Abstract
Background:
Fruits of Lycium ruthenicum Murr is a health food and also used as a folk to treat heart disease, abnormal menstruation and menopause in Tibetan, China. However; whether L. ruthenicum Murr fruits methanolic extracts (LFME) protect LLC-PK1 porcine renal tubules cells from AAPH-induced oxidative damage has not been investigated.
Objective:
To investigate the protective effects of L. ruthenicum Murr fruits methanolic extracts (LFME) against 2, 2’- azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidative damage in renal proximal tubule LLC-PK1 cells.
Materials and Methods:
LLC-PK1 cells were co-incubated with AAPH (1mM) and different concentrations of LFMW together for 24 h. Cell viability was determined by MTT assay. Total intercellular reactive oxygen species (ROS) levels and lipid peroxidation were measured using a fluorescent probe 2’, 7’-dichlorfluorescein-diacetate (DCFH-DA) and the TBA reactive substance (TBARS) assay, respectively. The endogenous antioxidant enzymes including catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-px) and intercellular glutathione (GSH) levels were determined using commercial assay kits according to the manufacturer's instructions.
Results:
LFME did not show a significant cytotoxic effect and increased the viability of LLC-PK1 cells in a concentration-dependent manner. LFME also decreased the total intercellular levels of ROS, reduced lipid peroxidation and increased the GSH levels as well as the activities of endogenous antioxidant enzymes to protect LLC-PK1 cells against AAPH-induced oxidative damage.
Conclusion:
The results from the present study indicated that LFME is an effective ROS scavenger to protect LLC-PK1 cells against AAPH-induced oxidative damage through decreasing ROS generation, reducing lipid peroxidation and up-regulation of endogenous GSH levels and antioxidant enzymes.
Keywords: Antioxidant enzymes, LLC-PK1 cells, Lycium ruthenicum Murr, oxidative stress
INTRODUCTION
Chronic kidney disease (CKD) is a common and serious public health problem that adversely affects human health, limits longevity, increase costs to health-care system worldwide and poor prognosis of morbidity and mortality.[1] Some evidences indicated that the prevalence of CKD gradual increase is accounted for about 7 to 11.5% in general population worldwide.[2,3] Reactive oxygen species (ROS) are produced in mammalian cells during energy production in mitochondria by reducing oxygen during aerobic respiration, including superoxide (O2-), peroxide (O2-2), hydroxyl radical(·OH), hydrogen peroxide (H2O2) and peroxynitrite (ONOO-) play a key role in the pathogenesis of cell damage in renal-related diseases such as acute renal failure, rhabdomyolysis, obstructive nephropathy, hyperlipidemia and glomerular damage to chronic renal failure and hemodialysis.[1,2,3,4,5,6,7] In generally, the accumulated ROS are eliminated by endogenous antioxidant defense system including antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-px) and glutathione S-transferase (GST) and other low molecular weight antioxidants such as glutathione, vitamin C and vitamin E.[8] In addition, dietary intake of foods rich in antioxidants, such as phenolic compounds is able to increase the levels of antioxidants in the body and improve the antioxidant defense system to reduce the risk of renal dysfunction induced by ROS.[4,9]
Lycium ruthenicum Murr is a perennial frutex native to northwest part of China, which grows widely distributes in Qinghai-Tibet plateau, central Asia and Caucasia.[10] L. ruthenicum Murr is rich in proteins, polysaccharides, unsaturated fats, flavones, amino acids, essential elements, pigments, ascorbic acid and tocopherols.[11,12] The ripe fruit of L. ruthenicum Murr has been recorded as a folk medicine used for treatment of heart disease, abnormal menstruation and menopause in Tibetan medical classic “Jing Zhu Ben Cao” and “Si Bu Yao Dian”.[10,13] Recently, some studies have reported that the fruits of L. Murr was rich in anthocyanins, in particular pentunidin-3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside and petundin-3-O-galactoside-5-O-glucoside.[10,14] L. ruthenicum Murr also showed anti-tumor, hypolipidemic and antioxidant effects.[10,13,14] However, the beneficial activities of L. ruthenicum Murr have not been studied. The present study was designed to investigate the ability of L. ruthenicum Murr to provide protection against 2, 2′-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidative damage compared in LLC-PK1 cells, an epithelial cell line derived from pig kidney that has retained characteristics of the proximal tubular epithelium and is wildly used as a cell model to study ROS-induced renal damage.[15] Additionally, the mechanisms underlying these effects were elucidated.
MATERIALS AND METHODS
Chemicals and reagents
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT), 2’, 7’- dichlorodihydrofluorescin diacetate (DCFH-DA), epigallocatechin gallate (EGCG) and 2,2’- azobis (2-amidinopropane) dihydrochloride (AAPH) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin solution and 0.05% trypsin-0.53mM EDTA were purchased from Grand Island Biological Company (Grand Island, NY, USA). Other chemicals used were of standard analytical grade.
Plant extracts preparation
The wildly fresh fruits of Lycium ruthenicum Murr were purchased form a local market in Terim, Xinjiang, China in October 2012. The collected samples were stored in a heat preservation box with efficient ice bag in accordance with the method distributed by Zheng et al.[10] Two hundred grams of fresh L. ruthenicum Murr fruits were freeze dried and then ground to a fine powder. A tenth-fold volume of methanol (80%, v/v) was added to the powdered samples and extracted third by stirring overnight. L. ruthenicum Murr fruits methanolic extracts (LFME) were concentrated by heat evaporation, freeze-drying and stored at -20°C until further study.
Cell culture
LLC-PK1 porcine renal tubules cells (ATCC® CL-101™) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). The cells were routinely maintained in DMEM medium supplemented with FBS (10%, v/v) and 1% penicillin-streptomycin in a humidified CO2 incubator (model 3154; Forma Scientific, Inc., Marietta, OH, USA) with 5% CO2 at 37°C.
Cell treatment and viability assay
Cell viability was determined by an MTT assay. LLC-PK1 cells were seeded in 96-well plates (Nunc, Rochester, NY, USA) at a density of 1 × 104 cells/well. The cells were incubated with the LFME (10, 25, 50 and 100 μg/ml) and AAPH (1mM) together for 24 h. After incubation, 100 μl MTT reagent (0.5 mg/ml) was added to each well and the cells were incubated in a humidified incubator at 37°C to allow the MTT to be metabolized. After 4 h, 100 μl DMSO was added to each well to dissolve the formazan crystals. Absorbance of the samples was measured at a wavelength of 490 nm using a microplate reader (model 680; Bio-Rad, Hercules, CA, USA).
Measurement of lipid peroxidation
Lipid peroxidation was quantitated using a TBA reactive substance (TBARS) assay.[16] Briefly, the cultured cells were washed with cooled phosphate-buffered saline (PBS), scraped into TCA (2.8%, w/v) and sonicated 3 times for 10 second intervals at 40 V setting over ice. The suspension (200 μl) was mixed with 1 ml TBA (0.67%, w/v) and 1 ml TCA (25%, w/v), heated (30 min at 95°C) and centrifuged (3,000 × g for 10 min at 4°C). The TBA reacted with the products of lipid oxidative degradation, yielding red complexes. Absorbance was measured at 532 nm using a UV-2401PC spectrophotometer (Shimadzu, Kyoto, Japan). Protein contents were determined using a Thermo scientific pierce bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA) according to the manufacturer's instructions.
Assessment of total intracellular ROS generation
Intracellular ROS levels were measured using the fluorescent probe DCFH-DA. The treated LLC-PK1 cells were washed with calcium- and magnesium-free PBS and incubated in DCFH-DA (20 μM) containing serum- and phenol red-free DMEM at 37°C for 30 min. The medium was then removed and the cells were washed twice with PBS. Fluorescence was measured using a FLUOstar OPTIMA fluorescence plate reader (BMG Labtech, Ortenberg, Germany); excitation was read at 485 nm and emission was detected at 535 nm. Relative ROS production was expressed as the percentage of fluorescence for the treated samples over fluorescence for the appropriate controls: (fluorescence treatment/fluorescence control) ×100.
Antioxidant enzyme activity and GSH levels
LLC-PK1 cells grown in a 10-cm cell culture dish (Nunc, Rochester, NY, USA) were incubated with LFME and AAPH (1mM) for 24 h at 37°C. The cells were washed with PBS, removed by scraping and centrifuged (3,000 × g for 10 min at 4°C). The resulting cell pellet was stored at -80°C. The pellets were then thawed, resuspended in 300 μl cold lysis buffer (PBS and 1mM EDTA), homogenized and centrifuged (12,000 × g for 10 min at 4°C). Protein contents were determined using a Thermo scientific pierce BCA protein assay kit (Pierce, Rockford, IL, USA) according to the manufacturer's instructions. Cellular catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-px) and glutathione (GSH) levels were determined using commercial assay kits (Beyotime Institute of Biotechnology, Jiangsu, China) according to the manufacturer's instructions. Enzyme activities and GSH contents were expressed as units (U) of enzyme activity and μmol of GSH per mg protein, respectively.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Differences between the mean values for individual groups were assessed with a one-way ANOVA and Duncan's multiple range tests. P < 0.05 were considered statistically significant. The SAS v9.1 statistical software package (SAS Institute Inc., Cary, NC, USA) was used to conduct the analysis.
RESULTS
Cell viability
To determine the potential cytotoxic activity of LFME, LLC-PK1 cells were first treated with the LFME (10, 25, 50 and 100 μg/ml) for 24 h and cell viability was measured with an MTT assay. LFME did not showed significantly cytotoxicity in LLC-PK1 cells (cell viability >90%, data not shown). Therefore, concentrations of 10, 25, 50 and 100 μg/ml were used for further studies. As shown in Figure 1, AAPH (1mM) induced death of the LLC-PK cells (cell viability was 44.2%). LFME significantly reduced the AAPH-induced LLC-PK1 cell damage in a concentration-dependent manner (P < 0.05). At 100 μg/ml, LFME had showed the highest cell viability (81.61%) and it weaker than that in 10 μg/ml EGCG-treated cells (cell viability was 94.5%).
Figure 1.
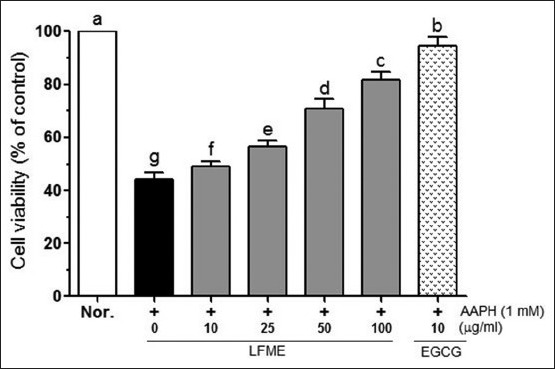
Effects of Lycium ruthenicum Murr fruits methanolic extracts (LFME) on cell viability in 1 mM 2, 2’-azobis(2-amidinopropane) dihydrochloride-treated LLC-PK1 cells. Data represent mean ± SD. a~fMean values with different letters on the bars are significantly different (P<0.05) according to Duncan's multiple range test. EGCG: Epigallocatechin gallate
Total intercellular ROS concentration
As shown in Figure 2, AAPH significantly increased the total intercellular ROS levels (to 250.9%) compared to that in normal cells. In the presence of AAPH, LFME significantly reduced ROS generation in a contraction-dependent manner (P < 0.05). Following treatment with 10 μg/ml of EGCG completely prevented the increased ROS levels induced by AAPH compared with that in 100 μg/ml LFME treated cells. These results suggest that LFME is an effective ROS scavenger.
Figure 2.
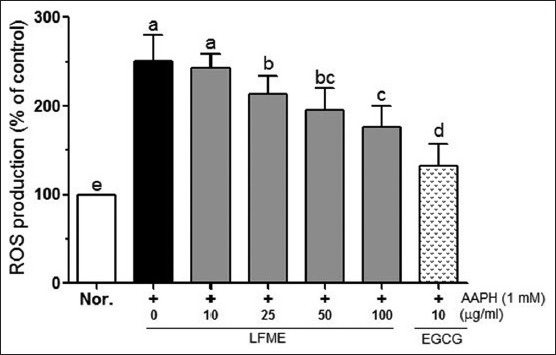
Effects of Lycium ruthenicum Murr fruits methanolic extracts (LFME) on reactive oxygen species in 1 mM 2, 2’-azobis (2-amidinopropane) dihydrochloride-treated LLC-PK1 cells. Data represent mean ± SD. a~eMean values with different letters on the bars are significantly different (P< 0.05) according to Duncan's multiple range test. EGCG: Epigallocatechin gallate
Lipid peroxidation levels
As shown in Figure 3, AAPH significantly increased the MDA generation (to 1.49 nmol/mg protein) compared to the untreated cells (0.35 nmol/mg protein). However, treatment with LFME reduced MDA generation in a concentration-dependent manner. Cells treated with 10 μg/ml EGCG exhibited significantly decreased MDA levels (0.62 nmol/mg protein) than that in 100 μg/ml LFME-treated LLC-PK1 cells (0.70 nmol/mg protein).
Figure 3.
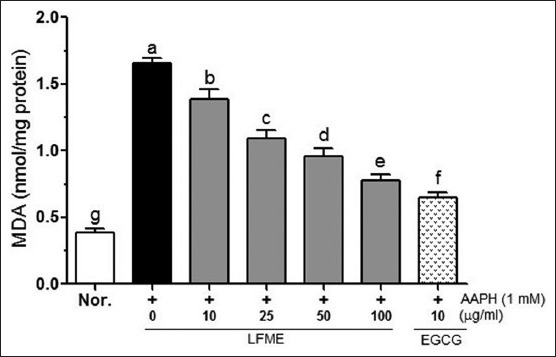
Effects of Lycium ruthenicum Murr fruits methanolic extracts on malondialdehyde in 1 mM 2, 2’-azobis (2-amidinopropane) dihydrochloride-treated LLC-PK1 cells. Data represent mean ± SD. a~gMean values with different letters on the bars are significantly different (P<0.05) according to Duncan's multiple range test. EGCG: Epigallocatechin gallate
Intercellular GSH levels
As shown in Figure 4, the intercellular levels of GSH were significantly (P < 0.05) decreased by 1 mM AAPH. Following treatment with different concentrations of LFME (10, 25, 50 and 100 μg/ml) was able to reduce the AAPH-induced decreasing of GSH in LLC-PK1 cells. 10 μg/ml EGCG (2.13 μmol/mg protein) showed a better activity to increase the GSH levels than that found in 100 μg/ml LFME-treated cells (1.98 μmol/mg protein).
Figure 4.
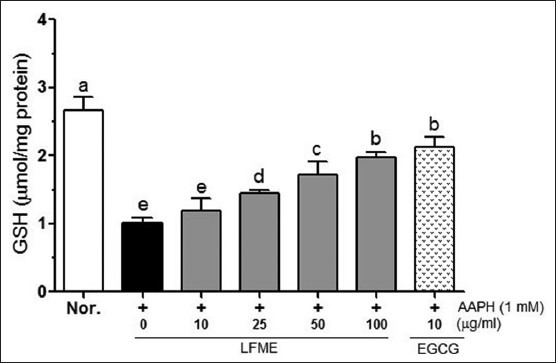
Effects of Lycium ruthenicum Murr fruits methanolic extracts on glutathione in 1 mM 2,2’-azobis(2-amidinopropane) dihydrochloride-treated LLC-PK1 cells. Data represent mean ± SD. a~eMean values with different letters on the bars are significantly different (P< 0.05) according to Duncan's multiple range test. EGCG: Epigallocatechin gallate
Antioxidant enzyme activities
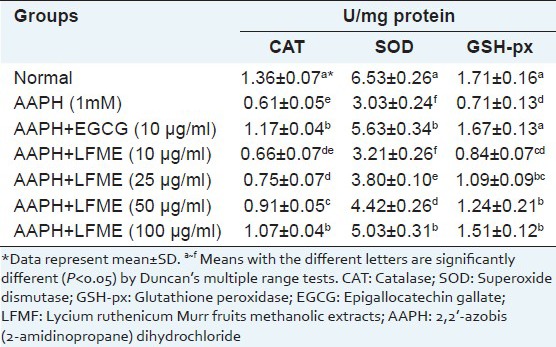
The effect of LFME on antioxidant enzyme activities in AAPH-treated LLC-PK1 cells are shown in Table 1. APPH (1 mM) significantly (P < 0.05) decreased CAT activity (to 0.61 U/mg protein) in LLC-PK1 cells compared to untreated cells (1.36 U/mg protein). Co-incubated with different concentrations of LFME effectively increased the CAT activity from 0.66 to1.07 U/mg protein in LLC-PK1 cells treated with AAPH. In addition, 10 μg/ml EGCG markedly increased the CAT activity (1.17 U/mg protein) than that in 100 μg/ml LFME-treated cells. SOD activity in the LLC-PK1 cells treated with 1mM AAPH was also significantly (P < 0.05) decreased (3.03 U/mg protein) compared to the untreated cells (6.53 U/mg protein). Co-incubated with the different concentrations of LFME for 24 h, the SOD activity was increased from 3.21 to 5.03 U/mg proteins in AAPH-treated LLC-PK1 cells. At a concentration of 100 μg/ml, LFME helped restore SOD activity (5.03 U/mg protein), but its activity was weaker than that in cells treated with 10 μg/ml EGCG (5.63 U/mg protein). GSH-px activity was significantly reduced by 1 mM AAPH (0.71 U/mg protein) compared to the untreated LLC-PK1 cells (1.71 U/mg protein). Treatment with LFME was concentration-dependently increased the GSH-px activity in AAPH-treated LLC-PK1 cells. 10 μg/ml EGCG was effectively increased the GSH-px (1.67U/mg protein) than that in cells treated with 100 μg/ml LFME (1.51 U/mg protein).
Table 1.
Effect of Lycium ruthenicum Murr fruits methanolic extracts on the levels of catalase, superoxide dismutase and glutathione peroxidase in 2,2’-azobis (2-amidinopropane) dihydrochloride-treated LLC-PK1 cells
DISCUSSION
The fruits of L. ruthenicum Murr are not only as a health food but also as a folk medicine used for treatment of heart disease, abnormal menstruation and menopause in Tibetan, China. The fruits of L. ruthenicum Murr are rich in vitamin C, γ-vitamin E, δ-vitamin E and antnocyannins.[11,12] Cheng et al.,[12] also reported the fruits of L. ruthenicum Murr contained high levels of poly-unstructured fatty acids (PUFAs) including linoleic acid and oleinic acid. However, whether L. ruthenicum Murr fruits methanolic extracts (LFME) protect LLC-PK1 porcine renal tubules cells from AAPH-induced oxidative damage has not been investigated. Therefore, the present study was to investigate the potential protective effect of LFME in AAPH-stimulated LLC-PK1 cells.
Many studies have suggested that ROS-induced oxidative stress plays an important role in the pathogenesis of chronic kidney disease.[1,6,7] AAPH is a well-known ROS donor, which generates peroxyl radicals and molecular oxygen, eventually causing the oxidation of some biomacromolecules (such as lipid, protein and DNA) in living organs.[17] In the present study, incubation with 24 h of AAPH (1 mM) significantly induced the oxidative damage and also increasing the total intercellular ROS generations and lipid peroxidation levels (MDA productions) in LLC-PK1 cells. However, co-incubated with different concentrations (10, 25, 50 and 100 μg/ml) of LFME significantly reduced the AAPH-induced oxidative damage in LLC-PK1 cells [Figure 1].
Attenuation of the intercellular ROS levels by treatment with some antioxidants such as vitamin E, omega-3 PUFAs, N-acetyl cysteine (NAC), allopurinol and coenzyme Q10 are able to prevent and control the oxidative stress-induced renal diseases.[1] In order to evaluate the role of the ROS in the protective activity of LFME, the effect on AAPH-induced ROS generations was analyzed using a H2DCF-DA assay. We observed LFME concentration-dependently reduced the total intercellular ROS levels in LLC-PK1 cells treated with AAPH [Figure 2]. Furthermore, treatment with EGCG, epigallocatechin (EGC), curcumin and Brassica rapa root extracts are able to reduce the ROS generation to protect oxidative stress-induced cell damage in LLC-PK1cells.[18,19,20,21] Our results suggested that the protective effect of LFME may associate with its great ROS scavenger activity.
3, 4-methylenedioxyamphetamine (MDA), a stable end product of the reaction between free radicals and cellular PUFAs, is an appropriate factor for evaluating the degree of ROS-induced cell damage.[22] Increasing of MDA levels has been reported frequently associated with the oxidative stress-induced renal cell damage in CKD such as end-stage renal failure.[23,24,25] Previous studies have demonstrated that AAPH is closely linked with increased peroxidation of lipid in cell membrane not only in renal cells[17] but also in other cells including rat basophile leukemia cells,[26] and human cell lines including red blood cells,[27] intestinal Caco-2 cells[28] and liver cells.[29] We found co-incubated with LFME concentration-dependently reduced the AAPH-induced MDA production in LLC-PK1 cells [Figure 3]. It is noticed that 10 μg/ml EGCG showed a better activity to inhibit MDA generation than that in 100 μg/ml LFME-treated LLC-PK1 cells. Inhibition of MDA generation is able to attenuate AAPH-induced oxidative damage in LLC-PK1 cells.[17] These results indicate that LFME reduced ROS-induced lipid peroxidation in AAPH-treated LLC-PK1 cells.
Kidney is an important organ to serve homeostatic functions such as maintaining acid-base balance, regulation of electrolytes and regulation of blood pressure. Under normal physiological conditions, the kidney is easy to generate ROS including O2-, H2O2, ONOO- and ·OH.[8] The accumulated ROS is able to eliminate by endogenous antioxidant enzymes such as CAT, SOD, GSH-px and GST and non-enzymatic low molecular weight antioxidants such as GSH, vitamin C and vitamin E.[8] GSH is a main endogenous non-enzyme antioxidant in renal proximal tubules.[30] The depletion of intercellular GSH may increase MDA generations and decreased the activities of endogenous antioxidant enzymes such as SOD, CAT, GSH reductase (GR) and GSH-px.[31,32] Maintaining the normal levels of GSH is able to restore cellular antioxidant defense system, block lipid peroxidation and protect renal cells to against nephrotoxic agents (such as cisplatin, H2O2)-induced oxidative stress.[21,33] In the present study, we found co-incubated with the different concentrations of LFME significantly increased the GSH levels in LLC-PK1 cells treated with AAPH [Figure 4]. The normal levels of intercellular GSH is useful to enhance the activity of protective enzymes NAD (P) H: Quinine oxidoreductase 1(NQO1) and γ-glutamyl cysteine ligase(γGCL) and maintaining the normal function of mitochondria to protect LLC-PK1 cells against ROS-induced oxidative damage.[34,35]
In addition, we also found LFME attenuated the decrease in the activity of endogenous antioxidant enzymes CAT, SOD and GSH-px in LLC-PK1 cell exposed to AAPH for 24 h [Table 1]. SOD catalyzes the conversion of O2- into H2O2; the H2O2 is further reduced to H2O by CAT and GSH-px.[36] Over expression of SOD was able to protect human embryonic kidney 293 (HEK 293) cells against ROS-induced oxidative damage.[37] Treatment with CAT and SOD helps prevent ROS-induced damage in LLC-PK1 cells.[38,39] Treatment with some nature plant extracts is able to increase the activity of antioxidants enzymes such as SOD, CAT, GSH-px and GR to against ROS-induced oxidative damage in LLC-PK1 cells and in rats, respectively.[21,40]
In conclusions, the results from the present study clearly demonstrated that LFME could protect LLC-PK1cells against AAPH-induced oxidative damage by modulating ROS generation, MDA production and GSH levels, as well as up-regulated endogenous antioxidant enzyme activities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology. 2012;17:311–21. doi: 10.1111/j.1440-1797.2012.01572.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen N, Wang W, Huang Y, Shen P, Pei D, Yu H, et al. Community-based study on CKD subjects and the associated risk factors. Nephrol Dial Transplant. 2009;24:2117–23. doi: 10.1093/ndt/gfn767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: The AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55:660–70. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Singh D, Kaur R, Chander V, Chopra K. Antioxidants in the prevention of renal disease. J Med Food. 2006;9:443–50. doi: 10.1089/jmf.2006.9.443. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez F, Bonacasa B, Fenoy FJ, Salom MG. Reactive oxygen and nitrogen species in the renal ischemia/reperfusion injury. Curr Pharm Des. 2013;19:2776–94. doi: 10.2174/1381612811319150014. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigo R, Bosco C. Oxidative stress and protective effects of polyphenols: Comparative studies in human and rodent kidney. A review. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142:317–27. doi: 10.1016/j.cbpc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;74:S4–9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- 8.Araujo M, Welch WJ. Oxidative stress and nitric oxide in kidney function. Curr Opin Nephrol Hypertens. 2006;15:72–7. doi: 10.1097/01.mnh.0000191912.65281.e9. [DOI] [PubMed] [Google Scholar]
- 9.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng J, Ding C, Wang L, Li G, Shi J, Li H, et al. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem. 2011;126:859–65. [Google Scholar]
- 11.Jia XL, Chi XF, Dong Q, Xiao YC, Hu FZ. Analysis of the nutritional components of lycium ruthenicum. Amino Acids Biotic Resour. 2011;33:60–2. [Google Scholar]
- 12.Cheng HJ, Hou XJ, Bai HJ, Kong XY. Analysis of several nutritional compositions from lycium ruthenicum. Chin Wild Plant Resour. 2002;21:55. [Google Scholar]
- 13.Chen C, Yun S, Tao Y, Mei L, Shu Q, Wang L. Main anthocyanins compositions and corresponding H-ORAC assay for wild Lycium ruthenicum Murr. fruits from the Qaidam Basin. J Pharm Tech Drug Res. 2013;2:1–5. [Google Scholar]
- 14.Liu Z, Dang J, Wang Q, Yu M, Jiang L, Mei L, et al. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int J Biol Macromol. 2013;61:127–34. doi: 10.1016/j.ijbiomac.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 15.Perantoni A, Berman JJ. Properties of Wilms’ tumor line (TuWi) and pig kidney line (LLC-PK1) typical of normal kidney tubular epithelium. In vitro. 1979;15:446–54. doi: 10.1007/BF02618414. [DOI] [PubMed] [Google Scholar]
- 16.Fraga CG, Leibovitz BE, Tappel AL. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: Characterization and comparison with homogenates and microsomes. Free Radic Biol Med. 1988;4:155–61. doi: 10.1016/0891-5849(88)90023-8. [DOI] [PubMed] [Google Scholar]
- 17.Yokozawa T, Cho EJ, Hara Y, Kitani K. Antioxidative activity of green tea treated with radical initiator 2, 2’-azobis (2-amidinopropane) dihydrochloride. J Agric Food Chem. 2000;48:5068–73. doi: 10.1021/jf000253b. [DOI] [PubMed] [Google Scholar]
- 18.Yokozawa T, Nakagawa T, Lee KI, Cho EJ, Terasawa K, Takeuchi S. Effects of green tea tannin on cisplatin - induced nephropathy in LLC-PK1 cells and rats. J Pharm Pharmacol. 1999;51:1325–31. doi: 10.1211/0022357991776912. [DOI] [PubMed] [Google Scholar]
- 19.Cohly HH, Taylor A, Angel MF, Salahudeen AK. Effect of turmeric, turmerin and curcumin on H2O2-induced renal epithelial (LLC-PK1) cell injury. Free Radic Biol Med. 1998;24:49–54. doi: 10.1016/s0891-5849(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 20.Costa S, Utan A, Cervellati R, Speroni E, Guerra M. Catechins: Natural free-radical scavengers against ochratoxin A-induced cell damage in a pig kidney cell line (LLC-PK1) Food Chem Toxicol. 2007;45:1910–7. doi: 10.1016/j.fct.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Kim YH, Kim YW, Oh YJ, Back NI, Chung SA, Chung HG, et al. Protective effect of the ethanol extract of the roots of Brassica rapa on cisplatin-induced nephrotoxicity in LLC-PK 1 cells and rats. Biol Pharm Bull. 2006;29:2436–41. doi: 10.1248/bpb.29.2436. [DOI] [PubMed] [Google Scholar]
- 22.Salahudeen AK. Role of lipid peroxidation in H2O2-induced renal epithelial (LLC-PK1) cell injury. Am J Physiol. 1995;268:F30–8. doi: 10.1152/ajprenal.1995.268.1.F30. [DOI] [PubMed] [Google Scholar]
- 23.Dounousi E, Papavasiliou E, Makedou A, Ioannou K, Katopodis KP, Tselepis A, et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis. 2006;48:752–60. doi: 10.1053/j.ajkd.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Atamer A, Kocyigit Y, Ecder SA, Selek S, Ilhan N, Ecder T, et al. Effect of oxidative stress on antioxidant enzyme activities, homocysteine and lipoproteins in chronic kidney disease. J Nephrol. 2008;21:924–30. [PubMed] [Google Scholar]
- 25.Karamouzis I, Sarafidis PA, Karamouzis M, Iliadis S, Haidich AB, Sioulis A, et al. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am J Nephrol. 2007;28:397–404. doi: 10.1159/000112413. [DOI] [PubMed] [Google Scholar]
- 26.Imai H, Sumi D, Sakamoto H, Hanamoto A, Arai M, Chiba N, et al. Overexpression of phospholipid hydroperoxide glutathione peroxidase suppressed cell death due to oxidative damage in rat basophile leukemia cells (RBL-2H3) Biochem Biophys Res Commun. 1996;222:432–8. doi: 10.1006/bbrc.1996.0762. [DOI] [PubMed] [Google Scholar]
- 27.Zou CG, Agar NS, Jones GL. Oxidative insult to human red blood cells induced by free radical initiator AAPH and its inhibition by a commercial antioxidant mixture. Life Sci. 2001;69:75–86. doi: 10.1016/s0024-3205(01)01112-2. [DOI] [PubMed] [Google Scholar]
- 28.Elisia I, Kitts DD. Anthocyanins inhibit peroxyl radical-induced apoptosis in Caco-2 cells. Mol Cell Biochem. 2008;312:139–45. doi: 10.1007/s11010-008-9729-1. [DOI] [PubMed] [Google Scholar]
- 29.Kusumoto C, Kinugawa T, Morikawa H, Teraoka M, Nishida T, Murawaki Y, et al. Protection by exogenously added coenzyme Q9 against free radical-induced injuries in human liver cells. J Clin Biochem Nutr. 2010;46:244–51. doi: 10.3164/jcbn.09-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnellmann RG. Renal mitochondrial glutathione transport. Life Sci. 1991;49:393–8. doi: 10.1016/0024-3205(91)90447-j. [DOI] [PubMed] [Google Scholar]
- 31.Mistry P, Merazga Y, Spargo DJ, Riley PA, McBrien DC. The effects of cisplatin on the concentration of protein thiols and glutathione in the rat kidney. Cancer Chemother Pharmacol. 1991;28:277–82. doi: 10.1007/BF00685535. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JG, Lindup WE. Role of mitochondria in cisplatin-induced oxidative damage exhibited by rat renal cortical slices. Biochem Pharmacol. 1993;45:2215–22. [PubMed] [Google Scholar]
- 33.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 34.Santos NA, Catão CS, Martins NM, Curti C, Bianchi ML, Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 2007;81:495–504. doi: 10.1007/s00204-006-0173-2. [DOI] [PubMed] [Google Scholar]
- 35.Guerrero-Beltrán CE, Calderón-Oliver M, Martínez-Abundis E, Tapia E, Zarco-Márquez G, Zazueta C, et al. Protective effect of sulforaphane against cisplatin-induced mitochondrial alterations and impairment in the activity of NAD (P) H: Quinone oxidoreductase 1 and γ glutamyl cysteine ligase: Studies in mitochondria isolated from rat kidney and in LLC-PK1 cells. Toxicol Lett. 2010;199:80–92. doi: 10.1016/j.toxlet.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, et al. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 37.Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates cisplatin-induced renal injury: Importance of superoxide. J Am Soc Nephrol. 2001;12:2683–90. doi: 10.1681/ASN.V12122683. [DOI] [PubMed] [Google Scholar]
- 38.Thamilselvan S, Byer KJ, Hackett RL, Khan SR. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol. 2000;164:224–9. [PubMed] [Google Scholar]
- 39.Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res. 2005;33:349–57. doi: 10.1007/s00240-005-0492-4. [DOI] [PubMed] [Google Scholar]
- 40.Kim YH, Choi JH, Rim HK, Kang HJ, Chang SG, Park JH, et al. 23-Hydroxytormentic acid and Niga-Ichgoside F 1 isolated from rubus coreanus attenuate cisplatin-induced cytotoxicity by reducing oxidative stress in renal epithelial LLC-PK 1 Cells. Biol Pharm Bull. 2011;34:906–11. doi: 10.1248/bpb.34.906. [DOI] [PubMed] [Google Scholar]


