Abstract
Background and Objective:
Hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) grafts have shown to be effective in promoting the clinical signs of periodontal regeneration in intrabony defects. The aim of our study was to clinically and radiographically evaluate the efficacy of HA and β-TCP composite bone graft material in the treatment of intrabony three-wall defect.
Materials and Methods:
Twenty patients participated in this study. Interproximal bony defects were surgically treated with a combination of HA–βTCP (biphasic calcium phosphate). Changes in clinical parameters such as gingival status, probing pocket depth, clinical attachment, and radiographic estimation of the amount of bone fill were evaluated after 6 months postoperatively.
Statistical Analysis Used:
Student's “t” test.
Results:
This treatment modality resulted in significant pocket depth reduction and clinical attachment gain which were observed to be 2.938 mm (47.04%) and 3.188 mm (29.09%), respectively. The defect fill as seen radiographically was 3.204 mm (63.195%). All the differences were highly significant and in favor of postoperative group.
Conclusion:
The results of this study suggest that HA–βTCP (biphasic calcium phosphate) provides an added regenerative effect in promoting the clinical resolution of intrabony three-wall defects in patients with periodontitis.
Keywords: HA–βTCP bone graft, intrabony three-wall defects, periodontal flap, periodontal regeneration
INTRODUCTION
Periodontitis can be defined as an inflammatory disease of the supporting tissues of the teeth caused by specific micro-organisms or a group of specific micro-organisms, resulting in progressive destruction of the periodontal ligament and alveolar bone with pocket formation, recession, or both. The ultimate goal of periodontal therapy is to regenerate the lost periodontal tissue for optimal function and esthetic.[1]
For regeneration, growth, and differentiation, cells and intercellular substances are required to form new tissues or parts; to achieve tissue regeneration, the cells in the wound must be of the same type and must be oriented in the same pattern as the original cells.
Biomaterials suitable for the restoration of periodontal osseous defects are a subject of particular interest and challenge. Materials ranging from bone grafts to alloplastic implants have been used with varying degrees of success. Autogenous bone graft was most popularly used for grafting, but the availability of the donor site and the limited quantity of the material caused some problems when the intraoral autogenous bone graft was used.[2]
To overcome the limitation of autogenous graft allograft, xenografts were introduced in the field of periodontics. Although these materials offer a solution to some of the above problems, the question of immunogenicity and disease transfer had often been raised.
Therefore, considering all the above-mentioned problems, alloplastic materials were introduced. These materials are synthetic, inorganic, biocompatible bone graft substitutes which represent a possible alternative for the treatment of intrabony defects. These materials have the advantages of easier availability, eliminating the need of a donor site, and carrying no risk for disease transmission.[2]
Among the various alloplastic materials available today, calcium phosphate ceramic is widely used for periodontal regeneration. Recently, a variety of calcium phosphate ceramics have become available as an off-the-shelf artificial grafting material for the restoration of periodontal osseous defects. Few materials like hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) have shown significant clinical improvement at grafted sites compared to non-grafted sites in controlled clinical studies.[2]
It has been reported that HA failed to show evidence of new periodontal tissue attachment, osteogenesis, or cementogenesis in the treatment of periodontal osseous defects. Therefore, it is believed that this material produces a response like a well-tolerated foreign body within the host connective tissue.[3]
β- TCP was reported to form bone within the periodontal osseous defects, but the new attachment was questionable. The material was also found to be resorbed unpredictably in biologic fluids and a variety of solvents. Therefore, it was concluded that β-TCP may not provide a scaffold for a predictable time period which is required for the growth of new bone.[3]
Thus, development of a two-phased calcium phosphate or biphasic calcium phosphate (BCP) ceramic made it possible to control the resorbability of the material and at the same time maintain its osteoconductive property. This ceramic might be classified as resorbable because of the β-TCP content (it is known that β-TCP resorbs much faster); the presence of HA in the structure retards the resorbability of the material.[3]
BCP with >99% crystalline structure consists of 60% HA and 40% β-TCP in particulate form and was introduced as a grafting material in periodontal, peri-implant, and various types of bone defects. Preclinical evidence suggested that the use of this ratio of HA and β-TCP may allow better control of the bioabsorbable ability of the graft material resulting in accelerated new bone formation.[4]
The aim of our study was to clinically and radiographically evaluate the efficacy of HA and β-TCP composite bone graft material in the treatment of intrabony periodontal defect.
MATERIALS AND METHODS
A total of 20 patients of both sexes (14 females and 6 males) were selected from the periodontics outpatient department, Kothiwal Dental College and Research Centre, Moradabad to be included in the study. After getting written acceptance from the patients to be a part of the study, the details of the consent form according to “Helsinki declaration” were given to be read thoroughly.[5]
Selection criteria
Inclusion criteria
Subjects having good general health (in the age group 29-47 years)
Subject should be a non-smoker
Subjects between 28 and 50 years of age
Subjects with pocket depth (PD) of ≥5 mm
Subjects with at least one intrabony three-wall defect of ≥4 mm (randomly selected).
Exclusion criteria
Patient having a history of any antibiotic therapy in the past 6 months
Patient with a history of smoking
Patient having a history of systemic disease (cardiovascular disease, diabetes, blood disorders, hepatitis, renal disorders) that could influence the course of periodontal diseases
Pregnant women or nursing mothers
Inability of the person to cooperate because of their physical or mental status or daily routine.
Clinical parameters
Prior to scaling and root planing, each selected site was subjected to assessment of the following clinical parameters:
Plaque index (Silness and Loe, 1964)
Gingival index (Loe and Silness, 1963)
Pocket depth PD
Clinical attachment level (CAL).
Periodontal treatment
All participants received oral hygiene instructions before the periodontal therapy. The treatment consisted of conventional periodontal therapy, i.e., full mouth scaling and root planing. Four weeks following scaling and root planing, a periodontal re-evaluation was performed to confirm the suitability of the sites for this periodontal surgical study.
Presurgical clinical measurement
With the acrylic stent in position, the periodontal probe was inserted into the pocket (surgical site) specify to reach the deepest portion of the interproximal pocket. A pencil mark was made where the probe made contact with the acrylic stent and a groove was made on the pencil mark area with a cylindrical low-speed bur. Using the groove as guide, the periodontal probe was reinserted into the pocket, and the PD, CAL, and gingival recession were recorded. Measurements were performed with a University of North Carolina Probe (UNC-15) and recorded to the nearest millimeter.
The following measurements were appropriately utilized to evaluate the effects of regenerative procedures on soft tissues:
Probing (clinical) attachment level (PAL/CAL): Vertical distance from the landmark (stent) to the base of the periodontal pocket
Probing PD: Vertical distance from the gingival margin to the base of the periodontal pocket.
Radiographic measurement
Standardized intraoral periapical radiographs of the test site were taken with the RINN XCP system™ (Dentsply, USA) at baseline and 6 months postoperatively [Figure 1]. The radiographs were digitized using a scanner. All the images were saved in JPEG format and stored in internal hard disk of the laptop.
Figure 1.
IOPA taken with XCP technique
For interpretation, the digitized radiographic images were first opened within the Image-Tool program (XVa3 software) of rvg. The following landmarks were identified on the radiographs at baseline and 6 months postoperatively[5,6,7,8] [Figures 2 and 3]:
Figure 2.
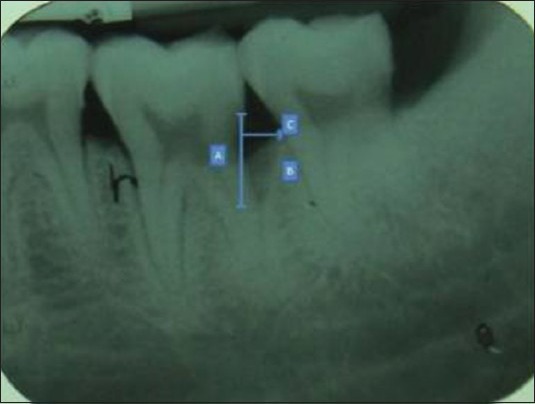
Technique demonstrating preoperative measurement taken with digital software
Figure 3.
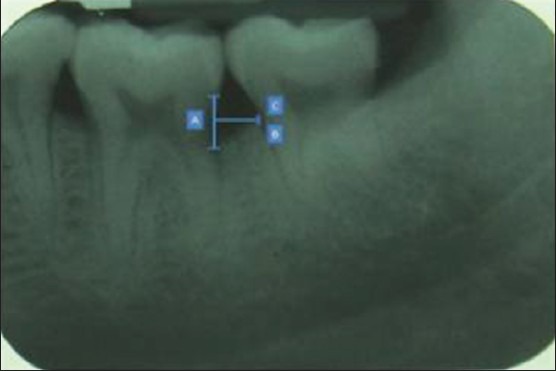
Technique demonstrating postoperative measurement taken after 6 months
Distance from the cemento-enamel junction (CEJ) to the base of the defect (BD) (CEJ–BD)
Distance from the CEJ to the alveolar crest (AC) (CEJ–AC)
Distance from the AC to the BD (AC–BD)
The above mentioned parameter were then calculated at 6 months following treatment. Linear measurements were taken with the measurement label tools in the action menu tab in XVa3 software. The cursor was placed on a fixed point from where the measurements were to be made. Then the mouse was dragged clicking the button to another fixed reference point. The measurements were made to the nearest millimeter and recorded. The same procedure was repeated for the postoperative radiographs and others. The distortion between the sets of radiographs was compensated for by calculation of the ratios of root length from baseline and follow-up radiographs.[6,7,8,9]
Surgical procedures
The surgical procedure was performed under local anesthesia with 2% lignocaine containing adrenaline at a concentration of 1:80,000. Intrasulcular incisions with reflection of full-thickness flaps were used to retain as much soft tissue as possible to attain primary closure. Debridement and root planing were done with hand instruments (Gracey curettes). After cleaning, the surgical area was irrigated with sterile saline. The surgical area was carefully inspected to ensure that the debridement procedure had been completed satisfactorily.
HA–β-TCP composite graft (Ossifi™) (Equinox Medical Technologies BV, Amersfoort, the Netherlands) [Figure 4] was emptied into a sterile dappen dish and four to six drops of saline were added. Graft material was added in increment and was condensed with an amalgam condenser until the defect was completely filled. The soft-tissue flap was then repositioned at the original level and closed with interrupted direct-loop 3-0 silk sutures. The surgical site was protected with a periodontal dressing [Figures 5-10].
Figure 4.
Ossifi graft material
Figure 5.
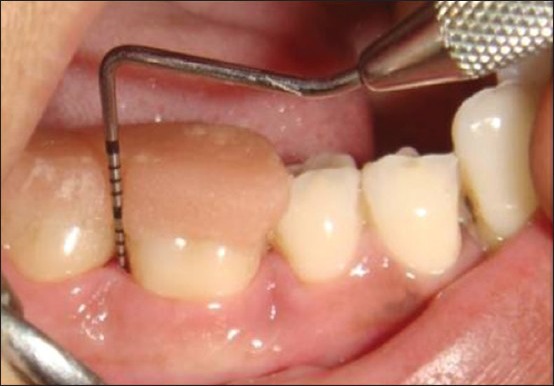
Preoperative pocket depth measurement
Figure 10.
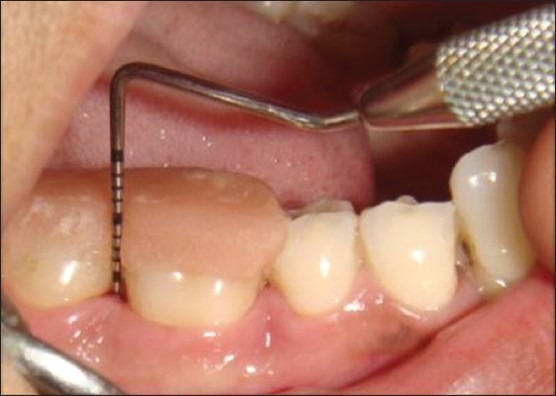
Postoperative pocket depth after 6 months
Figure 6.
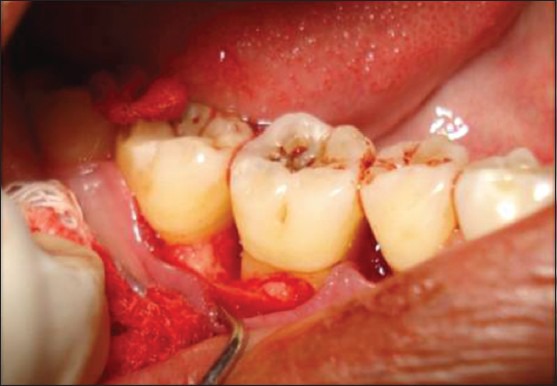
Three-wall defect after elevation of flap
Figure 7.
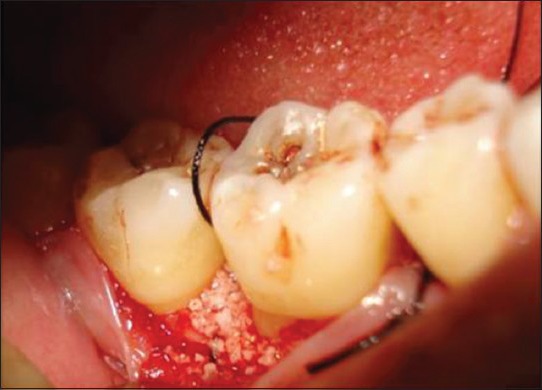
Bone graft placed after passing suture from the flap
Figure 8.
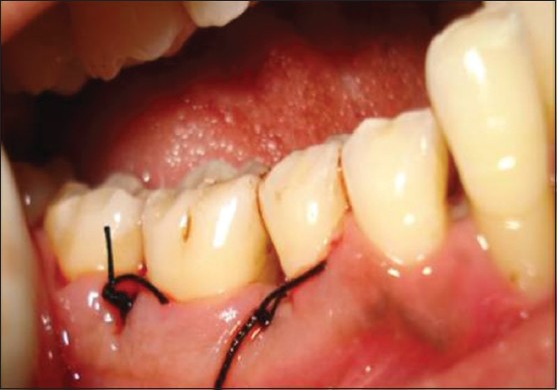
Flap suture after placement of graft
Figure 9.
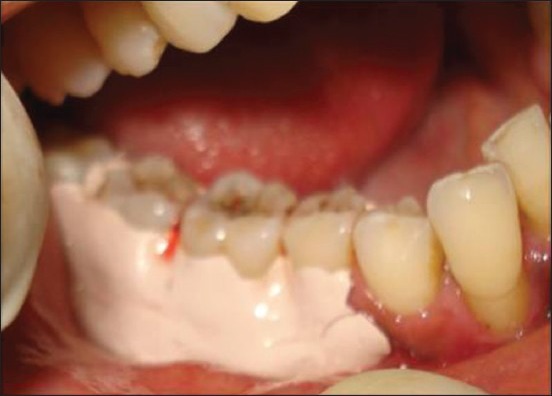
Coe-pak placed
Instructions were given to all subjects as a part of their postoperative regimen. The patients were advised to rinse with 10 ml of 0.2% chlorhexidine mouth wash twice daily for 14 days to help plaque control. They were advised to avoid chewing on the surgical site and told not to brush at the site or manipulate it for 10 days. The medications prescribed were antibiotics (500 mg Amoxicillin every 6 h for 5 days) and analgesics (400 mg Ibuprofen every 8 h). After 6 months postoperative radiograph was taken with the same mentioned technique and compared with preoperative radiograph. [Figures 11 and 12].
Figure 11.
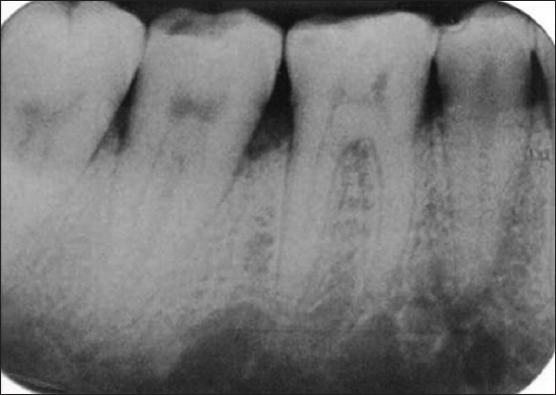
Preoperative radiograph of the defect
Figure 12.
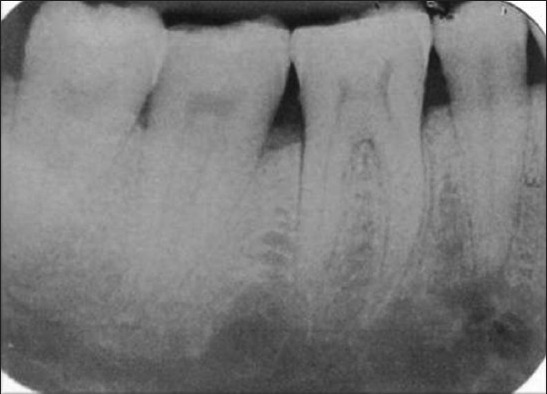
Postoperative radiograph of the defect
Statistical analysis
Statistical analysis was done using Statistical Package for Social Sciences (SPSS) Version 15.0 statistical analysis software. The values were represented as number (%) and Mean ± SD. The data were first tested for symmetry. It was found that for most of the parameters, the data were symmetrical. Hence, parametric analysis was performed. Analysis of variance was performed to see differences among groups, while post-hoc tests using Tukey's honestly significant difference (HSD) was performed to see intergroup differences.
RESULTS
Out of 20 patients, 16 (5 males and 11 females) returned for the postoperative evaluation. Four patients could not turn up for the 6th month postoperative follow-up as one female got pregnant, two others were forced to leave the city, and one male patient met with an accident, and therefore, they were dropped from the study. Remaining 16 patients completed the program and attended all the follow-up appointments.
Clinical measurements
The mean plaque index at baseline was 1.60 ± 0.41 (mean ± SD) which declined to 0.71 ± 0.28 after 6 months of periodontal regeneration therapy. The difference in reduction was statistically highly significant (P < 0.001) with a mean difference of 0.891 ± 0.217 and the t-value was 16.420 [Tables 1-3].
Table 1.
Baseline parameters
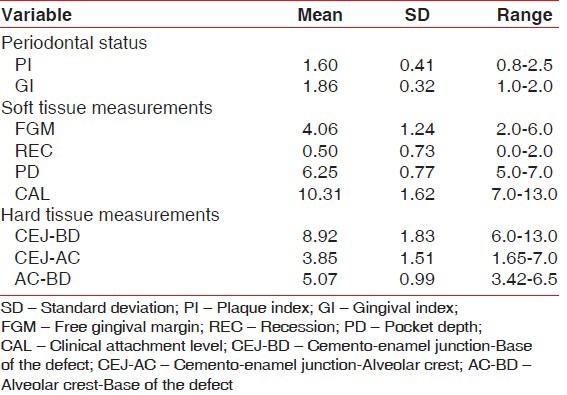
Table 3.
Comparison of change after treatment
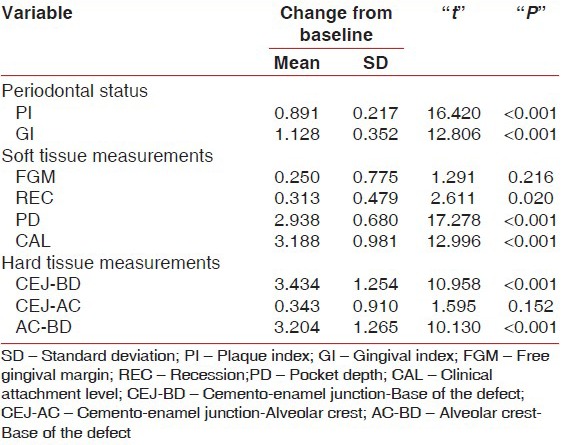
Table 2.
Parameters after treatment
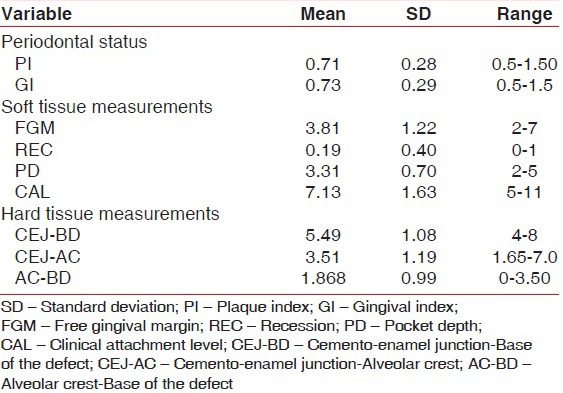
The mean gingival index at baseline was 1.86 ± 0.32 (mean ± SD) which declined to 0.73 ± 0.29 after 6 months of periodontal regeneration therapy. The difference in reduction was statistically highly significant (P < 0.001) with a mean difference of 1.128 ± 0.352 and the t-value was 12.806 [Tables 1-3].
The mean probing PD was 6.25 ± 0.77 mm at baseline with a range of 5.0-7.0 mm, which reduced to a mean of 3.31 ± 0.70 mm at 6 months with the range being 2.0-5.0 mm after periodontal regeneration therapy. The difference in reduction was 2.938 ± 0.68 mm which was found to be highly significant (P < 0.001) with a t-value of 17.278. Positive correlation was found between preoperative PD and CAL, with “r” being 0.677 which was significant at P < 0.01 [Tables 1-3].
The mean CAL was 10.31 ± 1.62 mm at baseline with a range of 7.0-13.0 mm, which reduced to a mean of 7.13 ± 1.63 mm at 6 months with the range being 5-11 mm after periodontal regeneration therapy. The difference in reduction was 3.188 ± 0.981 mm, which was found to be highly significant (P < 0.001) with a t-value of 12.996. Positive correlation was found between postoperative PD and CAL, with “r” being 0.720 which was significant at P < 0.01 [Tables 1-3].
Radiographic measurements
The mean CEJ–BD at baseline was 8.92 ± 1.83 mm with a range of 6.0-13.0, which reduced to 5.49 ± 1.08 mm with a range of 4-8 mm after the periodontal regeneration therapy. The difference in reduction of CEJ–BD was 3.434 ± 0.1.254 mm, which was statistically highly significant (P < 0.001) with a t-value of 10.958 [Tables 1-3].
The mean CEJ–AC at baseline was 3.85 ± 1.51 mm with a range of 1.65-7.0, which reduced to 3.51 ± 1.19 mm with a range of 1.65-7.0 mm after the periodontal regeneration therapy. The difference in reduction of CEJ–AC was 0.343 ± 0.910 mm, which was statistically not significant (P > 0.05) with a t-value of 1.595 [Tables 1-3].
The mean AC–BD at baseline was 5.07 ± 0.99 mm with a range of 3.42-6.5, which reduced to 1.868 ± 0.99 mm with a range of 3.42-6.5 mm after the periodontal regeneration therapy. The difference in reduction of AC–BD was 3.204 ± 1.265 mm, which was statistically highly significant (P < 0.001) with a t-value of 10.130 [Tables 1-3].
DISCUSSION
The field of periodontics is flooded with a variety of materials targeted as bone replacement grafts; however, the search for the optimal graft material continues. Autogenous bone has often been viewed as the gold standard against which other materials have been compared. Yet, autogenous bone may not be readily available in the surgical site, requiring a second site for harvesting.[10]
Alloplastic materials like HA and TCP have drawn considerable attention. Although HA and TCP have similar chemical composition, they differ in their biological resorbing capacity. The dense HA ceramics when used as bone implant are almost nonresorbable and bioinert. While the porous β-TCP containing ceramics display affinity for high-speed biological degradation, they are bioactive and bioresorbable.
The ideal graft material must be bioabsorbable to allow for host bone growth. The graft must be osteoconductive and must allow for host bone replacement in the graft space. So, BCP (HA–TCP) alloplast may be suitable as a bone fill material, as it potentially possesses both osteoconductive bone forming properties and the ability to be bioabsorbed.
The main feature of bioactive bone graft materials such as BCP ceramics is their ability to form a strong direct bond with the host bone, resulting in a strong interface, compared to bioinert or biotolerant materials which form a fibrous interface. Histologically, it was found that this bone substitute was completely integrated into a secondarily formed network of spongy bone, resulting in nearly complete osseous organization of the former defect area.[11]
Nery et al. studied the tissue response to BCP ceramic with different ratios of HA/β-TCP in periodontal osseous defects. They found that among the seven “active” treatment groups, two (65/35 and 85/15) had significantly higher gain in probing attachment levels than those in three groups (50/50, 100/0, and 0/100) (P < 0.05). Histologically, higher HA ratio (but not 100% HA) showed accelerated new bone formation and new attachment levels. Based on histological results, the 85 HA/15 β-TCP ratio appears to demonstrate greater gain in attachment level and bone regeneration in the treatment of periodontal osseous defects.[12]
The present study, therefore, envisaged to determine the efficacy of HA and β-TCP composite graft (BCP) in the treatment of periodontal intrabony defects. The patients included in this study were systemically healthy and presented with a PD and CAL greater than 5 mm and radiographic evidence of vertical bone loss.
There was no adverse reaction reported by any patient to the BCP during or after the surgery. All patients included in this study showed good oral hygiene throughout the length of the study, which was necessary for the success of any periodontal therapy.
In the present study, oral hygiene status was assessed by taking full-mouth plaque index at baseline and 6 months postoperatively. The plaque index and gingival index showed reduction over a period of 6 months. The mean plaque reduction from baseline to 6 months was from 1.6 ± 0.41 to 0.71 ± 0.28, which was statistically significant, and the mean gingival index reduction from baseline to 6 months was from 1.86 ± 0.32 to 0.77 ± 0.29, and the results were similar to those of Stein et al.[13]
Results of this clinical study demonstrated that BCP is beneficial in the treatment of intrabony defects. A mean reduction in PD of 2.938 mm (47.04%) was observed, while the clinical attachment gain was 3.188 mm (29.04%), which were statistically and clinically highly significant. Comparatively, Stein et al. reported a PD reduction of 3.6 mm and a mean clinical attachment gain of 3.0 mm as compared to baseline.[13] Sculean et al. demonstrated a reduction in mean PD of 3.3 mm and a reduction in mean CAL of 3.0 mm, which were stastically significant.[4] The present study demonstrated a bone gain of 63.195% after 6 months from baseline. The mean percentage resolution of defect from baseline to 6 months was statistically significant and was similar to other studies.[14]
Radiographically, the mean CEJ–AC difference was 0.343 mm (8.9%) which was statistically not significant; the mean CEJ–BD gain was 3.434 mm (38.45%) and the mean AC–BD gain was 3.204 (63.195%), which were highly significant similar to the study results of Zafiropoulos et al.[14]
Nery et al.[3] found that patients in the ceramic group had a gain in attachment level of 1.0 mm, those in the curettage group had a gain of 0.9 mm, and those in the bone implant group had a gain of 0.4 mm. Although the BCP patients had a greater gain, the difference was not statistically significant. In this veteran population, not only did BCP patients fail to outperform those in the control groups but also all three treatment groups were similarly ineffective.
Zafiropoulos et al.[15] treated the intrabony defects using guided tissue regeneration and autogenous spongiosa, alone or combined with HA/β-TCP bone substitutes or bovine-derived xenograft (BDX), and found that at baseline, no statistically significant differences in any of the clinical parameters were observed between the groups. At 12 months, HA/β-TCP and BDX treatment produced similar improvement in intrabony tissue regeneration.
In the present study, three-wall defects were selected. Bone fill was almost complete in few cases, while others showed partial bone fill. Ellegaard and Loe (1971), in their study on 191 defects, found that complete regeneration had occurred in 70% of three-walled defects and 45% of two-walled defects.
This conforms to the prevalent opinion of clinicians about the number of walls of bony defects that is required to have a better degree of bone fill. Intrabony 3 wall defects provide a good scaffold as they remain surrounded by bone from three sides and tooth from the fourth, thus providing a good stability to the graft material. It can, therefore, be concluded that the defect configuration plays an important role in the outcome of bone graft as the biomechanics of regeneration.
The clinical measurements were made using the UNC-15 probe and recorded to the nearest of 1 mm. The radiographic measurements were made with XVa3 software.
Digitized imaging has become an important tool in determining the subtle alterations seen on images of bone defects, not only because of the magnification of the image on the screen but also due to the possibility of adjusting its brilliance and contrast. Moreover, with improvements in the resources of digitized imaging programs, further advancement can be achieved in this field.[16]
A re-entry procedure, besides being unethical, could have provided information regarding the quality of newly formed tissues. Histological evaluation would have no doubt provided details about the actual healing process that would have occurred, but could not be done due to ethical and other concerns of morbidity.
The use of advanced diagnostic techniques such as third-generation probe and subtraction radiography would have perhaps made a more accurate estimation of the values possible.
Though HA–TCP (BCP) has shown promising results on clinical and radiographic evaluation in the present study, it would be appropriate to draw definite conclusions regarding the nature of defect fill. So, BCP (HA–TCP) can be an alternative to the bovine-derived materials if the patient is opposed to the use of bovine-derived graft materials. Further additional studies are required to compare its efficacy with other regenerative graft materials in human beings to analyze the maximum potential of BCP as a periodontal regenerative material.
CONCLUSION
The results of this study indicate that HA–TCP (BCP) may elicit significant new bone formation due to combination of the available pore size and rigid space for maintaining the scaffold. The slow bioabsorption may be an advantage in those in whom bone healing is slow, and is well tolerated by host tissues. It can only be speculated that the synthetic particle has a bone matrix maintenance influence on the crestal bone which allows regeneration of this bone, if it does indeed resorb, and possibly further allows the bone to grow around any adjacent particles. This is additionally supported by the radiographic findings that show the material to be most stable in areas adjacent to the osseous boundaries of the periodontal defects. Therefore, BCP was found to be a very effective material in the treatment of intrabony three-wall periodontal defect.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Novak MJ. Classification of diseases and conditions affecting the periodontium. In: Newman MG, Takei HH, Carranza FA, editors. Clinical Periodontology. 9th ed. Philadelphia: WB Saunders; 2002. pp. 64–72. [Google Scholar]
- 2.Nery EB, Lee KK, Czajkowski S, Dooner JJ, Duqqan M, Ellinger RF, et al. A veteran administration cooperative study of biphasic calcium phosphate ceramic in periodontal osseous defects. J Periodontol. 1990;61:737–44. doi: 10.1902/jop.1990.61.12.737. [DOI] [PubMed] [Google Scholar]
- 3.Nery EB, LeGeros RZ, Lynch KL, Lee K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/βTCP in periodontal osseous defects. J Periodontol. 1992;63:729–35. doi: 10.1902/jop.1992.63.9.729. [DOI] [PubMed] [Google Scholar]
- 4.Sculean A, Windisch P, Szendroi-Kiss D, Horváth A, Rosta P, Becker J, et al. Clinical and histologic evaluation of an enamel matrix derivative combined with a biphasic calcium phosphate for the treatment of human intrabony periodontal defects. J Periodontol. 2008;79:1991–9. doi: 10.1902/jop.2008.080009. [DOI] [PubMed] [Google Scholar]
- 5.William JR. The declaration of Helsinki and public health. Bull World Health Organ. 2008;86:650–2. doi: 10.2471/BLT.08.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch SE. Methods for evaluation of regenerative procedure. J Periodontol. 1992;63:1085–92. doi: 10.1902/jop.1992.63.12s.1085. [DOI] [PubMed] [Google Scholar]
- 7.Mayfield L, Soderholm G, Hallstorm H, Kullendorff B, Edwardsson S, Bratthall G, et al. Guided tissue regeneration for the treatment of intraosseous defects using a bioabsorbable membrane. J Clin Periodontol. 1998;25:585–95. doi: 10.1111/j.1600-051x.1998.tb02492.x. [DOI] [PubMed] [Google Scholar]
- 8.Persson GR, Falk H, Laurell L. A retrospective radiographic outcome assessment study of intra-bony defects treated by osseous surgery or by bone graft procedures. J Clin Periodontol. 2000;27:104–8. doi: 10.1034/j.1600-051x.2000.027002104.x. [DOI] [PubMed] [Google Scholar]
- 9.Eickholz P, Horr T, Klein F, Hassfeld S, Kim TS. Radiographic parameters for prognosis of periodontal healing of infrabony defects: Two different definitions of defect depth. J Periodontol. 2004;75:399–407. doi: 10.1902/jop.2004.75.3.399. [DOI] [PubMed] [Google Scholar]
- 10.Fleckenstein KB, Cuenin MF, Peacock ME, Billman MA, Swiec GD, Buxton TB, et al. Effect of a hydroxyapatite tricalcium phosphate alloplast on osseous repair in the rat calvarium. J Periodontol. 2006;77:39–45. doi: 10.1902/jop.2006.77.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz F, Herten M, Ferrari D, Wieland M, Schmitz L, Engelhardt E, et al. Guided bone regeneration at dehiscence- type defects using biphasic hydroxyapatite + beta tricalcium phosphate or a collagen coated natural bone mineral: As immunohistochemical study in dogs. Int J Oral Maxillofac Surg. 2007;36:1198–206. doi: 10.1016/j.ijom.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Nery EB, LeGeros RZ, Lynch KL, Lee K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/βTCP in periodontal osseous defects. J Periodontol. 1992;63:729–35. doi: 10.1902/jop.1992.63.9.729. [DOI] [PubMed] [Google Scholar]
- 13.Stein JM, Fickl S, Yekta SS, Hoischen U, Ocklenburg C, Smeets R. Clinical evaluation of a biphasic calcium composite grafting material in the treatment of human periodontal intrabony defects: A 12-month randomized controlled clinical trial. J Periodontol. 2009;80:1774–82. doi: 10.1902/jop.2009.090229. [DOI] [PubMed] [Google Scholar]
- 14.Zafiropoulos GG, Hoffmann O, Kasaj A, Willershausen B, Weiss O, Van Dyke TE. Treatment of intrabony defects using guided tissue regeneration and autogenous spongiosa alone or combined with hydroxyapatite/β tricalcium phosphate bone substitute or bovine derived xenograft. J Periodontol. 2007;78:2216–25. doi: 10.1902/jop.2007.070146. [DOI] [PubMed] [Google Scholar]
- 15.Zafiropoulos GG, Hoffmann O, Kasaj A. Treatment of intrabony defects using guided tissue regeneration and autogenous spongiosa alone or combined with hydroxyapatite/β tricalcium phosphate bone substitute or bovine derived xenograft. J Periodontol. 2007;78:2216–2225. doi: 10.1902/jop.2007.070146. [DOI] [PubMed] [Google Scholar]
- 16.Gomes-Filho IS, Sarmento VA, de Castro MS, da Costa NP, da Cruz SS, Trindade SC, et al. Radiographic features of periodontal bone defects: Evaluation of digitized images. Dentomaxillofac Radiol. 2007;36:256–62. doi: 10.1259/dmfr/25386411. [DOI] [PubMed] [Google Scholar]


