Abstract
Spinal cord injury (SCI) is a devastating type of neurological trauma with limited therapeutic opportunities. The pathophysiology of SCI involves primary and secondary mechanisms of injury. Among all the secondary injury mechanisms, the inflammatory response is the major contributor and results in expansion of the lesion and further loss of neurologic function. Meanwhile, the inflammation directly and indirectly dominates the outcomes of SCI, including not only pain and motor dysfunction, but also preventingneuronal regeneration. Microglia and macrophages play very important roles in secondary injury. Microglia reside in spinal parenchyma and survey the microenvironment through the signals of injury or infection. Macrophages are derived from monocytes recruited to injured sites from the peripheral circulation. Activated resident microglia and monocyte-derived macrophages induce and magnify immune and inflammatory responses not only by means of their secretory moleculesand phagocytosis, but also through their influence on astrocytes, oligodendrocytes and demyelination. In this review, we focus on the roles of microglia and macrophages in secondary injury and how they contribute to the sequelae of SCI.
Keywords: astrocytes, cytokines, chemokines, demyelination, inflammation, oligodendrocytes, M1/M2 activation, macrophages, microglia, secondary damage, spinal cord injury
Introduction
Spinal cord injury (SCI) is associated with devastating neurological outcomes and limited therapeutic opportunities. It has three phases: acute, secondary and chronic (Oyinbo, 2011). The outcomes of SCI are mainly influenced by the secondary phase. SCI causes inflammatory responses through the activation of innate immune responses that contribute to secondary injury (Fehlings and Nguyen, 2010). Macrophages accumulated within the epicenter and the hematoma of the injured spinal cord play a significant role in this inflammation (Zhang et al., 2013). Microglia/macrophages associated inflammation appears to be a significant mechanism related to neuronal degeneration and regeneration. Macrophages in the central nervous system (CNS) derived from blood monocytes and resident microglia, are pervasive in the injured spinal cord and change their phenotypes and functions in response to signals in the lesion environment.
In this review, we discuss the behavior and influence of microglia/macrophages during secondary damage from following perspectives: (1) the pathophysiology of spinal cord injury, and (2) how microglia and macrophages affect secondary injury, and the subsets of microglia/macrophages and their interrelationships in secondary injury mechanisms.
Characteristics and stages/phases of SCI
SCI, which is characterized by primary physical damage and secondary damage, results in severe sequelae such as paralysis, intense pain, and progressive neurological damage. The primary injury is typically restricted to the specific area of vertebral fracture and is characterized by acute hemorrhage and ischemia, which serve as the foci from which secondary mechanisms of injury are induced (Ray et al., 2002; Simon et al., 2009). The secondary injury, which is characterized by further destruction of neuronal and glial cells, leads to a significant expansion of the injury site, and allows paralysis to extend to adjacent spinal cord segments.
SCI has different characteristics. From the aspect of morphology, primary mechanical damage to SCI is characterized by direct destruction of spinal tissues, including the blood-spinal cord barrier, while secondary damage is characterized by inflammation that may cause reactive gliosis, edema, and cavitation of spinal parenchyma (Fleming et al., 2006). From the aspect of biological response, SCI can be divided into three phases: (1) acute (seconds to minutes after the injury), (2) secondary (minutes to weeks after the injury), and (3) chronic (months to years after the injury) (Oyinbo, 2011) (Table 1). Based on some previous SCI studies (Dumont et al., 2001; Rossignol et al., 2007; Fehlings and Nguyen, 2010; Oyinbo, 2011; Shin et al., 2013), SCI can be divided into four processes: (1) a primary mechanical insult, characterized by vasospasm and cell death from direct impact; (2) spreading of the injury, owing to vascular insults such as hemorrhage and ischemia-reperfusion; (3) immune/inflammatory reactions, characterized by apoptosis, demyelination of surviving axons and immune-mediated cell death; (4) stabilization, characterized by central cavitationand chronic scar formation.
Table 1.
The phases of spinal cord injury

Secondary damage following SCI
As summarized from some previous SCI research (Dumont et al., 2001; Ramer et al., 2005; Liu et al., 2006; Tanhoffer et al., 2007; Fehlings and Nguyen, 2010; Varnum and Ikezu, 2012; Zhang et al., 2012), secondary damage/injury after SCI has the following aspects: (1) timing: secondary damage mechanisms initiate within minutes after injury and last for weeks or months; (2) location: secondary damage is not only restricted to the area of the vertebral fracture, but also extends to adjacent segments and even influences the whole body; (3) mechanisms of damage: secondary injury following spinal cord trauma is multifactorial (McCormick, 1998; Ramer et al., 2005) (Table 1); (4) morphology: secondary damage after SCI is characterized by hematoma, edema, glial/axon scarring, and central cavitation; (5) cytokine secretion: pro-inflammatory cytokines and chemokines such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1β (IL-1β), IL-6, IL-23, leukemia inhibitory factors (LIF) and inducible nitric oxide synthase (iNOS); and anti-inflammatory cytokines such as IL-10, IL-4, IL-13, and transforming growth factor β (TGF-β).
Among all the mechanisms of secondary damage, inflammation is the most important, and directly or indirectly controls the sequelae after SCI. The inflammatory responses can be spatially and temporally subdivided into several phases: immediate neutrophil invasion and activation of resident microglia at 0–2 days, recruitment of blood monocytes to the lesion at 3–7 days, and resolution of the scar by anti-inflammatory macrophages and axonal regrowth from day 7 onward (Sroga et al., 2003; Fleming et al., 2006; Kigerl et al., 2009; Jiang et al., 2013). The release of inflammatory cytokines and chemokines from spinal cord cells in or near the lesion starts the inflammatory responses (Carlson et al., 1998; Donnelly and Popovich, 2008; Popovich and Longbrake, 2008). These mediators lead to the sequentially orchestrated activation and migration of microglia towards thelesion and recruitment of circulating leukocytes to the injury (Carlson et al., 1998; Taoka and Okajima, 2000).
The potential benefits and the tissue-damaging consequences of the inflammatory response after central nervous system injury have long been disputed. But now, there is a consensus that inflammation has both beneficial and tissue-damaging effects. Obviously, inflammation causes destructive activities such as widespread cell damage and deterioration of the extracellular matrix (Gutteridge and Halliwell, 1989; Pratico et al., 2001; Pratico and Sung, 2004; Sinescu et al., 2010). These early inflammatory events in the first week after SCI also create a hostile microenvironment for various SCI treatments, thus creating obstacles for transplantation-based therapies (Okano et al., 2003; Coyne et al., 2006). Local and systematic inflammatory responses not only result in the pathogenesis of neurodegenerative events during acute and chronic phases of SCI, but also subsequently lead to the death of glia and neurons, forming glial scar and a cavity in the spinal parenchyma (Fleming et al., 2006; Kigerl et al., 2009). Recent studies have shown that inflammation benefits neuronal regeneration and functional recovery. Activated macrophages play an important role in this period. The inflammation mediated by activated microglia/macrophages plays an important role in clearance of damaged and degenerating tissues(Greenhalgh and David, 2014). As summarized by Ren and Young, abrogating the pro-inflammatory environment in the injured spinal cord has therefore become a major therapeutic target to reduce secondary cell death and promote neuronal regeneration (Gensel et al., 2011; Ren and Young, 2013).
Microglia and macrophages in secondary injury
As a hallmark of SCI pathology and a pivotal inflammatory cell in the central nervous system, the macrophage has become a topic of intense research interest (Fleming et al., 2006; Donnelly and Popovich, 2008). Two macrophage populations, namely resident microglia and monocyte-derived macrophages, participate in and respond to degeneration and regeneration of spinal tissues after SCI (Beuche and Friede, 1984; Heumann et al., 1987; Perry et al., 1987; Stoll and Muller, 1999; Horie et al., 2004). Resident microglia are located in the immune-privileged CNS tissues, including the brain, the eye, and the spinal cord, which are secluded from the peripheral circulation by a complex of barriers (Hanisch and Kettenmann, 2007; Ransohoff and Perry, 2009; Rivest, 2009; Graeber, 2010; Ransohoff and Cardona, 2010; David and Kroner, 2011; Prinz et al., 2011; Saijo and Glass, 2011). Macrophages/microglia were once thought to play a negative role in secondary tissue damage following CNS injury (Bracken et al., 1990). Popovich et al. (1999) showed that reducing the infiltration of macrophages could diminish secondary tissue damage. However, because there are no reliable morphological or specific antigenic markers that can differentiate between the resident microglia and bone marrow derived macrophages, it is difficult to clarify the respective roles of these two macrophage populations. By taking advantage of bone marrow chimeras, infiltrating bone marrow derived macrophages can be distinguished from resident microglia (Hickey and Kimura, 1988; Mueller et al., 2003). Shechter et al. (2009) showed that monocyte-derived macrophages are often localized mainly in the margins of the lesion site following SCI, while the resident microglia are distributed in the lesion core and its margins (Rolls et al., 2008; Shechter et al., 2009). However, our unpublished data have suggested that macrophages and microglia cells have unique phenotypes and locations. Residential microglia can be distinguished from recently recruited bone marrow derived macrophages based on the expression of Mac-2 (galactin-3). After injury, infiltrating bone marrow-derived macrophages (CX3CR1low/Mac-2high) migrate to the epicenter of injury, while microglia (CX3CR1high/Mac-2low) localize to the edges of lesion (Figure 1). In other words, a vast majority of macrophages in the lesion site arecirculating bone marrow cells rather than locally activated microglia cells after 4 weeks. These two populations of macrophages may have different functions. Residential microglial cells form a border that seems to seal the lesion and block the spread of damage (Hines et al., 2009). In contrast, bone marrow derived macrophages (BMDMs) enter the epicenter of injured spinal cord andphagocytize apoptotic and necrotic cells and clear tissue debris such as myelin debris (David and Kroner, 2011). Greenhalgh and David (2014) recently showed that microglia play a major role to clear damaged and degenerate tissues at 3 days after injury and BMDMs predominantly contribute to the phagocytosis, which persists for up to 42 days.
Figure 1.
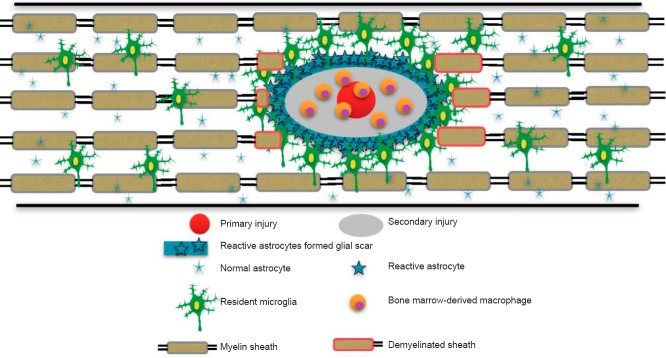
After primary injury, the nearby astrocytes and resident microglia are activated and migrate towards the injury site.
Activated astrocytes and other glial cells including fibroblasts form the glia scar. Microglial cells are mainly present in the marginal and uninjured areas. Bone marrow-derived macrophages accumulate at the epicenter of injured spinal cord.
Subsets of microglia/macrophages
Several macrophage subsets have been classified based on the expression of cell surface markers, intracellular enzymes, and secreted molecules, including M1 (classical activation), M2 (alternative activation), regulatory macrophages, tumor-associated macrophages (TAM), myeloid-derived suppressor cells (MDSC), and so forth (Classen et al., 2009; Menzies et al., 2010; Cassetta et al., 2011; Murray and Wynn, 2011; Vereyken et al., 2011; Comalada et al., 2012; Shechter and Schwartz, 2013). M1 and M2 are often seen as the two primary subsets of macrophages at the injured site. Depending on the phenotypes and activation status of macrophages, they may not only initiate secondary damage, but also initiate repair. The phenotypes and functions of macrophages in the injured spinal cord are dynamic and can change according to the microenvironment in the spinal lesion (Stout and Suttles, 2004; Menzies et al., 2010). As reported, M1 (CD86-positive) and M2 (arginase-1-positive) macrophages coexist at the lesion epicenter during the first week after SCI, but only M1 macrophages persist until day 28 post-injury in mice (Kigerl et al., 2009). Our unpublished data showed that macrophages phagocytosis of myelin debris are detected in injury site from 1–2 weeks after SCI. These myelin-laden macrophages exhibit M1 like phenotype and persist for long period of time (Figure 2). It was also reported that M1 microglia appear immediately after injury and secrete pro-inflammatory cytokines and chemokines that both lead to further damage following primary mechanical injury (Nakajima et al., 2012). The appearance of M2 macrophages and secreted anti-inflammatory cytokines and chemokines lead to the suppression of excessive inflammatory responses around the injured spinal cord and regeneration of injured spinal tissues (Gratchev et al., 2008; Varnum and Ikezu, 2012; Shechter and Schwartz, 2013; Weisser et al., 2013). In addition, a switch from M1 to M2 in the injured spinal cord, induced by transplantation of stem cells (neural and other), prevents axonal damage and improves locomotor function (Busch et al., 2011; Cusimano et al., 2012).
Figure 2.
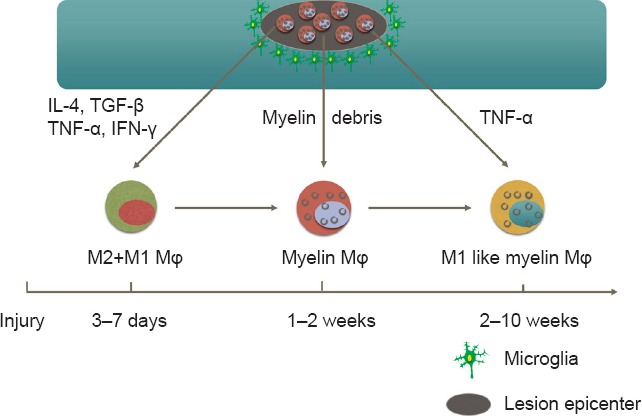
Bone-marrow derived macrophages (BMDMs) are recruited to lesion epicenter at 3–7 days after injury.
Both M1 and M2 macrophages are detected in lesion epicenter during the first week after spinal cord injury (SCI). Macrophages phagocytosis of myelin debris (myelin Mϕ) are detected in injury site from 1–2 weeks after SCI. These myelin-laden macrophages (myelin Mϕ) exhibit M1 like phenotype and persist for long period of time. IL-4: Interleukin-4; TGF-β: transforming growth factor β; TNF-α: tumor necrosis factor-α; IFN-γ: interferon-γ.
However, it is not clear which factors in the injured spinal cord result in a phenotype switch of macrophages. Although the expression of cytokines is a major determinant of macrophage activation (Gordon and Taylor, 2005; Mosser and Edwards, 2008), this can be driven by lesion-related factors as well because rapid increases in pro-inflammatory cytokines are only detected before macrophages influx (Pineau and Lacroix, 2007; David and Kroner, 2011). Kroner et al. (2014) demonstrated that iron accumulated in macrophages in SCI increases TNF-α expression that prevents myelin phagocytosis-medicated conversion from M1 to M2. Our unpublished data demonstrated that myelin debris is one of lesion-associated factors altering the M2 phenotype. To clearly illustrate the role of macrophages in secondary damage after SCI, the differences between M1 and M2 macrophages during secondary injury isdetailed in Table 2.
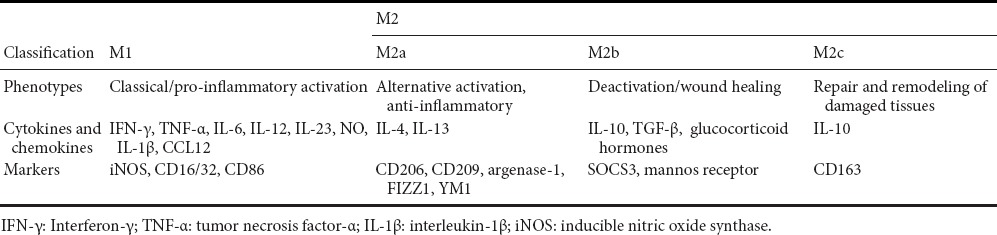
Table 2.
Classification of macrophages
Classically activated microglia/macrophages (M1)
Classical activation involves the induction of M1 macrophages by Th1 cell-derived cytokines. Generally, the properties of M1 macrophages in inflammation during secondary damage are neurotoxic and growth inhibitory. Because of these properties, M1 macrophages contribute to the formation of axonal growth-inhibitory glial scar and production of pro-inflammatory radicals/mediators, leading to a hostile environment at the lesion site, which results in the limited regenerative nature of the injured spinal cord (Shechter and Schwartz, 2013). Markers for M1 macrophages in the inflammation phase of secondary damage include NOX, NOS2, CD16/32 and CD86 (Gutteridge and Halliwell, 1989; Brown, 2007; Kigerl et al., 2009) (Table 2). Activated M1 macrophages produce a high level of pro-inflammatory molecules such as IL-1β, IL-6, IL-12, IL-23, TNF-α, IFN-γ, chemokine (C-C motif) ligand 5 (CCL5), nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, inducible nitric oxide synthase (iNOS), toxic intermediates, and opsonic receptors such as immunoglobulin Fcγ receptors (Unkeless et al., 1988; Gutteridge and Halliwell, 1989; Ravetch and Kinet, 1991; Heinrich et al., 1998; Akiyama et al., 2000; Pratico et al., 2001; Gordon and Taylor, 2005; Patel et al., 2005; Brown, 2007; Bellora et al., 2010). Classical activation also causes the release of proteolytic enzymes that can lead to deterioration of the extracellular matrix, such as metalloproteinases, collagenases, and furin, thus degrading cellular integrity and leading to easier destruction of the cell (Chandler et al., 1995; Maeda and Sobel, 1996; Rosenberg et al., 2001; Rosenberg, 2009; Shiryaev et al., 2009). Meanwhile, these cells secrete low levels of anti-inflammatory mediators (Kigerl et al., 2009; Shechter et al., 2013). M1-polarized macrophages show the ability to induce neuron death directly through iNOS activity and the capacity to obliquely contribute to secondary degradation (Kigerl et al., 2009).
Alternatively activated macrophages (M2)
Some researchers believe that M2 macrophages are generated from the phenotypic switch of M1 macrophages/activated microglia for inflammation resolution, but the detailed conditions and the timing of this generation are still unknown (Gratchev et al., 2008; Varnum and Ikezu, 2012; Weisser et al., 2013). We do know that M2 macrophages in SCI play an important role in resolving pro-inflammatory milieus produced by M1 macrophages and some CNS glia (both resident microglia and astrocytes), thereby supporting neuroprotection and regeneration of spinal tissues, and promoting renewal of damaged cells from progenitors. The properties of M2 macrophages are resolving/anti-inflammatory function, neuro/axonal-trophic support and scar-degrading capacities (Shechter and Schwartz, 2013). Markers for M2 macrophages are arginase-1, Ym1, found in inflammatory zone 1 (FIZZ1), CD206, CD209, CD163, and mannose receptor (MR) and IL-1 receptor antagonist (IL-1Ra) (Table 2) (Simmons and Seed, 1988; Ezekowitz et al., 1990; Law et al., 1993; Relloso et al., 2002; Menzies et al., 2010; Komori et al., 2011; Andrade et al., 2012; Rodriguez Guerrero et al., 2012; Varnum and Ikezu, 2012). Kigerl et al. (2009) showed a comparatively smaller and transient M2 macrophage response in SCI, which probably explains the prolonged pro-inflammatory response, with detrimental effects on tissue viability if not terminated on time (Shechter and Schwartz, 2013). Importantly, a deregulated M2 response might be detrimental as well when its timing, dosing or location is not optimally controlled.
Alternative activation of macrophages is induced by Th2 cell-derived cytokine activation. M2 macrophages can be further divided into three subsets: M2a, M2b, and M2c. The three subsets all play important roles in anti-inflammation and repair of a spinal lesion during secondary damage. M2a macrophages promoted by IL-4, IL-13, and arginase-1 mainly participate in reducing inflammation, enhancing phagocytosis and differentiation of neural stem cells (NSCs) (Varnum and Ikezu, 2012). Arginase-1 antagonizes iNOS and contributes to the anti-inflammatory response (Ahn et al., 2012). As an essential cytokine for M2a skewing (Maher et al., 2005; Nolan et al., 2005; Lyons et al., 2007) and a ligand of M2a, IL-4 has many functions during secondary inflammation. Through stimulating microglia/macrophages, IL-4 could up-regulate IGF-1 to induce neurogenesis, or down-regulate TNF-α and enhance neural differentiation (Butovsky et al., 2005; Butovsky et al., 2006; Kiyota et al., 2010). An in vivo study shows that gene delivery of IL-4 can directly enhance neurogenesis and restore impaired motor function (Kiyota et al., 2010). M2b macrophages contribute to the clearance of reactive nitrogen and oxygen species released during M1 activation, and take part in the production of CCL1 and IL-10. Immune complexes and TLR or IL-1R agonists are responsible for M2b skewing (Mantovani et al., 2004). The phenotype of M2c macrophages is deactivation/wound healing. These cells enhance proliferation of NSCs and deactivation of glial inflammation (Maher et al., 2005; Kiyota et al., 2010) through expressing CCR2, CCR5, chemokine (C-X-C motif) ligand 13 (CXCL13), and CCL16, CCL17, and CCL18 (Mantovani et al., 2004). IL-10 is a M2c ligand (Ekdahl et al., 2003) and an important cytokine for M2c skewing (Mantovani et al., 2004). Through stimulating microglia, IL-10 could enhance proliferation but not differentiation of NSCs (Kiyota et al., 2010), and promote anti-inflammatory responses including reducing pro-inflammatory cytokines and preventing glial activity (Plunkett et al., 2001) (Table 2).
It has been shown that administration of M2 macrophages is beneficial to noninfectious CNS inflammation including SCI (Weber et al., 2007; Shechter and Schwartz, 2013). As summarized by Shin et al. (2013), alternatively activated M2 macrophages over the M1 phenotype is a common phenomenon associated with functional recovery of SCI regardless of treatment regimens. Importantly, apart from the functions of M2 macrophages, the therapies summarized by Shin et al. are all associated with reduction of pro-inflammatory molecules and increase of anti-inflammatory molecules (Shin et al., 2013). These data suggest thatsome therapies to improve recovery from SCI may rely on the anti-inflammatory of M2 macrophages.
As mentioned above, the function of microglia/macrophages in SCI cannot be regarded as a simple dichotomy of bad–good or M1–M2; a much more complex scenario should be considered. To solve this problem, some researchers point out that we should classify macrophage phenotypes in another way. With this idea, macrophages are classified into six subsets according to their activities: inflammation, phagocytosis, vascular remodeling, matrix rebuilding, regeneration, and immune regulation (Hanahan and Weinberg, 2000; Condeelis and Pollard, 2006).
Microglia/macrophages and glial scar comprised of astrocytes
The formation of a glial scar is mainly mediated by activated astrocytes and other glia (Schwab and Bartholdi, 1996). A glial scar builds a barrier around the lesion epicenter (Figure 1). The infiltration of macrophages contributes to axonal diebacks, which represent the phenomenon where axons retract from a spinal lesion. A study utilizing a model of glial scar has demonstrated that macrophages are associated with unhealthy axons and directly lead to long-distance retraction of axons (Horn et al., 2008; Busch et al., 2009). Activation of astrocytes follows and is promoted by the microglial response (Kreutzberg, 1996; Popovich et al., 1997). Inhibition of microglia has been shown to reduce damage to oligodendrocytes, inhibit axonal dieback, change the formation of glial scar, and improve recovery of locomotive function (Stirling et al., 2004; Festoff et al., 2006; Yune et al., 2007).
Microglia/macrophages and oligodendrocytes
During secondary damage after SCI, the oligodendrocytes not only influence signaling efficiency and conductance of axons, but also contribute to neuronal survival and preservation of axonal structural integrity (McTigue et al., 2006). Oligodendrocytes are injured by macrophages at the lesion epicenter after the injury and continue to undergo apoptosis in the spinal parenchyma for many weeks after SCI (Classen et al., 2009; Comalada et al., 2012). Oligodendrocytes are responsible for the myelination of multiple axons. The results of the loss of oligodendrocytes are the demyelination of many spared axons and the loss of conduction of action potential by ascending and descending lateral axons (Hains et al., 2003). As axons provide essential connections between brain and caudal spinal neurons, damage to spinal axons can cause many clinical problems (McTigue, 2008). Activated and resting macrophages and microglia secrete molecules such as IL-1β, glutamate, NOS and TNF-α which all contribute to secondary death of oligodendrocyte cells (Streit et al., 1998; Merrill and Scolding, 1999; Rosenberg et al., 1999).
Microglia/macrophages and demyelination
Astrogliosis is a pervasive response to different insults to the adult CNS, including trauma, toxicity, and genetic and degenerative diseases (Norton et al., 1992; Eddleston and Mucke, 1993; Sofroniew and Vinters, 2010). Astrogliosis is responsible for the failure of remyelination in many experimental models of demyelination and demyelinating pathologies (Sergott et al., 1985; Bunge et al., 1993; Butt and Berry, 2000; Holley et al., 2003; Keirstead et al., 2005; Frohman et al., 2006). The pathological process of demyelination due to the loss of oligodendrocytes is particularly active during the sub-acute (secondary) and chronic phase of SCI (Guest et al., 2005; Joy, 2005). Recent studies suggested that immunological demyelination is accompanied by a robust activation of macrophage/microglial cells without an astrogliosis response (Cloutier et al., 2013). The activities of macrophages and microglia following SCI are maximal between 3 and 7 days post-injury. Notably, activated macrophages and microglia were reported to exclusively locate to regions of immunological demyelination, with only a few of them outside of the region. In spinal lesions during secondary injury after SCI, the activities of microglia and macrophages were significantly higher within regions of immunological demyelination (Cloutier et al., 2013). Immunological demyelination creates a unique environment in which astrocytes do not form a glial scar and provides a unique model to understand the putative interaction between astrocytes and activated macrophage/microglial cells. However, during the process of demyelination, axons are directly exposed to damaging effects such as inflammatory cytokines and free radicals, leading to neuronal loss. As a result, demyelination leads to conduction delays and conduction block (McTigue, 2008; Hall and Traystman, 2009).
Conclusion
This review has explored the relationship between microglia/macrophages and the secondary damage that develops after SCI. By understanding how macrophages either promote or prevent secondary damage in spinal cord inflammation, we may be able to deduce new approaches for mitigating the currently poor outcomes after SCI and promoting the recovery of motor function in affected patients. Meanwhile, it is critically important to understand how current or planned therapies influence and/or interact with macrophages, even if macrophages are not the designated therapeutic target.
Footnotes
Funding: This work was supported by grants from National Institutes of Health (R01GM100474) and the New Jersey Commission on Spinal Cord Research (CSCR13IRG005.
References
- Ahn M, Lee C, Jung K, Kim H, Moon C, Sim KB, Shin T. Immunohistochemical study of arginase-1 in the spinal cords of rats with clip compression injury. Brain Res. 2012;1445:11–19. doi: 10.1016/j.brainres.2012.01.045. [DOI] [PubMed] [Google Scholar]
- Akiyama H, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade MR, Amaral EP, Ribeiro SC, Almeida FM, Peres TV, Lanes V, D’Imperio-Lima MR, Lasunskaia EB. Pathogenic Mycobacterium bovis strains differ in their ability to modulate the proinflammatory activation phenotype of macrophages. BMC Microbiol 12. 2012 doi: 10.1186/1471-2180-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellora F, Castriconi R, Dondero A, Reggiardo G, Moretta L, Mantovani A, Moretta A, Bottino C. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proc Natl Acad Sci U S A 107. 2010:21659–21664. doi: 10.1073/pnas.1007654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuche W, Friede RL. The role of non-resident cells in wallerian degeneration. J Neurocytol. 1984;13:767–796. doi: 10.1007/BF01148493. [DOI] [PubMed] [Google Scholar]
- Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochemical Society Transactions. 2007:1119–1121. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury: A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Advances in Neurology; Neural injury and regeneration (Seil FJ, ed) 1993:75–89. [PubMed] [Google Scholar]
- Busch SA, Horn KP, Silver DJ, Silver J. Overcoming macrophage-mediated axonal dieback following CNS injury. J Neurosci. 2009;29:9967–9976. doi: 10.1523/JNEUROSCI.1151-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Hamilton JA, Horn KP, Cuascut FX, Cutrone R, Lehman N, Deans RJ, Ting AE, Mays RW, Silver J. Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J Neurosci. 2011;31:944–953. doi: 10.1523/JNEUROSCI.3566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Mol Cell Neurosci. 2005;29:381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Butt AM, Berry M. Oligodendrocytes and the control of myelination in vivo: New insights from the rat anterior medullary velum. J Neurosci Res. 2000;59:477–488. doi: 10.1002/(SICI)1097-4547(20000215)59:4<477::AID-JNR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- Cassetta L, Cassol E, Poli G. Macrophage polarization in health and disease. ScientificWorldJournal. 2011;11:2391–2402. doi: 10.1100/2011/213962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler S, Coates R, Gearing A, Lury J, Wells G, Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201:223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- Classen A, Lloberas J, Celada A. Macrophage activation: classical vs. alternative. Methods in Molecular Biology (Reiner NE, ed) 2009:29–43. doi: 10.1007/978-1-59745-396-7_3. [DOI] [PubMed] [Google Scholar]
- Cloutier F, Sears-Kraxberger I, Keachie K, Keirstead HS. Immunological demyelination triggers macrophage/microglial cells activation without inducing astrogliosis. Clin Dev Immunol 2013. 2013:812456–812456. doi: 10.1155/2013/812456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comalada M, Yeramian A, Modolell M, Lloberas J, Celada A. Leucocytes: Methods and Protocols (Ashman RB, ed); 2012. Arginine and macrophage activation; pp. 223–235. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24:2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- Cusimano M, Biziato D, Brambilla E, Donega M, Alfaro-Cervello C, Snider S, Salani G, Pucci F, Comi G, Manuel Garcia-Verdugo J, De Palma M, Martino G, Pluchino S. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135:447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont RJ, Okonkwo DO, Verma RS, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254–264. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Mucke L. Molecular profile of reactive astrocytes - implication for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz RAB, Sastry K, Bailly P, Warner A. Molecular characterization of the human macrophage mannose receptor - demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in cos-1 cells. J Exp Med. 1990;172:1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlings MG, Nguyen DH. Immunoglobulin G: A Potential Treatment to Attenuate Neuroinflammation Following Spinal Cord Injury. J Clin Immunol. 2010;30:S109–112. doi: 10.1007/s10875-010-9404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006;97:1314–1326. doi: 10.1111/j.1471-4159.2006.03799.x. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Medical progress: Multiple sclerosis - The plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Donnelly DJ, Popovich PG. Spinal cord injury therapies in humans: an overview of current clinical trials and their potential effects on intrinsic CNS macrophages. Expert Opin Ther Targets. 2011;15:505–518. doi: 10.1517/14728222.2011.553605. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Gratchev A, Kzhyshkowska J, Kannookadan S, Ochsenreiter M, Popova A, Yu X, Mamidi S, Stonehouse-Usselmann E, Muller-Molinet I, Gooi L, Goerdt S. Activation of a TGF-beta-specific multistep gene expression program in mature macrophages requires glucocorticoid-mediated surface expression of TGF-beta receptor II. J Immunol. 2008;180:6553–6565. doi: 10.4049/jimmunol.180.10.6553. [DOI] [PubMed] [Google Scholar]
- Greenhalgh AD, David S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J Neurosci. 2014;34:6316–6322. doi: 10.1523/JNEUROSCI.4912-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–393. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Gutteridge JM, Halliwell B. Iron toxicity and oxygen radicals. Baillieres Clin Haematol. 1989;2:195–256. doi: 10.1016/s0950-3536(89)80017-4. [DOI] [PubMed] [Google Scholar]
- Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Na(v)1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J Neurosci. 2003;23:8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Traystman RJ. Role of animal studies in the design of clinical trials. Clinical Trials in the Neurosciences (Woodbury-Harris KM, Coull BM, eds) 2009:10–33. doi: 10.1159/000209470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanisch U-K, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H. Differential regulation of messenger-RNA encoding nerve growth-factor and its receptor in rat sciatic-nerve during, development, degeneration, and regeneration - role if macrophages. Proc Natl Acad Sci U S A. 1987;84:8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines DJ, Hines RM, Mulligan SJ, Macvicar BA. Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia. 2009;57:1610–1618. doi: 10.1002/glia.20874. [DOI] [PubMed] [Google Scholar]
- Holley JE, Gveric D, Newcombe J, Cuzner ML, Gutowski NJ. Astrocyte characterization in the multiple sclerosis glial scar. Neuropathol Appl Neurobiol. 2003;29:434–444. doi: 10.1046/j.1365-2990.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- Horie H, Kadoya T, Hikawa N, Sango K, Inoue H, Takeshita K, Asawa R, Hiroi T, Sato M, Yoshioka T, Ishikawa Y. Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J Neurosci. 2004;24:1873–1880. doi: 10.1523/JNEUROSCI.4483-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28:9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MH, Lim JE, Chi GF, Ahn W, Zhang M, Chung E, Son Y. Substance P reduces apoptotic cell death possibly by modulating the immune response at the early stage after spinal cord injury. Neuroreport. 2013;24:846–851. doi: 10.1097/WNR.0b013e3283650e3d. [DOI] [PubMed] [Google Scholar]
- Joy JE, Altevogt BM, Liverman CT, Johnson RT. Spinal cord injury: progress, promise, and priorities. 2005 [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Okuyama S, Swan RJ, Jacobsen MT, Gendelman HE, Ikezu T. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer's disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J. 2010;24:3093–3102. doi: 10.1096/fj.10-155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Morikawa Y, Inada T, Hisaoka T, Senba E. Site-specific subtypes of macrophages recruited after peripheral nerve injury. Neuroreport. 2011;22:911–917. doi: 10.1097/WNR.0b013e32834cd76a. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Law SKA, Micklem KJ, Shaw JM, Zhang XP, Dong Y, Willis AC, Mason DY. A new macrophage differentiation antigen which is a member of the scavenger receptor superfamily. Eur J Immunol. 1993;23:2320–2325. doi: 10.1002/eji.1830230940. [DOI] [PubMed] [Google Scholar]
- Liu NK, Zhang YP, Titsworth WL, Jiang XY, Han S, Lu PH, Shields CB, Xu XM. A novel role of phospholipase A(2) in mediating spinal cord secondary injury. Ann Neurol. 2006;59:606–619. doi: 10.1002/ana.20798. [DOI] [PubMed] [Google Scholar]
- Lyons A, Griffin RJ, Costelloe CE, Clarke RM, Lynch MA. IL-4 attenuates the neuroinflammation induced by amyloid-beta in vivo and in vitro. J Neurochem. 2007;101:771–781. doi: 10.1111/j.1471-4159.2006.04370.x. [DOI] [PubMed] [Google Scholar]
- Maeda A, Sobel RA. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:300–309. doi: 10.1097/00005072-199603000-00005. [DOI] [PubMed] [Google Scholar]
- Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- McCormick PC. Biology of neurological recovery and functional restoration after spinal cord injury - Comments. Neurosurgery. 1998;42:707–707. doi: 10.1097/00006123-199804000-00007. [DOI] [PubMed] [Google Scholar]
- McTigue DM. Potential therapeutic targets for PPAR gamma after spinal cord injury. PPAR Res. 2008:517162. doi: 10.1155/2008/517162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Tripathi R, Wei P. NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. J Neuropathol Exp Neurol. 2006;65:406–420. doi: 10.1097/01.jnen.0000218447.32320.52. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Henriquez FL, Alexander J, Roberts CW. Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation. Clin Exp Immunol. 2010;160:369–379. doi: 10.1111/j.1365-2249.2009.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE, Scolding NJ. Mechanisms of damage to myelin and oligodendrocytes and their relevance to disease. Neuropathol Appl Neurobiol. 1999;25:435–458. doi: 10.1046/j.1365-2990.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WEB, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, Bolton AE, Mills KHG, Lynch MA. Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. J Biol Chem. 2005;280:9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- Norton WT, Aquino DA, Hozumi I, Chiu FC, Brosnan CF. Quantitative aspects of reactive gliosis - a review. Neurochem Res. 1992;17:877–885. doi: 10.1007/BF00993263. [DOI] [PubMed] [Google Scholar]
- Okano H, Ogawa Y, Nakamura M, Kaneko S, Iwanami A, Toyama Y. Transplantation of neural stem cells into the spinal cord after injury. Semin Cell Dev Biol. 2003;14:191–198. doi: 10.1016/s1084-9521(03)00011-9. [DOI] [PubMed] [Google Scholar]
- Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- Patel NS, Paris D, Mathura V, Quadros AN, Crawford FC, Mullan MJ. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer's disease. J Neuroinflammation. 2005;2:9. doi: 10.1186/1742-2094-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral-nerve injury - A possible role for macrophages in regeneration. J Exp Med. 1987;165:1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol. 2001;168:144–154. doi: 10.1006/exnr.2000.7604. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Longbrake EE. Perspectives - Opinion - Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Pratico D, Sung S. Lipid peroxidation and oxidative imbalance: Early functional events in Alzheimer's disease. J Alzheimers Dis. 2004;6:171–175. doi: 10.3233/jad-2004-6209. [DOI] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanowswki JQ, Lee VMY. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- Ramer LM, Ramer MS, Steeves JD. Setting the stage for functional repair of spinal cord injuries: a cast of thousands. Spinal Cord. 2005;43:134–161. doi: 10.1038/sj.sc.3101715. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: Unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Kinet JP. Fc-receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Ray SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol. 2002;17:1137–1152. doi: 10.14670/HH-17.1137. [DOI] [PubMed] [Google Scholar]
- Relloso M, Puig-Kroger A, Pello OM, Rodriguez-Fernandez JL, de la Rosa G, Longo N, Navarro J, Munoz-Fernandez MA, Sanchez-Mateos P, Corbi AL. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J Immunol. 2002;168:2634–2643. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- Ren Y, Young W. Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plast 2013. 2013:945034. doi: 10.1155/2013/945034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Rodriguez Guerrero A, Uchida K, Nakajima H, Watanabe S, Nakamura M, Johnson WEB, Baba H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J Neuroinflammation. 2012;9:40. doi: 10.1186/1742-2094-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Segev Y, Jacob-Hirsch J, Amariglio N, Rechavi G, Schwartz M. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: A role in microglia/macrophage activation. PLoS Med. 2008;5:1262–1277. doi: 10.1371/journal.pmed.0050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Sullivan N, Esiri MM. White matter damage is associated with matrix metalloproteinases in vascular dementia. Stroke. 2001;32:1162–1167. doi: 10.1161/01.str.32.5.1162. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci. 1999;19:464–475. doi: 10.1523/JNEUROSCI.19-01-00464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Schwab M, Schwartz M, Fehlings MG. Spinal cord injury: time to move? J Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Sergott RC, Brown MJ, Polenta RMD, Lisak RP, Silberberg DH. Optic-nerve demylination induced by hunam-serum-patients with multiple-sclerosis or optic neuritis and normal subjects. Neurology. 1985;35:1438–1442. doi: 10.1212/wnl.35.10.1438. [DOI] [PubMed] [Google Scholar]
- Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. J Pathol. 2013;229:332–346. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim K-W, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, Ahn M, Moon C, Kim S, Sim K-B. Alternatively activated macrophages in spinal cord injury and remission: another mechanism for repair? Mol Neurobiol. 2013;47:1011–1019. doi: 10.1007/s12035-013-8398-6. [DOI] [PubMed] [Google Scholar]
- Shiryaev SA, Remacle AG, Savinov AY, Chernov AV, Cieplak P, Radichev IA, Williams R, Shiryaeva TN, Gawlik K, Postnova TI, Ratnikov BI, Eroshkin AM, Motamedchaboki K, Smith JW, Strongin AY. Inflammatory proprotein convertase-matrix metalloproteinase proteolytic pathway in antigen-presenting cells as a step to autoimmune multiple sclerosis. J Biol Chem. 2009;284:30615–30626. doi: 10.1074/jbc.M109.041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, Seed B. The fc-gamma receptor of natural-killer cells is a phospholipid-linked membrane-protein. Nature. 1988;333:568–570. doi: 10.1038/333568a0. [DOI] [PubMed] [Google Scholar]
- Simon CM, Sharif S, Tan RP, LaPlaca MC. Spinal cord contusion causes acute plasma membrane damage. J Neurotraum. 2009;26:563–574. doi: 10.1089/neu.2008.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinescu C, Popa F, Grigorean VT, Onose G, Sandu AM, Popescu M, Burnei G, Strambu V, Popa C. Molecular basis of vascular events following spinal cord injury. J Med Life. 2010;3:254–261. [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Muller HW. Nerve injury, axonal degeneration and neural regeneration: Basic insights. Brain Pathol. 1999;9:313–325. doi: 10.1111/j.1750-3639.1999.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- Tanhoffer RA, Yamazaki RK, Nunes EA, Pchevozniki AI, Pchevozniki AM, Nogata C, Aikawa J, Bonatto SJ, Brito G, Lissa MD, Fernandes LC. Glutamine concentration and immune response of spinal cord-injured rats. J Spinal Cord Med. 2007;30:140–146. doi: 10.1080/10790268.2007.11753925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka Y, Okajima K. Role of leukocytes in spinal cord injury in rats. J Neurotrauma. 2000;17:219–229. doi: 10.1089/neu.2000.17.219. [DOI] [PubMed] [Google Scholar]
- Unkeless JC, Scigliano E, Freedman VH. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer's Disease brain. Arch Immunol Ther Exp (Warsz) 2012;60:251–266. doi: 10.1007/s00005-012-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereyken EJF, Heijnen PDAM, Baron W, de Vries EHE, Dijkstra CD, Teunissen CE. Classically and alternatively activated bone marrow derived macrophages differ in cytoskeletal functions and migration towards specific CNS cell types. J Neuroinflammation. 2011;8:58. doi: 10.1186/1742-2094-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MS, Prod’homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, Zamvil SS. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- Weisser SB, McLarren KW, Kuroda E, Sly LM. Generation and characterization of murine alternatively activated macrophages. Methods Mol Biol. 2013;946:225–239. doi: 10.1007/978-1-62703-128-8_14. [DOI] [PubMed] [Google Scholar]
- Yune TY, Lee JY, Jung GY, Kim SJ, Jiang MH, Kim YC, Oh YJ, Markelonis GJ, Oh TH. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27:7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Yin Y, Xu SJ, Wu YP, Chen WS. Inflammation & apoptosis in spinal cord injury. Indian J Med Res. 2012;135:287–296. [PMC free article] [PubMed] [Google Scholar]
- Zhang YK, Liu JT, Peng ZW, Fan H, Yao AH, Cheng P, Liu L, Ju G, Kuang F. Different TLR4 expression and microglia/macrophage activation induced by hemorrhage in the rat spinal cord after compressive injury. J Neuroinflammation. 2013;10:112. doi: 10.1186/1742-2094-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]


