Abstract
Background:
In traditional Chinese medicine (TCM), raw and processed herbs are used to treat the different diseases. Fructus Arctii, the dried fruits of Arctium lappa l. (Compositae), is widely used in the TCM. Stir-frying is the most common processing method, which might modify the chemical compositions in Fructus Arctii.
Materials and Methods:
To test this hypothesis, we focused on analysis and identification of the main chemical constituents in raw and processed Fructus Arctii (PFA) by high-performance liquid chromatography/diode array detection-electrospray ionization-mass spectrometry.
Results:
The results indicated that there was less arctiin in stir-fried materials than in raw materials. however, there were higher levels of arctigenin in stir-fried materials than in raw materials.
Conclusion:
We suggest that arctiin reduced significantly following the thermal conversion of arctiin to arctigenin. In conclusion, this finding may shed some light on understanding the differences in the therapeutic values of raw versus PFA in TCM.
Keywords: Chemical constituents, Fructus Arctii, high-performance liquid chromatography/diode array detection-electrospray ionization-mass spectrometry, stir-frying
INTRODUCTION
Fructus Arctii (Niubangzi in Chinese), the dried fruits of Arctium lappa L. (Compositae) is one of the most popular traditional Chinese medicines (TCMs) and is officially listed in the Chinese Pharmacopoeia.[1] It has been widely used in herbal medicine for dispelling pathogenic wind-heat, promoting eruption, relieving sore throat, removing toxic substances and subduing swelling.[1] The major components of Fructus Arctii are lignans, i.e., arctiin and arctigenin.[2] Pharmacological functions of Fructus Arctii are closely related to these lignans such as the antagonistic effect on the platelet activating factor receptor,[3] anti-proliferative,[4] and anticarcinogenesis[5] activities.
The processing of TCMs is a common practice before most herbs are prescribed.[6] There are a variety of traditional ways for processing herbs such as stir-frying with sand or oil, sauteing with rice wine or wheat bran, steaming with water or rice wine, and braising with rice wine or licorice liquids, etc.[7] According to the theory of TCM, the main purposes of the herb processing are to increase activity, reduce toxicity and alter effects.[8] The main mechanisms underlying herb processing were found to be related to changes in the composition and/or activity of the components in the herbs.[9] One typical example is frying Semen Strychni (Maqianzi), the seeds of Strychnos nux-vomica L., with sand or oil, to reduce its toxicity and increase the analgesic potency, which has been proved by modern phytochemical, toxicological, and the pharmacological investigations.[10]
The dried fruits of A. lappa L. has been used in two forms in TCM: Dried, called raw Fructus Arctii (RFA), and processed with the stir-frying method, called processed Fructus Arctii (PFA). PFA has been reported to exhibit more potent pharmacological activities than RFA.[11] These enhanced biological activities of PFA were thought to be mediated by changes in the chemical constituents by stir-frying. However, the chemical changes of Fructus Arctii by stir-frying are not yet fully elucidated. To reveal the chemical changes of processing Fructus Arctii, a high-performance liquid chromatography/diode array detection-electrospray ionization-mass spectrometry (HPLC/DAD-ESI-MS) method was developed for the identification of chemical constituents in raw and PFA. In total, 13 compounds in raw and the PFA were analyzed and characterized [Figure 1]. The amounts of these compounds greatly changed during the stir-frying process.
Figure 1.
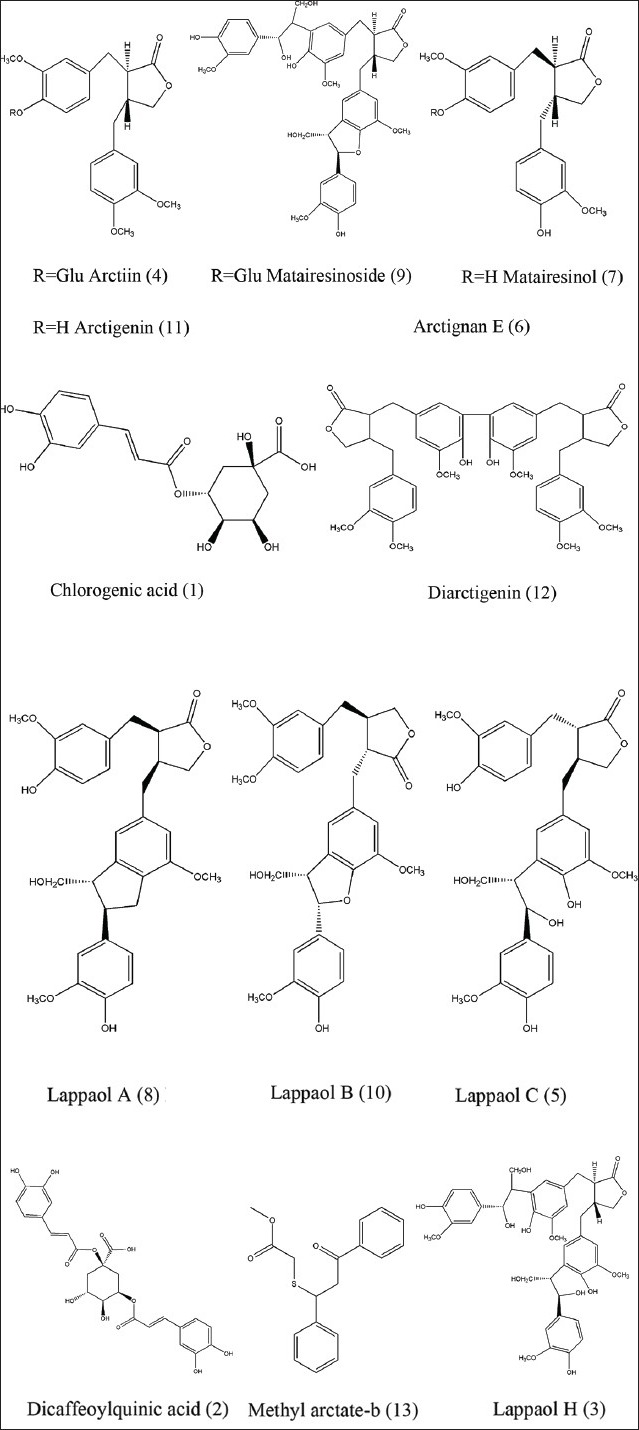
The chemical structures of the main constituents identified in Fructus Arctii
EXPERIMENTAL
Reagents and materials
Acetonitrile (HPLC-grade) was purchased from Nanjing Chemical Reagent Co. Ltd. (Nanjing, China) and deionized water was from Hangzhou Wahaha Co. Ltd. (Hangzhou, China). Formic acid was purchased from Nanjing Chemical Reagent Co. Ltd. (Nanjing, China). Other solvents from Nanaohuabo Co. Ltd. (Nanjing, China) were of analytical grade.
Fructus Arctii was collected from Cangshan County, Shandong Province, one of its indigenous cultivating regions in China. The sample was identified by Professor Jianwei Chen (Nanjing University of Chinese Medicine). RFA was obtained by the sun-drying fruits of A. lappa L. The PFA was produced by stir-frying RFA for 15 min under 150°C, according to the processing method described in Chinese Pharmacopoeia.[1]
The reference standards of arctiin and arctigenin were isolated previously from the crude Fructus Arctii by authors, and the structures were established based on spectroscopic analyses compared with the reference data.[12,13] The purities of these lignans were determined to be above 98.5% by normalization of the peak areas detected by HPLC-DAD and confirmed by ESI-MS and NMR spectroscopy.
Apparatus and chromatographic conditions
The HPLC system consisted of a Shimadzu LC-20AD solvent delivery pump, a DGU-20A3 degasser, a CTO-20A column oven, a SPD-M20A photodiode array detector, and a SIL-20A auto sampler (Shimadzu, Kyoto, Japan). The mass spectrometer was a Shimadzu LCMS-2020 single quadrupole equipped with a ESI source interface. At the end of data collection, the chromatograms were processed with the software LCMS Solution (Shimadzu, Kyoto, Japan).
For chromatographic analysis, an YMC-ODS C18 column (250 mm × 4.6 mm, 5 μm) was used. HPLC separation was performed using a linear gradient and a flow rate of 1.0 mL/min. The column temperature was 35°C. The mobile phase consisted of acetonitrile (A) and water containing 0.1% formic acid (B) using the elution gradient 5-25% A at 0-35 min, 25-35% A at 35-65 min, 35-50% A at 65-80 min, 50-70% A at 80-90 min, 70-5% A at 90-95 min and 5% A at 95-100 min. Detection wave-length was set at 254 nm. The ESI-MS spectra were acquired in both negative and positive modes scanning from 100 to 800. The typical ion source parameters were as follows: ESI probe temperature 350°C, CDL temperature 280°C, heat block temperature 320°C, ESI probe voltage 4.5 kV, detector voltage 1.5 kV, and nebulizing gas flow 1.5 L/min.
Sample preparation
The standard solutions of arctiin and arctigenin were prepared with 80% (v/v) methanol. The pulverized samples of raw and PFA were accurately weighed (approximately 1.0 g) and ultrasonic-extracted with 15.0 mL methanol for 30 min. Then, 15.0 mL water was added and the mixture was ultrasonic-extracted for another 30 min. The resulting solutions were centrifuged at 3000 r/min, the supernatants were filtered through a 0.2 μm PTFE syringe filter, and an aliquot (2 μL) of each filtrate was subjected to HPLC/DAD-ESI-MS analysis.
RESULTS AND DISCUSSION
Two organic solvents, methanol and acetonitrile, with different gradient programs were compared to optimize the HPLC separation condition. Finally, acetonitrile-0.1% formic acid water solution with a linear gradient program was chosen for its well baseline resolution and suitable analysis duration.
Figure 2 showed the HPLC/DAD chromatograms of RFA and PFA under optimal separation conditions. As can be seen, the separation could be completed within 100 min and all peaks were well-separated from each other. Some peaks were found in both samples, including peak 1 to peak 13. However, most of the peak areas in RFA and PFA change a lot [Table 1]. For example, chlorogenic acid (peak 1), arctiin (peak 4), peak S1, peak S2 and peak S3 reduced significantly upon stir-frying, but dicaffeoylquinic acid (peak 2) and arctigenin (peak 11) increased significantly after stir-frying.
Figure 2.
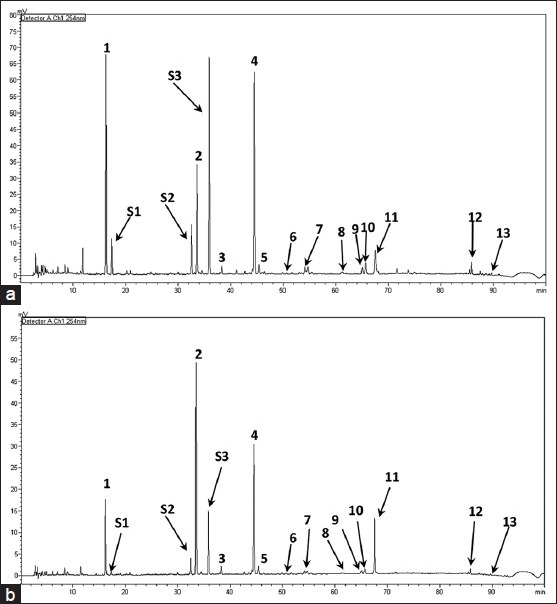
High-performance liquid chromatography/diode array detection chromatograms (254nm) of raw Fructus Arctii (a) and processed Fructus Arctii (b)
Table 1.
Retention time, MW and MS spectra data for constitutes from RFA and PFA
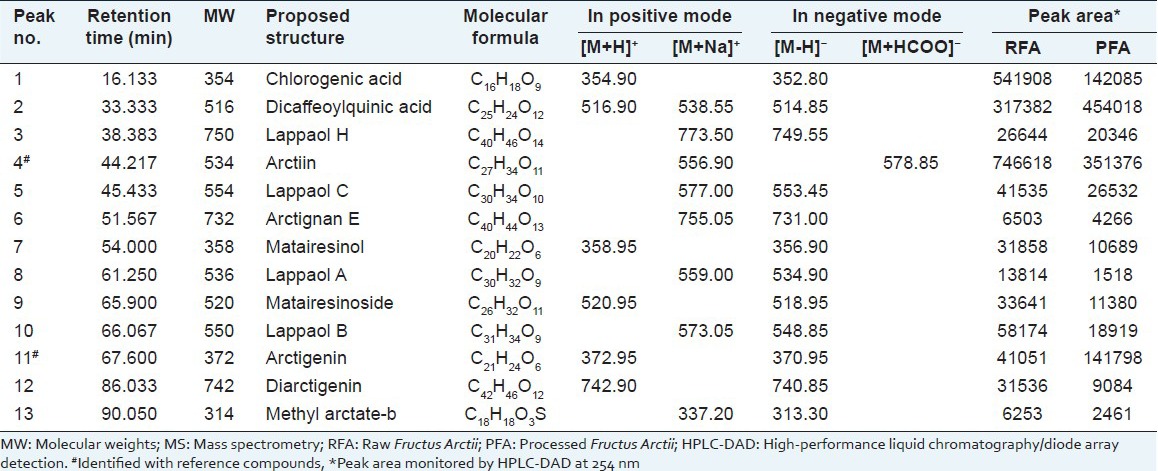
The structural identification of each peak was carried out by the on-line DAD and ESI-MS techniques. In this study, both positive and negative ion modes were selected, the results showed that ESI base peak chromatogram in negative mode was particularly sensitive [Figure 3]. In the ESI-MS experiment, the molecular weight of each peak could be obtained. The detail information of each peak was listed in Table 1.
Figure 3.
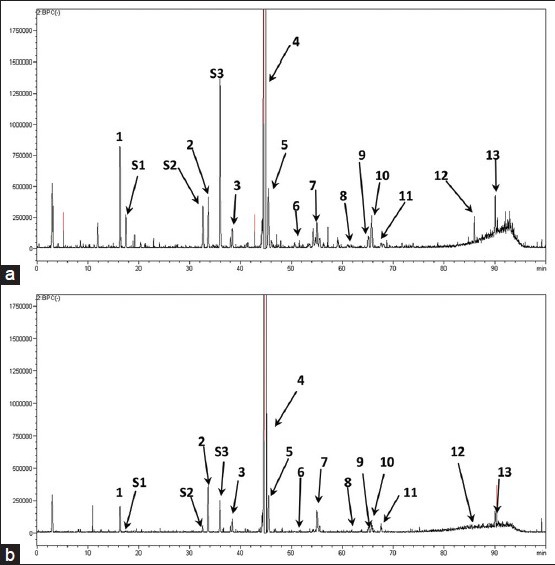
Base peak chromatograms of high-performance liquid chromatography/diode array detection-electrospray ionization-mass spectrometry analysis of raw Fructus Arctii (a) and processed Fructus Arctii (b) in the negative ion mode
The MS spectrum of peak 4 showed [M + Na]+ ion at m/z 556.90 in positive modes and [M + HCOO]- ion at m/z 578.85 in negative mode. The MS spectra, retention behaviors and the MWs of peak 4 agreed with the standard marker arctiin. Hence, peak 4 was identified as arctiin [Figure 4a, b]. Peak 11 was identified as arctigenin based on MS spectrum, ultraviolet (UV) spectrum and retention behavior, which was the same with the standard arctigenin. The MS spectrum of arctigenin showed [M + H]+ ion of m/z 372.95 in positive mode and [M - H]- ion of m/z 370.95 in negative mode [Figure 4c, d]. Peaks 5, 8 and 10 had [M + Na]+ ions at m/z 577.00, 559.00 and 573.05 in positive mode and had [M - H]− ions at m/z 553.45, 534.90 and 548.85 in negative mode, respectively. Their UV spectra were very similar and all exhibited the characteristic absorbance of lignans with two maximum absorbance bands: B and I (300-400 nm) and band II (270-295 nm). Thus, peak 5, 8 and 10 were tentatively identified as lappaol A, B and C by comparing the retention time, UV and MS spectrum with the reported literature.[12,13] Another 8 compounds were identified in the same way.[14]
Figure 4.
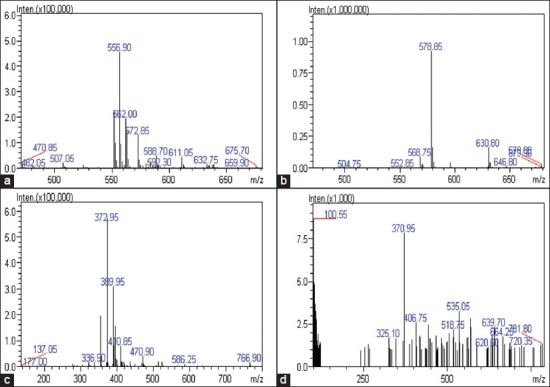
Mass spectrometry spectra of arctiin and arctigenin (positive ion and negative ion detection mode)
As shown in the Table 1, all the compounds were found in both RFA and PFA, including chlorogenic acid, dicaffeoylquinic acid, lappaol (A, B, C, H), arctiin, arctignan E, matairesinol, matairesinoside, arctigenin, diarctigenin, and methyl arctate-b. However, there were also many changes between the RFA and PFA. For instance, some peak areas varied much during the stir-frying process. Among the 13 identified compounds, the areas of 11 compounds (peak 1, 3, 4, 5, 6, 7, 8, 9, 10, 12) reduced although the areas of peak 2 and 11 increased significantly. From these results, it can be concluded that substantial differences exist between the RFA and PFA. Although, several relevant researches about other herbs have revealed that processing methods can change the chemical composition between the raw and processed herbs,[15,16,17] the mechanism for chemical changes between RFA and PFA still need further investigation.
It can be seen that peak S1, peak S2 and peak S3, three unknown compounds in RFA have been changed during the stir-frying process. The results indicate that the conditions of processing may offer the more favorable factor for the decomposition of peak S1, peak S2 and peak S3. Peak S1, peak S2 and peak S3 gave the base peaks at m/z 506.95 [M + H]+, 632.82 [M + H]+ and 642.65 [M + H]+ in positive mode respectively. In the negative mode, peak S1, peak S2 and peak S3 showed the abundant ion at m/z 505.05 [M - H]-, 630.95 [M - H]- and 641.50 [M - H]- in their MS spectra. Therefore, 506, 632 and 642 were considered as their molecular weight respectively. However, we did not identify them through the published data, which indicated that they are unknown compounds in Fructus Arctii. Furthermore, we are trying to gain and identify the three important compounds with traditional chemical isolation method, which is still in progress in our laboratory.
CONCLUSION
In this study, a new HPLC/DAD-ESI-MS method was proposed to rapidly screen and analyze the chemical constituents of RFA and PFA. Based on this method, 13 chemical components were tentatively identified. We found that arctiin reduced significantly following the thermal conversion of arctiin to arctigenin. This result will be helpful for the degradation studies of processing mechanisms on TCMs. In conclusion, the method made it possible to rapidly and simultaneously screen and analyze the chemical constituents during processing of TCMs. This research will also be helpful to the study of the processing mechanisms of TCMs and give a better understanding of the pharmacological effect of this drug.
ACKNOWLEDGMENTS
This study was supported by the Jiangsu province Natural Science Foundation (No. BK2011135), Fund Project for Transformation of Scientific and Technological Achievements of Jiangsu Province (No. BA2011024) the International Science and Technology Co-operation Project of Jiangsu Province (No. BZ2011053) and The Open Project of National First Class Key Discipline for Science of Chinese Materia Medica, Nanjing University of Chinese Medicine (No. 2011ZYX2-013).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.National Commission of Chinese Pharmacopoeia. Beijing: China Medical Science Press; 2010. Pharmacopoeia of Peoples Republic of China. [Google Scholar]
- 2.Ichikawa K, Kinoshita T, Nishibe S, Sankawa U. The Ca2+antagonist activity of lignans. Chem Pharm Bull (Tokyo) 1986;34:3514–7. doi: 10.1248/cpb.34.3514. [DOI] [PubMed] [Google Scholar]
- 3.Iwakami S, Wu JB, Ebizuka Y, Sankawa U. Platelet activating factor (PAF) antagonists contained in medicinal plants: Lignans and sesquiterpenes. Chem Pharm Bull (Tokyo) 1992;40:1196–8. doi: 10.1248/cpb.40.1196. [DOI] [PubMed] [Google Scholar]
- 4.Ryu SY, Ahn JW, Kang YH, Han BH. Antiproliferative effect of arctigenin and arctiin. Arch Pharm Res. 1995;18:462–3. [Google Scholar]
- 5.Hirose M, Yamaguchi T, Lin C, Kimoto N, Futakuchi M, Kono T, et al. Effects of arctiin on PhIP-induced mammary, colon and pancreatic carcinogenesis in female Sprague-Dawley rats and MeIQx-induced hepatocarcinogenesis in male F344 rats. Cancer Lett. 2000;155:79–88. doi: 10.1016/s0304-3835(00)00411-0. [DOI] [PubMed] [Google Scholar]
- 6.Li SL, Song JZ, Qiao CF, Zhou Y, Qian K, Lee KH, et al. A novel strategy to rapidly explore potential chemical markers for the discrimination between raw and processed Radix Rehmanniae by UHPLC-TOFMS with multivariate statistical analysis. J Pharm Biomed Anal. 2010;51:812–23. doi: 10.1016/j.jpba.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Z, Liang Z, Chan K, Lu G, Lee EL, Chen H, et al. A unique issue in the standardization of Chinese materia medica: Processing. Planta Med. 2010;76:1975–86. doi: 10.1055/s-0030-1250522. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang E, Zhang C, Li X. Simultaneous determination of geniposide, chlorogenic acid, crocin1, and rutin in crude and processed Fructus Gardeniae extracts by high performance liquid chromatography. Pharmacogn Mag. 2011;7:267–70. doi: 10.4103/0973-1296.90391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai BC, Gong QF. Beijing: People Health Press; 2009. Processing of Chinese Medicinal Herbs. [Google Scholar]
- 10.Cai B, Nagasawa T, Kadota S, Hattori M, Namba T, Kuraishi Y. Processing of nux vomica VII Antinociceptive effects of crude alkaloids from the processed and unprocessed seeds of Strychnos nux-vomica in mice. Biol Pharm Bull. 1996;19:127–31. doi: 10.1248/bpb.19.127. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Sun JY, Wu HY, Zhong Y. Advances in studies on chemical constituents and pharmacological effects of the fruits of Arctium lappa L. Qilu Pharm Aff. 2009;28:738–40. [Google Scholar]
- 12.Liu S, Chen K, Schliemann W, Strack D. Isolation and identification of arctiin and arctigenin in leaves of burdock (Arctium lappa L.) by polyamide column chromatography in combination with HPLC-ESI/MS. Phytochem Anal. 2005;16:86–9. doi: 10.1002/pca.816. [DOI] [PubMed] [Google Scholar]
- 13.Ferracane R, Graziani G, Gallo M, Fogliano V, Ritieni A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J Pharm Biomed Anal. 2010;51:399–404. doi: 10.1016/j.jpba.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Zheng YF, Peng GP. The study development of the chemieal constituents of Arctium lappa L. Lishizhen Med Mater Med Res. 2005;16:153–5. [Google Scholar]
- 15.Wang KT, Chen LG, Yang LL, Ke WM, Chang HC, Wang CC. Analysis of the sesquiterpenoids in processed Atractylodis Rhizoma. Chem Pharm Bull (Tokyo) 2007;55:50–6. doi: 10.1248/cpb.55.50. [DOI] [PubMed] [Google Scholar]
- 16.Cao G, Zhang Y, Feng J, Cai H, Zhang CR, Ding MJ, et al. A rapid and sensitive assay for determining the main components in processed Fructus Corni by UPLC-Q-TOF-MS. Chromatographia. 2011;73:135–41. [Google Scholar]
- 17.Boldizsár I, Füzfai Z, Tóth F, Sedlák E, Borsodi L, Molnár-Perl I. Mass fragmentation study of the trimethylsilyl derivatives of arctiin, matairesinoside, arctigenin, phylligenin, matairesinol, pinoresinol and methylarctigenin: Their gas and liquid chromatographic analysis in plant extracts. J Chromatogr A. 2010;1217:1674–82. doi: 10.1016/j.chroma.2010.01.019. [DOI] [PubMed] [Google Scholar]


