each) for employing Y-maze test (6 groups) and passive avoidance test (6 groups) separately. WSE (100, 200 and 400 mg/kg) was administered intragastrically for 12 successive days for learning and memory studies. Physiologic saline (N.S., 20 ml/kg) was administered in the same way for the blank control and model group. As an established nootropic agent, piacetam (1520 mg/kg) was administered to positive control group. On the 12th day, 30% alcohol (10 ml/kg) was used in mice (except blank control) to induce the memory retrieval disorders after continuous administration of N.S., WSE or piacetam. All the dosages were selected on the basis of human doses and the results of the probe trials.
Y-maze test
In this research, a modified Y-maze test[24] was performed to evaluate learning and memory in mice by a MG-2C/3C maze stimulator (Zhangjiagang, China). During the testing course, the entry of mice into darken arm (with electricity) was recorded as a “discrimination error” and the decrease of number of errors (NOE) indicated enhanced performance of mice. On the 11th day, the trained animals were tested with 15 trials after 60 min of the previously described treatment, and the number of errors was recorded as learning results. On the 12th day (the same period), the animals (except blank control) were treated with alcohol (50 min after administration of of different drugs), and were tested again with 15 trials 10 min later. The records were referred to as memory results.
Passive avoidance test (PAT)
According to the previous reports,[25,26] the mice were subjected to the passive avoidance test (PAT) with a BA-200 automatic mouse passive avoidance test apparatus (Chengdu, China). In this research, transfer latency time (TLT), electrical stimuli time (EST) and number of errors (NOE) were measured for evaluation of learning and memory in mice. TLT was the time taken by the animal to step from the bright room into dark room (with 0.3mA electricity) in the testing trial period (5 min). EST was total time of the experimental mouse suffering the electric shock and NOE was defined as the number of entry into the dark room in the period. Just as the treatment described in the Y-maze test, the mice were tested with PAT. The determined data of 11th day and 12th day were recorded as the learning and memory results respectively.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 4™ and comprehensive statistical software SPSS (version 11.0, SPSS Corporation, USA). One-way analysis of variance (ANOVA) was used for statistical evaluation of comparing the bioactivity values. Differences between the values were considered to be statistically significant with P < 0.05. Extreme values were excluded by means of Dixon's principles of exclusion of extreme values. All determinations in chemical analysis were performed at least triplicate and represented as mean ± SD.
RESULTS
Optimization of MS/MS conditions
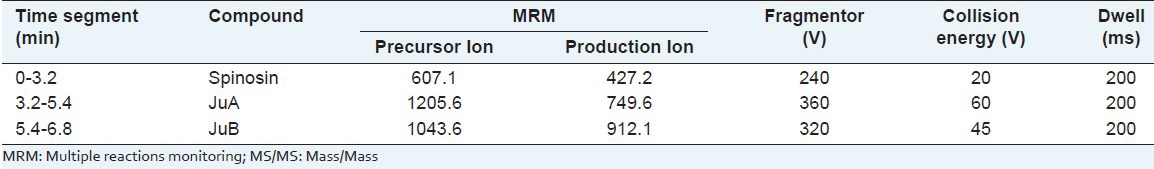
In this study, the mass spectral conditions were investigated in both positive and negative ion modes, and the negative ion mode was found to be more sensitive. So, the components were identified and quantified by MS on negative ion mode with a time-segment program. Different fragmentors from 200 to 360V and collision energies from 30 to 70V were investigated with standard solutions. As the fragmentor and collision energy increased, the peak areas varied obviously. When the fragmentor and collision energy were higher than the optimum value, the intensity of pseudo molecule ions decreased because of producing more fragments. The optimized MS conditions of each component are shown in Table 1.
Table 1.
Optimized MS/MS parameters and time-segment program of the three components
Validation of the assay
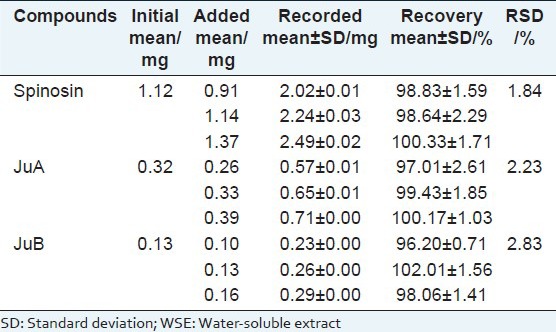
For determination, a calibration curve for each marker was constructed and tested for linearity. The linearity was determined by triplicate analyses of standard solutions. The peak area (y) was plotted against concentration (x, ng/ml) for calibration and all calibration curves showed good linear correlation (Spinosin: y = 196.97x + 2175.2; JuA: y = 56.622x + 87.802; JuB: y = 95.19x + 264.71) with the linear range of 25-500ng/ml, and all the correlation coefficients (r) ranged from 0.9996 to 0.9999. The values of LOD (1.2-4.1 ng/ml) and LOQ (4.7-14.2 ng/ml) indicated that developed method was very sensitive for assaying the three compounds. The assayed intra and inter-day (n = 3) variations (R.S.D.) of the standard solution were all less than 2.1% and R.S.D. values of reproducibility were all lower than 2.3% [Table 2], which demonstrated good precision and reproducibility of the proposed method. The recoveries ranged from 96.20 to 102.01% with appropriate R.S.D. values (1.84% - 2.83%), indicating perfect accuracy and little matrix effect in the method [Table 3].
Table 2.
Precision, reproducibility and assayed contents of the three components in WSE
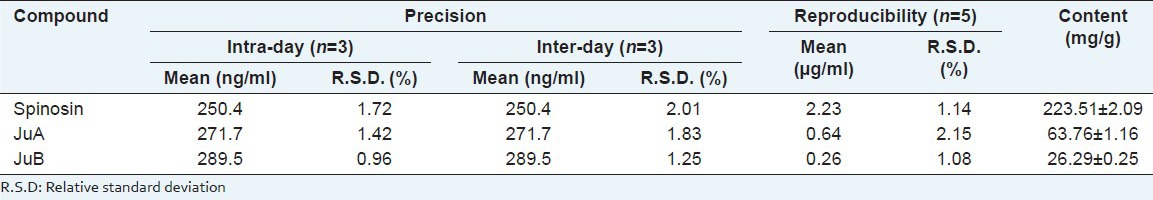
Table 3.
Recoveries of the three major components in WSE (n=3)
Analysis of the components by HPLC-ESI-MS/MS
The three biomarkers were identified by comparing the retention time and MS spectrum of the reference standards. Just as shown in Figure 1, the three biomarkers were simultaneously found in WSE. Under the optimum HPLC-ESI-MS/MS conditions described previously, spinosin, JuA and JuB were separated very well. Their characters in MS/MS were consistent with the previous reports.[27,28,29]
With the aid of MRM, the three components were assayed successfully. As shown in Table 2, spinosin was the predominant flavonoid component in WSE, and accounted for over 22% of the extract weight, which was congruent with the result of previous report.[23] By contrast, the total content of JuA and JuB was up to over 9% in WSE, and were the main representative saponins in ZSS.[3]
Effect of WSE on learning and memory in Y-maze test
In order to examine the effects of WSE on learning and memory related behavior, the experimental mice were subjected to the Y-maze test and passive avoidance test (PCT).
The Y-maze test is a common method to investigate how the rodents function with memory and spatial learning through applying various stimuli. In this experiment, the NOE was measured to evaluate the learning and memory in mice, and the significant decrease of NOE values denoted improving effect on learning and memory. On the 11th day, compared with N.S group, three dosages of WSE (100, 200 and 400 mg/kg, i.g.) decreased NOE significantly in the normal mice (P < 0.05), and this effect showed perfect dose-dependent correlation. Among them, the effect of high dosage WSE (400 mg/kg) was even better than piracetam (P < 0.01). These results demonstrated that WSE could play a promising role in improving the learning performance of the normal mice [Figure 2A].
Figure 2.
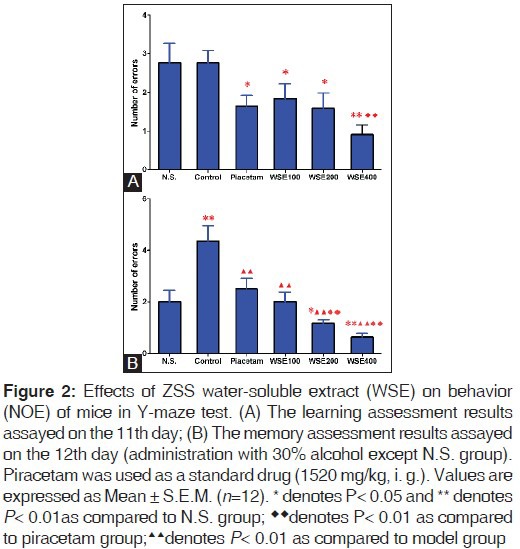
Memory retrieval is an essential process of memory, and controlling accessible memory through modulation of retrieval could help humans to adapt to their environment better.[30,31] In this research, the memory retrieval disorders mouse model induced by alcohol was used to investigate the effect of WSE on memory. As shown in Figure 2 B, on the 12th day, 30% alcohol (10 ml/kg, i.g.) significantly increased (P < 0.01, compared to N.S. group) NOE indicating successful inducement of memory retrieval disorders in mice. Piracetam (positive control) reversed this alcohol induced effect in mice as expected (P < 0.01, compared to model group). WSE (100, 200 and 400 mg/kg, i.g.) showed significant dose-dependent reduction (P < 0.01 compared to model group) of NOE in mice, which indicated that WSE exerted high activities in ameliorating the memory. Moreover, the moderate dosage (200 mg/kg) and high dosage of ZF (400 mg/kg) exhibited stronger effect than the piracetam at the dose of 1520 mg/kg (P < 0.01). In conclusion, WSE could ameliorate the memory in mice with alcohol induced memory retrieval disorders.
Effect of WSE on learning and memory in PCT
The passive avoidance test (PCT) was commonly conducted to investigate the long-term memory of mice.[32] To evaluate the effect of WSE, the values of TLT, NOE and EST were assayed in this research. The significant increase of TLT meant the ameliorating effect on the behavior in mice, and so did the significant decrease of NOE and EST.
As shown in Figure 3A (1-3), three dosages of WSE as well as piracetam increased the TLT significantly (P < 0.05), indicating some improving effect of WSE on learning capacity in normal mice. However, this effect was not verified by the assayed data of NOE and EST. As a result, it was yet not able to draw the conclusion that WSE could really exert specific effect on learning of normal mice in PCT.
Figure 3.
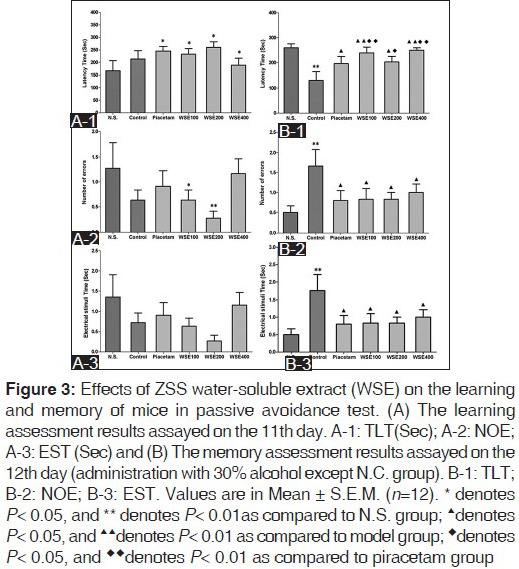
Figure 3B (1-3) showed the effect of WSE on the memory of mice with alcohol induced memory retrieval disorders. On the 12th day, 30% alcohol significantly shortened the TLT and increased of NOE and EST in model group (P < 0.01, compared to N.S. group) and piracetam could resist this impairment on memory of mice (P < 0.05, compared to model group). The low and high dosages of WSE (100 and 400 mg/kg) exerted remarkable improving effect on memory as indicated by the significant increase in the TLT (P < 0.01, compared to model group) and the effect was stronger than piracetam (P < 0.01). In addition, moderate dosage (200 mg/kg) could also increase the TLT (P < 0.05), which is equal to piracetam.
As to NOE and EST, WSE exhibited the same remarkable reduction effect on them (P < 0.05, compared to the model group). Moreover, there was no significant difference between WSE groups and piracetam group (P > 0.05), which illustrated three dosages of WSE exerted attenuating effect on learning capacity equal to piracetam.
DISCUSSION
From the present results, it was seen that WSE exerted specific ameliorating effect on the mice memory behavior both in Y-maze test and PCT. WSE showed a great potential as a new botanical dietary supplement for some memory disorders. Spinosin, JuA and JuB are demonstrated as the main constituents in the extract. It has been reported that they are the main bioactive components in ZSS, however, there has been no reports on their effect on ameliorating learning and memory. Therefore, the bioactivity mechanism of this extract requires further investigation to be elucidated. In addition, cyclopetide alkaloids (such as Sanjoine K and ZiZyphusine) and other saponin compounds (including Jujuboside A1 and Jujuboside B1) were the minor constituents responsible for the sedative bioactivity in ZSS.[3,27,33] These constituents might also contribute to the effect of ameliorating learning and memory.
In the study, the effect of ZSS water soluble extract on learning and memory in mice was investigated by Y-maze and passive avoidance test for the first time. The results indicated that water soluble extract had a promising benefit for ameliorating memory function in mice with alcohol induced memory retrieval disorders. In addition, with a new efficient HPLC-ESI-MS/MS method, three predominant components (spinosin, JuA and JuB) were assayed in the extract. Their total contents accounted for over 30% of the extract weight and they might be mainly responsible for this bioactivity. Collectively, the ZSS water soluble extract has a great prospect as a new botanical dietary supplement for certain memory disorders.
ACKNOWLEDGMENTS
The present research was financially supported by the National Natural Science Foundation of China (No.: 31101235, 31000749), and National Undergraduate Training Programs for Innovation and Entrepreneurship (No.: 201310069010).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Beijing: Press of Chemical Industry; 2010. Pharmacopoeia Commission of People's Republic of China. Pharmacopoeia of People's Republic of China (part 1; pp. 343–4. [Google Scholar]
- 2.Zhang MC, Zhang YQ, Xie JB. Simultaneous determination of jujuboside A, B and betulinic acid in semen Ziziphi spinosae by high performance liquid chromatography-evaporative light scattering detection. J Pharm Biomed Anal. 2008;48:1467–70. doi: 10.1016/j.jpba.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Cao JX, Zhang QY, Cui SY, Cui XY, Zhang J, Zhang YH, et al. Hypnotic effect of jujubosides from Semen Ziziphi Spinosae. J Ethnopharmacol. 2010;130:163–6. doi: 10.1016/j.jep.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Fang XS, Hao JF, Zhou HY, Zhu LX, Wang JH, Song FQ. Pharmacological studies on the sedative-hypnotic effect of Semen Ziziphi spinosae (Suanzaoren) and Radix et Rhizoma Salviae miltiorrhizae (Danshen) extracts and the synergistic effect of their combinations. Phytomedicine. 2010;17:75–80. doi: 10.1016/j.phymed.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Qiao W, Yang Y, Ren L, Sun Y, Wang S. Antidepressant-like effect of the ethanolic extract from Suanzaorenhehuan Formula in mice models of depression. J Ethnopharmacol. 2012;141:257–64. doi: 10.1016/j.jep.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Xie J, Guo L, Pang G, Wu X, Zhang M. Modulation effect of Semen Ziziphi Spinosae extracts on IL-1β, IL-4, IL-6, IL-10, TNF-α and IFN-γ in mouse serum. Nat Prod Res. 2011;25:464–7. doi: 10.1080/14786419.2010.534474. [DOI] [PubMed] [Google Scholar]
- 7.Xie J, Zhang Y, Wang L, Qi W, Zhang M. Composition of fatty oils from semen ziziphi spinosae and its cardiotonic effect on isolated toad hearts. Nat Prod Res. 2012;26:479–83. doi: 10.1080/14786419.2010.516433. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Lee HJ, Koh SB, Ban JY, Seong YH. Protection of NMDA -induced neuronal cell damage by methanol extract of Zizyphi Spinosi Semen in cultured rat cerebellar granule cells. J Ethnopharmacol. 2004;95:39–45. doi: 10.1016/j.jep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer's disease. J Intern Med. 2004;256:195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- 10.Crichton GE, Bryan J, Murphy KJ. Dietary antioxidants, cognitive function and dementia-A systematic review. Plant Foods Hum Nutr. 2013;68:279–92. doi: 10.1007/s11130-013-0370-0. [DOI] [PubMed] [Google Scholar]
- 11.Li BL, Fu ZY, Hu R, Chen YH, Zhang ZX. Semen Ziziphi Spinosae and Fructus Gardeniae extracts synergistically improve learning and memory of a mouse model. Biomed Rep. 2013;1:247–50. doi: 10.3892/br.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang JG, Huang XJ, Chen J, Lin QS. Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus jujube. Nat Prod Res. 2007;21:310–20. doi: 10.1080/14786410701192827. [DOI] [PubMed] [Google Scholar]
- 13.Wang LE, Cui XY, Cui SY, Cao JX, Zhang J, Zhang YH. Potentiating effect of spinosin, a C-glycoside flavonoid of Semen Ziziphi spinosae, on pentobarbital-induced sleep may be related to postsynaptic 5-HT1A receptors. Phytomedicine. 2010;17:404–9. doi: 10.1016/j.phymed.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 14.You ZL, Xia Q, Liang FR, Tang YJ, Xu CL, Huang J, et al. Effects on the expression of GABAA receptor subunits by jujuboside A treatment in rat hippocampal neurons. J Ethnopharmacol. 2010;128:419–23. doi: 10.1016/j.jep.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Yang B, Zhang A, Sun H, Yan G. Potential drug targets on insomnia and intervention effects of Jujuboside A through metabolic pathway analysis as revealed by UPLC/ESI-SYNAPT-HDMS coupled with pattern recognition approach. J Proteomics. 2012;75:1411–27. doi: 10.1016/j.jprot.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, You ZL, Xia Q, Xiong T, Xia Y, Yao DZ. Upregulation of Mark3 and Rpgrip1 mRNA expression by jujuboside A in mouse hippocampus. Acta Pharmacol Sin. 2007;28:334–8. doi: 10.1111/j.1745-7254.2007.00497.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Ning G, Shou C, Lu Y, Hong D, Zheng X. Inhibitory effect of jujuboside A on glutamate-mediated excitatory signal pathway in hippocampus. Planta Med. 2003;69:692–5. doi: 10.1055/s-2003-42786. [DOI] [PubMed] [Google Scholar]
- 18.Lu YJ, Zhou J, Zhang SM, Zhang HY, Zheng XX. Inhibitory effects of jujuboside A on EEG and hippocampal glutamate in hyperactive rat. J Zhejiang Univ Sci B. 2005;6:265–71. doi: 10.1631/jzus.2005.B0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang Y, Zhang K, Ma G, Zhang M, Xie J. Degradation kinetics of Jujuboside B by rat intestinal flora in vitro with an RRLC-MS-MS method. J Chromatogr Sci. 2014;52:691–6. doi: 10.1093/chromsci/bmt100. [DOI] [PubMed] [Google Scholar]
- 20.Haider S, Batool Z, Tabassum S, Perveen T, Saleem S, Naqvi F, et al. Plant effects of walnuts (Juglans regia) on learning and memory functions. Foods Hum Nutr. 2011;66:335–40. doi: 10.1007/s11130-011-0260-2. [DOI] [PubMed] [Google Scholar]
- 21.Yau SY, Lau BW, Tong JB, Wong R, Ching YP, Qiu G, et al. Hippocampal neurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PLoS One. 2011;6:e24263. doi: 10.1371/journal.pone.0024263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Kim JM, Park SJ, Lee S, Shin CY, Cheong JH, et al. Hippocampal extracellular signal-regulated kinase signaling has a role in passive avoidance memory retrieval Induced by GABAA receptor modulation in mice. Neuropsychopharmacology. 2012;37:1234–44. doi: 10.1038/npp.2011.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao KD, Li P, Qi LW, Li HJ, Yi L, Wang W, et al. Characterization of flavonoid metabolites in rat plasma, urine, and feces after oral administration of Semen Ziziphi Spinosae extract by HPLC-diode-array detection (DAD) and ion-trap mass spectrometry (MS (n)) Chem Pharm Bull. 2009;57:144–8. doi: 10.1248/cpb.57.144. [DOI] [PubMed] [Google Scholar]
- 24.Paris JJ, Walf AA, Frye CA. II. Cognitive performance of middle-aged female rats is influenced by capacity to metabolize progesterone in the prefrontal cortex and hippocampus. Brain Res. 2011;1379:149–63. doi: 10.1016/j.brainres.2010.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murnane KS, Perrine SA, Finton BJ, Galloway MP, Lee HL, Fantegrossi WE. Effects of exposure to amphetamine derivatives on passive avoidance performance and the central levels of monoamines and their metabolites in mice: Correlations between behavior and neurochemistry. Psychopharmacology (Berl) 2012;220:495–508. doi: 10.1007/s00213-011-2504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radahmadi M, Alaei H, Sharifi MR, Hosseini N. The effect of synchronized running activity with chronic stress on passive avoidance learning and body weight in rats. Int J Prev Med. 2013;4:430–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Chen B, Yao S. Simultaneous analysis and identification of main bioactive constituents in extract of Zizyphus jujuba var. sapinosa (Zizyphi spinosi semen) by high-performance liquid chromatography-photodiode array detection-electrospray mass spectrometry. Talanta. 2007;71:668–75. doi: 10.1016/j.talanta.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Choi SH, Ahn JB, Kozukue N, Levin CE, Friedman M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J Agric Food Chem. 2011;59:6594–604. doi: 10.1021/jf200371r. [DOI] [PubMed] [Google Scholar]
- 29.Ma JJ, Kang LP, Zhou WB, Yu HS, Liu P, Ma BP. Identification and characterization of saponins in extract of Ziziphi spinosae Semen (ZSS) by ultra-performance liquid chromatography-electrospray ionization-quadrupole time-of-ight tandem mass spectrometry(UPLCESIQTOFMSE) J Med Plants Res. 2011;5:61529. [Google Scholar]
- 30.Depue BE. A neuroanatomical model of prefrontal inhibitory modulation of memory retrieval. Neurosci Biobehav Rev. 2012;36:1382–99. doi: 10.1016/j.neubiorev.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews-Zwilling Y, Gillespie AK, Kravitz AV, Nelson AB, Devidze N, Lo I, et al. Hilar GABAergic interneuron activity controls spatial learning and memory retrieval. PLoS One. 2012;7:e40555. doi: 10.1371/journal.pone.0040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heo HJ, Kim DO, Shin SC, Kim MJ, Kim BG, Shin DH. Effect of Antioxidant flavanone, naringenin, from Citrus junos on neuroprotection. J Agric Food Chem. 2004;52:1520–5. doi: 10.1021/jf035079g. [DOI] [PubMed] [Google Scholar]
- 33.Han BH, Park MH, Park JH. Chemical and pharmacological studies on sedative cyclopeptide alkaloids in some Rhamnaceae plants. Pure Appl Chem. 1989;61:443–8. [Google Scholar]


