Abstract
Background:
In periodontal regeneration, several alloplastic materials are being used with a goal to reconstruct new osseous tissue in the infrabony defect sites. The present study was undertaken to evaluate the efficacy of hydroxyapatite–bioactive glass (HA:BG) composite granules in the management of periodontal bony defects.
Materials and Methods:
A randomized control study was conducted. Subjects with infrabony defects were divided into three groups. Test Group 1 (n = 10): Defect site was treated with HA:BG, with a biodegradable membrane. Test Group 2 (n = 10): Defect site was treated with HAP, with a biodegradable membrane. Control group (n = 10): Defect site was treated with open flap debridement with a biodegradable membrane
Results:
The healing of defects was uneventful and free of any biological complications. The gain in clinical attachment level, reduction of probing pocket depth, and defect fill were statistically significant in all three groups. TG1 sites showed significant defect fill than TG2 and CG sites.
Conclusion:
The performance of HA:BG was better compared to HAP and open flap debridement for the reconstruction of infrabony defects.
Keywords: Alloplastic bone graft, calcium hydroxyapatite and bioactive glass, periodontal bony defect
INTRODUCTION
Periodontal disease is a common multi-factorial chronic inflammatory disease that leads to progressive loss of connective tissue attachment and supporting alveolar bone of the periodontium.[1] In addition to reducing bone height it alters the morphologic features of alveolar bone leading to various osseous defects or deformities. These deformities of the alveolar bone unless corrected interfere with the eradication of the periodontal pockets, which make the oral hygiene maintenance difficult, thereby leading to recurrence of the disease.
Periodontal therapy is directed towards arresting the progression of periodontal tissue destruction with the goal of achieving long-term prognosis. Conventional periodontal therapy generally comprises the techniques that repair the diseased periodontium rather than regenerating the lost tissue.[2] Hence, regeneration of periodontium especially the alveolar bone lost due to disease is a matter of prime concern in clinical management of periodontal disease, though predictable regeneration of the same is one of the biggest challenges in modern day dentistry.
The osseous autografting[3] being the “gold standard” of bone grafting possesses inherent disadvantage in the sense of procurement of the same and patient discomfort.
Allografts[4] are being used in the form of Freeze Dried Bone Allograft (FBDA) and Decalcified Freeze Dried Bone Allograft (DFBDA) with a good amount of success over the years. However, the problems of antigenic reactivity, availability and added danger of transmitting of deadly diseases like HIV make these materials less accepted in modern day periodontal use.
Xenografts[5] are the possible alternatives, but again not encouraged because of the risk of immunogenic reactions and rejection by the host tissue.
So, alloplastic materials, used as fillers or scaffolds, are biocompatible, surgically convenient, osteoconductive and predictably promote periodontal regeneration. These materials include plaster of Paris, tricalcium phosphate,[6,7] calcium hydroxyapatite ceramics,[8] bioactive glasses,[9] and polymers.[10]
An ideal bone graft material should be the one which integrates with the host tissue rapidly and attains the optimum chemical and mechanical stability within the shortest period without provoking any untoward toxicity. After its bonding, it should again get resorbed fully and should be replaced and remodeled by new regenerated bone in the area of grafting. In this endeavor of developing such an ideal bio-material, the scientists and researchers have come up with several novel materials and compositions in recent times.
A unique synthetic bone graft material based on hydroxyapatite (HAP) and bioactive glass (BG) have been developed for periodontal osseous defect reconstruction and regeneration. The product named BioGraft®-HABG Active, (IFGL Refractories Ltd, Kolkata, Knowhow from Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum), is a new-generation composite bioactive material made through a ‘sol-gel’ synthesis method. The resultant product is a composite of calcium phosphate and silicate, which bonds with host bone faster than HAP ceramics and resorbs slower than pure BG products. These in vivo properties are ideal for the material to be used for periodontal regeneration.
The product is processed in the form of particle sizes of 150-700 μ with internal pore in a size range of 100-200 μ, specifically for periodontal applications. The material characterization has been done extensively to ensure suitable mechanical properties. The screening tests and toxicological tests are done according to international guidelines of ISO 10993. The biological activity and efficacy in bone defect healing have been tested through in vitro studies and animal experiments. The bone–bonding and resorption abilities of the material were excellent when compared to conventional HAP-based granules in the pre-clinical studies.
In the light of the favorable pre-clinical results, the Ethical committee of Guru Nanak Institute of Dental Sciences and Research, Kolkata has approved the use of the material named BioGraft®-HABG Active, (IFGL Refractories Ltd, Kolkata) [Figure 1] and BioGraft® HA, a HAP granules, (IFGL Refractories Ltd, Kolkata, technical know-how from Central Glass Ceramic Research Institute, Kolkata) [Figure 2], in human clinical trials to see the efficacy of hydroxyapatite–bioactive glass (HA: BG) composite granules in the management of periodontal bony defects, compare the postsurgical results with those treated with HAP and open flap debridement. In all the cases, GTR (PerioCol™–GTR, Eucare Pharmaceuticals (P) Ltd) [Figure 3] membranes were used.
Figure 1.
BioGraft® - HABG active, (IFGL Refractories Ltd, Kolkata)
Figure 2.

BioGraft® HA, a Hydroxyapatite granules, (IFGL Refractories Ltd, Kolkata)
Figure 3.

PerioColTM - GTR, Eucare Pharmaceuticals (P) Ltd.
MATERIALS AND METHODS
A total of 30 sites from 18 systemically healthy patients with chronic periodontitis, with at least one or two radiographically detectable infrabony defect and probing depth >5 mm, aged between 20 and 70 years, were selected from the Out Patient Department, Department of Periodontics, Guru Nanak Institute of Dental Sciences and Research, Kolkata. Subjects who failed to maintain adequate oral hygiene (Silness and Löe plaque score >1), with any deleterious habits such as smoking and tobacco chewing were excluded. A randomized control study was conducted. Subjects with infrabony defects were divided into three groups. Test Group 1 (n = 10): Defect site was treated with HA: BG (BioGraft®- Active), with a biodegradable membrane (PerioCol™–GTR). Test Group 2 (n = 10): Defect site was treated with HAP (BioGraft® HA), with biodegradable membrane. Control group (n = 10): Defect site was treated with open flap debridement with biodegradable membrane. Prior to the commencement of the study, the subjects were informed of the purpose and the design of this clinical study. Informed consents were signed by the subjects as a requirement for the study. A thorough medical and dental history was obtained. Blood investigations, which included TC, DC, BT, CT, Hb, HbsAg and HIV, were carried out for each subject. Each subject received phase–I therapy, which comprised of ultrasonic scaling and root planning by hand instruments. Selective coronoplasty was done to remove any traumatic occlusion wherever required. All subjects were instructed proper oral hygiene methods and plaque score was brought <1 (Silness and Löe 1964). To record the clinical parameters, a customized acrylic occlusal stent with a guide groove was fabricated for each site to fit over the selected tooth and two adjacent teeth, one mesial and one distal to the concerned tooth. This provided a fixed reference point (RP) and fixed angulation of measurements at each site over the entire duration of the study. The clinical parameters which included probing pocket depth (PPD), clinical attachment level (CAL) and gingival recession (GR) were recorded to the nearest millimeter with the help of a Williams graduated periodontal probe at baseline and 6 months post surgically. Radiographic evaluations of the study were carried out on IOPA radiographs taken using the long-cone paralleling technique, using film holder (XCP, RINN Denstply) [Figure 4] and were measured using 1 sq mm gridlines. Four weeks after the phase–1 therapy just prior to the surgical procedure, each subject received a re-evaluation examination and baseline data were recorded.
Figure 4.

XCP RINN film holder for parallel cone technique
All the probing measurements were recorded for the test and control teeth by a single investigator using a Williams graduated periodontal probe. The site representing the deepest point of the PPD [Figure 5] was included in the study. The cemento-enamel junction served as the fixed RP. PPD was calculated by subtracting the distance from the Gingival Margin (GM) to the base of the pocket (BOP). CAL was calculated by measuring the distance between the RP to the BOP.
Figure 5.
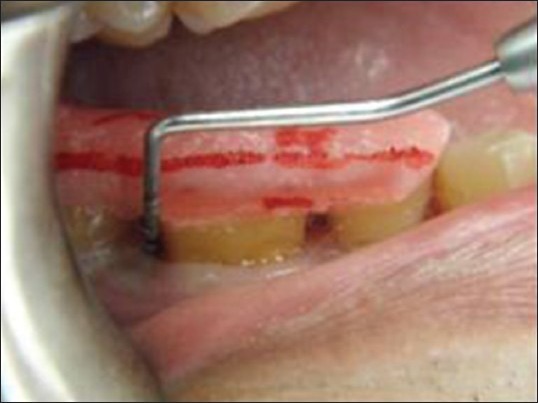
Pre-operative probing with stent in place
An IOPA radiograph was taken for each selected site to measure the radiographic depth of the defect (DD) and to calculate the percentage of bone defect fill. The radiographic measurements were carried out using indigenously made gridlines of 1 sqmm. [Figure 6a]. The measurements of the radiographic images were done from: CEJ to base of the bone defect, CEJ to crest of the bone. The radiographic DD was calculated as the linear distance (in mm) from the most coronal extension of the radiopaque crest to the most apical extension of the defect. Radiographic defect fill percentage was calculated as follows:
Figure 6a.
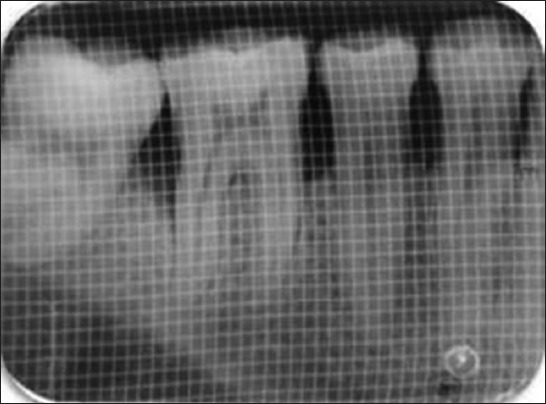
Pre-operative IOPA radiograph showing the defect in 1-mm sq grid
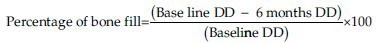
All the instruments used in the surgery were sterilized by autoclaving (temperature 121°C at 15 psi pressure for 15 minutes). The facial skin around oral cavity was scrubbed with 5% povidone iodine solution and subjects were asked to rinse with 0.2% chlorhexidine.
Area subjected to surgery was anesthetized by nerve block/infiltration depending on the surgical site using local anesthesia, Xylocaine 2% with adrenaline 1: 2,00,000. Sensitivity testing was done prior to surgery and nerve block and infiltration was administered to adequately anesthetize the surgical site.
Sulcular incisions were made using no 15 Bard Parker blade to the level of alveolar bone [Figure 7]. The incision was extended one tooth mesially and one tooth distally from the involved tooth. Mucoperiosteal flap was raised using Howarth periosteal elevator on both facial and palatal/lingual sides until the bony defect was exposed. Care was taken to preserve as much as inter proximal soft tissue as possible. Papilla preservation flap was done where diastema was present. This was to ensure adequate coverage of the graft and the membrane after suturing. Vertical releasing incisions were performed when needed for better access.
Figure 7.
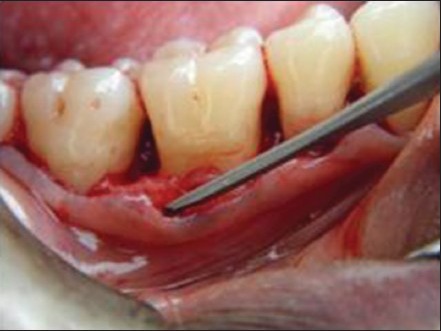
Flap reflected showing the osseous defect
In all the sites after raising the flap, the osseous defect was debribed of granulation tissue using surgical curettes to expose the root surface and alveolar bone [Figure 8]. The root surface was thoroughly scaled and planed with area specific Gracey curettes until smooth hard consistency was found. Then defect area was confirmed clinically.
Figure 8.
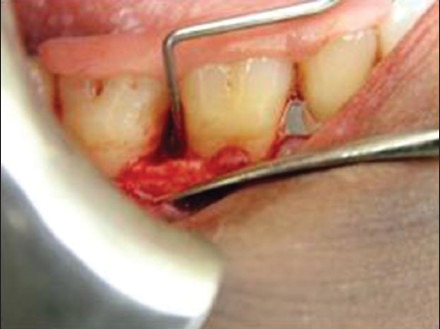
Pre-operative probing showing the depth of the defect
Once the debridement was over templates were made for the GTR membrane to see the adequacy of the same so that it covers the full defect below which the graft materials were to be placed and after that flaps were to be sutured back without any tension. Once the template satisfied the need then the GTR membrane was placed in the defect site and sling sutures were placed using 3-0 vicryl suture. Under the GTR, HA:BG graft was placed in the defect in test Group 1, HAP was placed in the defect in test Group 2, and the control group did not receive any [Figures 9 and 10]. The osseous defect was filled in increments to the most coronal level of the osseous walls using light pressure avoiding overfilling or under filling the defect.
Figure 9.
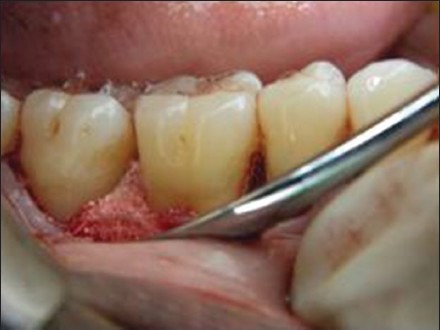
Alloplastic graft material placed in the defect
Figure 10.
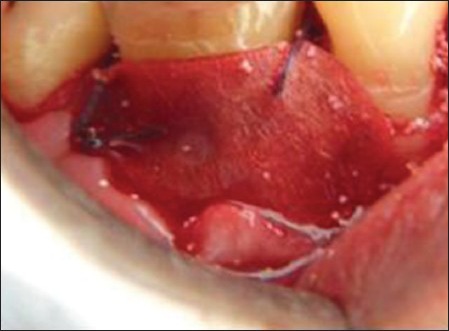
Resorbable GTR membrane covering the graft material
Primary soft tissue closure was done with interrupted sutures at the original level using 3-0 black silk sutures [Figure 11]. Periodontal dressing (Coe-Pack™) was applied over the surgical site [Figure 12].
Figure 11.
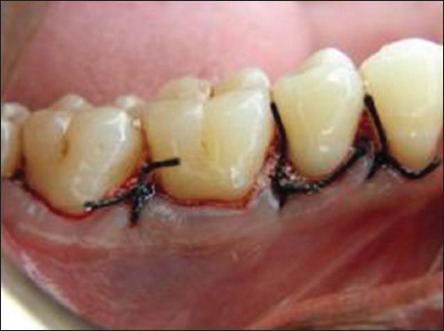
Flap apposed with 3’0 black silk suture
Figure 12.
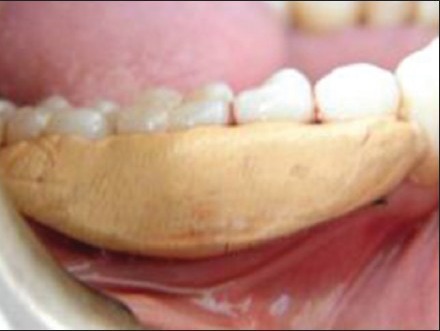
Periodontal dressing given
Post surgically, subjects were prescribed antibiotics (Amoxicillin 500 mg tid for 5 days), anti-inflammatory analgesics (Ibuprofen 400 mg + Paracetamol 500 mg bdpc for 3 days), H2 receptor blocker (Ranitidine 150 mg bdac for 3 days). Subjects were instructed to rinse with 0.2% chlorhexidine gluconate (10 ml 12 hourly until next visit) and were instructed proper brushing technique for the surgical site. At 1 week, periodontal dressing and sutures were removed. Recall appointments were then made at 1 month, 3 months and 6 months for additional follow up and plaque control and to collect the final data at 6 months time [Figures 13 and 6b].
Figure 13.
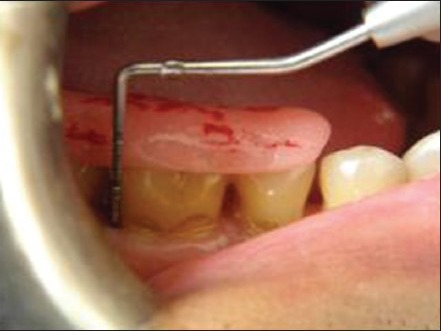
Post-operative probing (after 6 months)
Figure 6b.
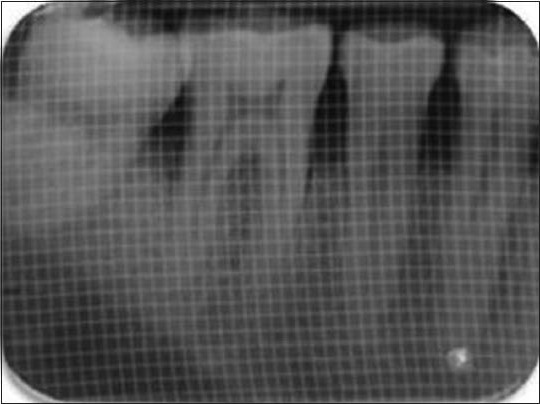
Post-operative IOPA radiograph showing the filled defect in 1-mm sq grid
RESULTS AND OBSERVATIONS
Inter-group comparison of soft tissue parameter and hard tissue parameter data at baseline and 6 months post surgery are depicted in Tables 1-3 through Table 4 and and Box plots 1-3.
Table 1.
Intergroup comparison of PPD at different observation periods
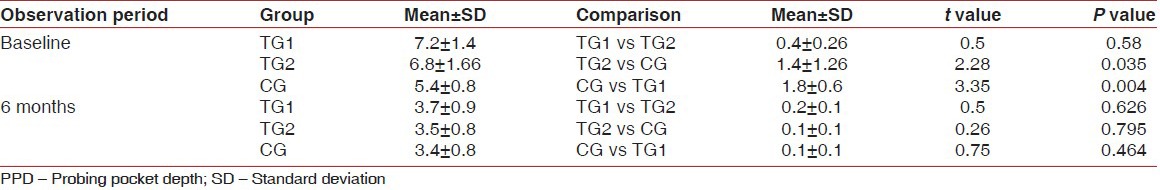
Table 3.
Intergroup comparison of defect fill after 6 months

Table 4.
Intergroup comparison of percentage of defect fill after 6 months
Box plot 1.
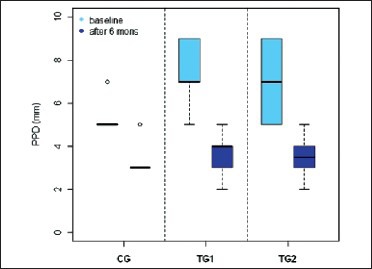
PPD (in mm) in three groups
Box plot 3.
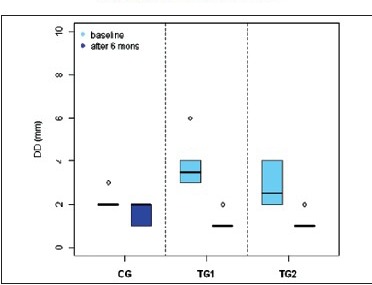
DD in three groups
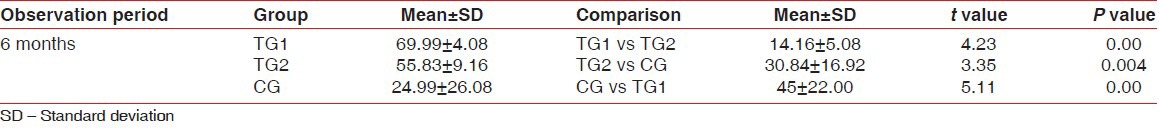
Table 2.
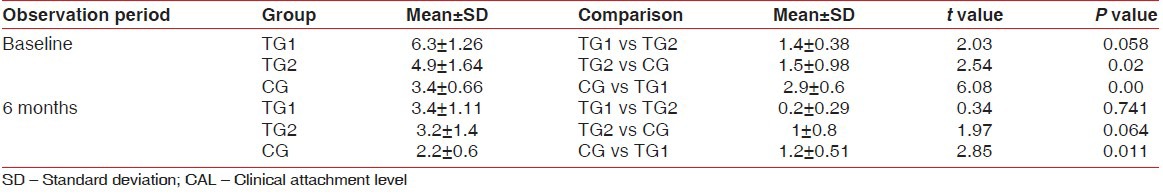
Intergroup comparison of CAL at different observation periods
Box plot 2.
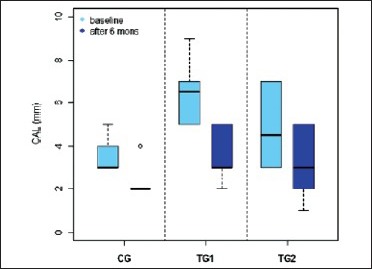
CAL (in mm) in three groups
There is highly significant difference in the test groups from the control group regarding defect fill after 6 months and percentage of bone fill. TG1 differ significantly from TG2 and CG in terms of defect fill.
DISCUSSION
Available literature regarding periodontal regeneration suggest, clinically significant reconstruction of human supporting periodontal tissues is possible in select sites and patients with the use of the proper graft materials, membranes, and their combination. In this clinical study, it has been found that, the usage of alloplastic regenerative materials such as HA:BG; is well accepted by the patients, in various types of intraosseous defects in slowly progressive type of periodontal diseases.
The selection of bone defects were not made on the basis of qualitative assessment of bony walls in this study. The cortical plate with a thin cancellous bone would render a better result than only the cortical plate in a defect. The bony defects in this study were assumed to have only the cortical part. Hence, the osteogenic potential of the cancellous bone could not take part in the new bone formation.
Zybutz et al.[11] compared clinical and radiographic measurements of interproximal vertical defects before and 1 year after surgical treatments to assess the association between clinical and radiographic measurements. They concluded that the standardized radiographs reliably and permanently describe the hard tissue changes and thus can serve as substitute for probing to bone or re-entry measurements of bone changes. Though in this study the bony defects were not clinically measured three dimensionally and the vertical depths taken into account as evidenced in the radiograph, statistical significant results were obtained in TG1 and TG2 in terms of stated parameters.
The physical characteristics of alloplastic bone replacement grafts are of paramount importance. The particle size and the pore size play an important role in osteoconductivity of bone replacement graft material. The size of particles of the test material ranged from 150 to 700 microns. In a re-entry study it was seen that smaller particles <300 microns undergo completely ionic dissolution and disappear by 1 year.[12] These particles were completely devoid of any cations. The larger sized particles were present for longer periods of time, but by 3 years the particles were found completely replaced by bone.[13]
Along with graft materials, new therapeutic approaches for periodontal regeneration have brought different biologic mediators into practice. The recognition and appreciation that new tissues are formed by cell populations have resulted in efforts to stimulate the cells that are located in the periodontal defect. One way to stimulate these cells is to use proteins (growth factors) that can bind to surface receptors on the cell membranes, which in turn trigger a series of events to occur that alter the genetic activity of the cell with the result that cell behavior is stimulated. These growth factors, primarily secreted by macrophages, endothelial cells, fibroblasts, and platelets, include platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), basic fibroblast growth factor (bfGF), BMP, and transforming growth factor (TGF). These biologic mediators have been used to stimulate periodontal wound healing (e.g., promoting migration and proliferation of fibroblasts for periodontal ligament formation) or to promote the differentiation of cells to become osteoblasts, thereby favoring bone formation.[14] In both the test groups and in the control group no growth factors were added.
Bony status can be evaluated by histological, clinical and radiographic methods. Camargo et al.[15] stated that the improvement in clinical parameter can result in gain in attachment; however, it should be remembered that the placement of the graft material into the defect may modify gingival tissue consistency and therefore interfere with the penetration of the periodontal probe without necessarily having induced any gain in CAL. Hence, surgical re-entry is important to substantiate the post-operative data for evaluating regeneration. However, it has certain inherent disadvantages like inducing further resorption at the treated site and inability to ascertain the exact histological nature of the hard tissue. The second surgical procedure is also time consuming and may interrupt the regenerative process if the healing is still going on.
Here in this study the different clinic-physiological factors are being observed, like physical characteristics of the material, chemical composition of the material, patient selection, defect selection, pre-operative preparation, flap design, defect or root debridement, graft management, flap closure, post-operative management and periodontal maintenance influence the treatment outcome after the use of bone replacement grafts. Some of these factors are beyond the control of the operator.
The soft tissue variables evaluated were position of the GM, PPD and CAL. These variables by themselves, following regenerative therapy, do not provide the direct proof of formation of new bone or new attachment. Nonetheless, such clinical measurements are routinely used as they assess the volume of subgingival area, which harbors the pathogenic microbiota and favor disease activity.
The results of this study show that the mean PPD in TG1 at baseline was 7.2 ± 1.4 and at 6 months reduced to 3.7 ± 0.9, in TG2 at baseline 6.8 ± 1.66 and at 6 months reduced to 3.5 ± 0.8. At the control sites mean PPD at baseline was 5.4 ± 0.8, and at 6 months reduced to 3.4 ± 0.8. These data indicates that there is a marked reduction in the PPD in both control and experimental sites from baseline to 6 months. And there was statistically significant difference in PPD reduction between the control group and TG1 and TG2. The clinical trials with various alloplastic bone grafts also have shown a significant reduction in PPD when compared to open flap debridement.[16] So the changes in PPD reflect the effect of the response of the gingival tissue to the surgical treatment, may not always denote the periodontal regeneration.
CAL gain following regenerative therapy is another commonly used soft tissue measurement to evaluate treatment outcome. The results of our study indicate that the mean CAL in TG1 was 6.3 ± 1.26 at baseline and 3.4 ± 1.11 at 6 months; in TG2 was 4.9 ± 1.64 at baseline and 3.2 ± 1.4. In the control group, the mean CAL at baseline was 3.4 ± 0.66 and 2.2 ± 0.6 at 6 months. This suggests that there is statistically significant attachment gain from baseline to 6 months in both test groups than in the control group. And among TG1 and TG2, TG1 shows more significant results than TG2. This confirms that both TG1 and TG2 have added advantage over open flap debridement and again TG1 shows significant results than TG2. The previous studies with other alloplastic bone grafts also have shown significant gain in CAL when compared to open flap debridement.[12]
To find out the defect fill after treatment surgical re-entry and histological evaluation can effectively measure the outcome. However, due to ethical reasons and patients concern, it is not possible in routine clinical trials. Hence, radiographic evaluation is most commonly used outcome measure for bone regeneration.[16] Radiographic assessment was done at 6 months post-operatively. The results of this study indicate that the defect fill at 6 months in TG1 and TG2 2.6 ± 0.66 and 1.6 ± 0.66, respectively, where as in the control group sites it was 0.9 ± 0.7. Both TG1 and TG2 show significant difference from the control group in terms of defect fill after 6 months. Previous studies using various autografts, allografts and xenografts also have shown a significant bone fill in human intrabony defects when compared to open flap debridement.[17]
In the present study, it has been identified that good bone defect fill, evidenced radiographically, occurs with the use of ‘BioGraft® HABG Active’ in the treatment of periodontal infrabony defects. The results show that ‘BioGraft® HABG Active’ improves the healing outcome when PPD reduction and gain in CAL are used as clinical parameters. Though the clinical advantages are obvious, it is not possible to give conclusive description on the biological process of healing. The literature to date considers alloplastic graft materials to have functioned primarily as biocompatible defect fillers. In order to establish the biological mechanism to support the observed efficacy of BG-based materials, long-term, controlled studies and histological evidences of regeneration are essential. The results of this study corroborate well with the reported clinical trials. However, a direct comparison of clinical results and in vivo material properties to draw a definite conclusion may not be appropriate. It seems evident by comparing the existing clinical works with this study that, attachment gain of such nature of periodontal defect cannot be made 100%. A narrow range of gain has been observed in all clinical works and the variation in success largely depends upon the type of the defects chosen for surgical intervention. The present study also experiences a gain in bone height using HA: BG, a new material system. Thus, it can be concluded that the biologic environment of the periodontal defect has a great influence in achieving the goal; probably the skill of the operator does not play an important role although the autograft is a gold standard and allogenic being next, the alloplast used in this study poses a significant and successful role.
There are certain limitations in this clinical study like the periodontal status of soft tissue is complex and probing force may result in inaccurate or inconsistent probing measurements. Surgical re-entry evaluation was excluded from the study. The population included in the study is relatively small and extrapolation of these results to a larger population would be inappropriate. The combination of Bioglass and calcium hydroxyapatite is relatively new material and the trial of which has not been done widely. So further study, using this material, is sought for.
CONCLUSION
The following conclusions were made from the study:
Clinical evaluation has proved the regenerative effect of Biograft®-HABG Active composite granules
There is gain in clinical attachment level 6 months post-operatively
There is greater reduction in probing pocket depth 6 months post-operatively with Biograft®- HABG active granules in comparison with HAP and the control
Radiographic evaluation demonstrated definite obliteration of infrabony defect and reduction in the defect depth 6 months post-operatively.
Both test groups (TG1, TG2) showed significant improvement over the control in both the clinical and radiological parameters. Histological evaluation and long-term clinical trials are required for further study. Applying the philosophy of tissue engineering to the healing of bone, it is recommended that future studies employ a greater number of patients as well as experimental studies be conducted to analyze the maximum potential of Bioceramics and Bioactive Glass in regenerative periodontal therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pihlstromn BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;66:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Bartold PM, McCulloch CA, Naryanan AS, Pitaru S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000;24:253–69. doi: 10.1034/j.1600-0757.2000.2240113.x. [DOI] [PubMed] [Google Scholar]
- 3.Nabers CL, O’Leary TJ. Autogenous bone transplant in the treatment of osseous defects. J Periodontol. 1965;36:5–14. doi: 10.1902/jop.1965.36.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Schallhorn RG, Hiatt WH. Human allografts of iliac cancellous bone and marrow in periodontal osseous defects. II. Clinical observations. J Periodontol. 1972;43:67–81. doi: 10.1902/jop.1972.43.2.67. [DOI] [PubMed] [Google Scholar]
- 5.Scoop IW, Morgan FH, Dooner JJ. Bovine bone (Boplant) implants for infrabony oral lesions (clinical trials in humans) Periodontics. 1966;4:169–78. [Google Scholar]
- 6.Snyder AJ, Levin MP, Cutright DE. Alloplastic implants of tricalcium phosphate ceramic in human periodontal osseous defects. J Periodontol. 1984;55:273–7. doi: 10.1902/jop.1984.55.5.273. [DOI] [PubMed] [Google Scholar]
- 7.Stahl SS, Froum S. Histologic evaluation of human intraosseous healing responses to the placement of Tricalcium phosphate ceramic implants. I. Three to eight months. J Periodontol. 1986;57:211–7. doi: 10.1902/jop.1986.57.4.211. [DOI] [PubMed] [Google Scholar]
- 8.Yukna R, Cassingham RJ, Caudill RF, Evans GF, Miller S, Mayer ET, et al. Six month evaluation of Calcitite (hydroxyapatite ceramics) in periodontal osseous defects. Int J Periodont Rest Dent. 1986;6:34–45. [PubMed] [Google Scholar]
- 9.Li R, Clark AE, Hench LL. An investigation of bioactive glass powders by sol-gel processing. J Appl Biomater. 1991;4:231–9. doi: 10.1002/jab.770020403. [DOI] [PubMed] [Google Scholar]
- 10.Yukna RA. HTR polymer grafts in human periodontal osseous defects. I. 6-month clinical results. J Periodontol. 1990;61:633–42. doi: 10.1902/jop.1990.61.10.633. [DOI] [PubMed] [Google Scholar]
- 11.Zybutz M, Rapoport D, Laurell L, Persson GR. Comparisons of clinical and radiographic measurements of interproximal vertical defects before and 1 year after surgical treatments. J Clin Periodontol. 2000;27:179–86. doi: 10.1034/j.1600-051x.2000.027003179.x. [DOI] [PubMed] [Google Scholar]
- 12.Froum SJ, Weinberg MA, Tarnow D. Comparison of bioactive glass synthetic bone graft particles and open flap debridement in the treatment of human periodontal defects. A clinical study. J Periodontol. 1998;69:698–709. doi: 10.1902/jop.1998.69.6.698. [DOI] [PubMed] [Google Scholar]
- 13.Hench LL, Wilson J. Surface-active biomaterials. Science. 1984;226:630–6. doi: 10.1126/science.6093253. [DOI] [PubMed] [Google Scholar]
- 14.Cochran DL, Wozney JM. Biological mediators for periodontal regeneration. Periodontol 2000. 2000;19:40–58. doi: 10.1111/j.1600-0757.1999.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 15.Camargo PM, Lekovic V, Weinlaender M, Klokkevold PR, Kenney EB, Dimitrijevic B, et al. Influence of bioactive glass changes in alveolar process dimensions after exodontia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:581–6. doi: 10.1067/moe.2000.110035. [DOI] [PubMed] [Google Scholar]
- 16.Canton JG. Over view of clinical trials of periodontal regeneration. Ann Periodontol. 1997;2:215–22. doi: 10.1902/annals.1997.2.1.215. [DOI] [PubMed] [Google Scholar]
- 17.Machtei EE. Outcome variables for the study of periodontal regeneration. Ann Periodontol. 1997;2:229–39. doi: 10.1902/annals.1997.2.1.229. [DOI] [PubMed] [Google Scholar]


