Abstract
The neuromuscular junction becomes progressively less receptive to regenerating axons if nerve repair is delayed for a long period of time. It is difficult to ascertain the denervated muscle's residual receptivity by time alone. Other sensitive markers that closely correlate with the extent of denervation should be found. After a denervated muscle develops a fibrillation potential, muscle fiber conduction velocity, muscle fiber diameter, muscle wet weight, and maximal isometric force all decrease; remodeling increases neuromuscular junction fragmentation and plantar area, and expression of myogenesis-related genes is initially up-regulated and then down-regulated. All these changes correlate with both the time course and degree of denervation. The nature and time course of these denervation changes in muscle are reviewed from the literature to explore their roles in assessing both the degree of detrimental changes and the potential success of a nerve repair. Fibrillation potential amplitude, muscle fiber conduction velocity, muscle fiber diameter, mRNA expression levels of myogenic regulatory factors and nicotinic acetylcholine receptor could all reflect the severity and length of denervation and the receptiveness of denervated muscle to regenerating axons, which could possibly offer an important clue for surgical choices and predict the outcomes of delayed nerve repair.
Keywords: nerve regeneration, denervation, reinnervation, fibrillation potential, muscle fiber conduction velocity, muscle fiber diameter, maximal isometric force, neuromuscular junction, gene expression, neural regeneration
Introduction
After denervation, the neuromuscular junction (NMJ) and muscles undergo several changes. At the NMJ level, instability results in remodeling that increases fragment number, gutter depth and plantar area (Ma et al., 2005). At the same time, target muscles gradually atrophy, and muscle wet weight and muscle fiber diameter (MFD) gradually decrease. In turn, muscle fiber conduction velocity (MFCV) decreases (Kraft, 1990). Denervated muscle fibers begin spontaneously discharge, thereby producing a fibrillation potential (Fib) (Kraft, 1990; Jiang et al., 2000; Burns et al., 2007). Finally, the mRNA expression of nicotinic acetylcholine receptor (nAChR) and myogenic regulatory factors (MRFs) is initially up-regulated before rebounding to normal or subnormal levels (Voytik et al., 1993; Adams et al., 1995; Weis et al., 2000; Farina and Merletti, 2004; Ma et al., 2005, 2007).
Following an immediate nerve repair, the target muscle Fib gradually disappears (Chuang et al., 2002; Kerns et al., 2003; Heaton and Kobler, 2005); the mean MFCV (Van der Hoeven et al., 1993; Cruz-Martinez and Arpa, 1999), muscle wet weight (Bain et al., 2001; Brown et al., 2002; Aydin et al., 2004) and MFD all increase (Van der Hoeven et al., 1993; Cruz-Martinez and Arpa, 1999; Bain et al., 2001; Brown et al., 2002; Chuang et al., 2002; Kerns et al., 2003; Aydin et al., 2004; Heaton and Kobler, 2005). Muscle experiences excellent functional recovery as it returns to its pre-injury state (Brunetti et al., 1985; Brown et al., 2002; Aydin et al., 2004). However, if repair is delayed, NMJs become progressively less receptive to regenerating axons (Aydin et al., 2004; Ma et al., 2007).
Post denervation changes complicate clinical scenarios in patients who do not present immediately. With a delayed presentation it is difficult to ascertain the denervated muscle's residual receptivity and to gauge the appropriateness of performing a neuroplasty (Wu et al., 2013). Although patients may be evaluated in terms of electrophysiological testing, age, extent of injury, and delay to presentation, there are no concrete criteria for choosing between treatment modalities. The current consensus is that neuroplasty can be considered for up to 1 year after injury (Totosy et al., 1992). Patients presenting beyond this cutoff are evaluated for functional reconstruction. This stems from multiple observations that nerve repairs performed after the 1-year mark produce poor recovery (Gregory, 2009). Nevertheless, multiple case reports have documented transected nerves regaining full function as late as 9 years post injury (Trail, 1985). These outliers suggest that time alone does not dictate target muscle receptivity. It follows that improving the criteria by which physicians assess denervation and receptivity to repair would enhance both clinical judgment and treatment planning. As previously mentioned, Fib, MFCV, MFD, muscle wet weight, Po, gene expression levels, and NMJ remodeling have all been correlated with the time course and extent of muscle denervation. These parameters may indirectly reflect muscle receptivity to regenerating axons, providing an index for judging the likelihood of a successful nerve repair. An overview of these indices, the techniques to detect them and their clinical significance are provided.
Fibrillation potentials (Fibs)
After denervation, individual muscle fiber cell membranes spontaneously depolarize creating an unstable resting membrane potential at the end-plate known as Fib (Thesleff and Ward, 1975; Wiechers, 1977).
Parameters of Fib
A Fib always presents as biphasic or triphasic waves, lasting 1–5 ms with peak amplitudes of 20–200 μV (Thesleff and Ward, 1975; Burns et al., 2007). Fib frequency or firing density can either be numerically quantified or semi-quantitatively accounted for on a scale of (+) to (++++) (Izumi et al., 1999). Fib frequency, which peaks 7–10 days after initial injury, is directly proportional to the number of denervated muscle fibers (Smith and Thesleff, 1976; Izumi et al., 1999). Fib recording morphology depends heavily on electrode placement. Maximal Fib recordings are obtained in close proximity to the muscle end plate and distancing the electrodes from the end plate attenuates the signal. Maximal Fib recordings demonstrate stable morphology and positively correlate with MFD (Jiang et al., 2000). Using this relationship Fib recording can indicate the degree of muscle denervation.
Fib changes in denervated muscles
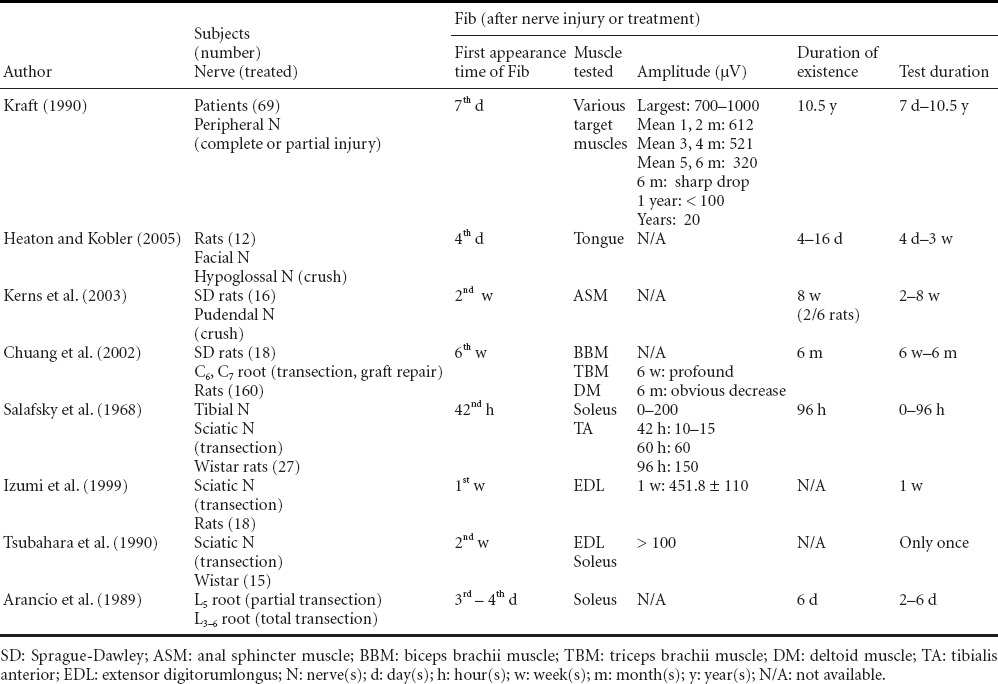
Table 1 shows the time of first Fib appearance, Fib persistent time and maximal amplitude of Fib in denervated muscles of humans and rats after nerve crush injury, transection, and repair. The time course of Fib development varies between species and individuals. Fib first manifests 1–3 weeks post denervation in humans and 3–7 days in rats (Salafsky et al., 1968; Arancio et al., 1989; Izumi et al., 1999; Burns et al., 2005; Heaton and Kobler, 2005). The literature lacks a standard timeline of Fib resolution as most studies do not use this as an end point (Desmedt and Borenstein, 1975; Heckmann and Ludin, 1982; Arancio et al., 1989; Kraft, 1990; Tsubahara et al., 1990). Some studies have reported resolution in as early as 8 weeks, while others document persistence up to 10 years after brachial plexus trauma. The mechanism by which Fibs are maintained for so long is unknown. One possible explanation is that proliferating connective tissue encircles denervated muscle preventing infiltration of the regenerative axons. As per this account, Fibs will persist as long as denervated muscles retain a blood supply (Dumitru and King, 1998). Likewise, Fib resolution might indicate total reinnervation (Chuang et al., 2002; Heaton and Kobler, 2005), substantial muscle atrophy or autonomic axon sprouting (Heaton et al., 2014). In humans, the maximal Fib amplitude can reach up to 1,600 μV in the first month (Kraft, 1990). While in rats, the maximal Fib amplitude is tested as 451 μV in extensor digitorum longus (EDL) muscle 1 week after the sciatic nerve transection (Izumi et al., 1999).
Table 1.
Fibrillation potential (Fib) in denervated muscles of humans and rats
Detection of Fibs
Detection of Fibs with needle electrodes
Fibs can be detected by inserting either concentric (Desmedt and Borenstein, 1975; Heckmann and Ludin, 1982; Arancio et al., 1989; Kraft, 1990; Jiang et al., 2000; Chuang et al., 2002; Kerns et al., 2003; Burns et al., 2007) or bipolar electrodes (Salafsky et al., 1968; Kraft, 1990; Heaton and Kobler, 2005) into the target muscle to a depth of 1–2 mm (Salafsky et al., 1968). Maximal Fib is a stable parameter, while Fib morphology is variable. Since Fib data are inherently variable, at least 20 Fibs from three different positions are needed to accurately calculate maximal Fib amplitude (Jiang et al., 2000). Each Fib is analyzed for 10 seconds (Heckmann and Ludin, 1982).
Detection of Fibs with surface electrode and high-density surface electromyography
Regular surface electrodes are inappropriate for measuring Fibs (Haig et al., 1996) as their large surface areas prohibit accurate detection of a single muscle fiber's membrane potential (Keller et al., 2002). This problem is exacerbated in deeper fibers. High density surface electrodes which are composed of a series of smaller electrodes, may offer a workable non-invasive alternative to needle electrodes, allowing them to pinpoint individual fiber potentials (Merletti et al., 2008).
Fib determinants
Fib is affected by factors including species, temperature and others as described below:
Species
The interval between injury and Fib onset varies by species (Table 1). Fib appears 1–3 weeks in humans (Kraft, 1990; Jiang et al., 2000) while 3–7 days in rats after nerve injury (Salafsky et al., 1968; Arancio et al., 1989; Izumi et al., 1999; Heaton and Kobler, 2005).
Temperature and metabolism
Fib firing rate and amplitude positively correlate with body temperature and metabolism (Izumi et al., 1999). Fib amplitude drops 42% for every ten degrees decrease in temperature (Lee and Kwon, 1997). Accordingly, accurate data collection in humans requires careful monitoring (Salafsky et al., 1968; Smith and Thesleff, 1976; Heckmann and Ludin, 1982; Kraft, 1990; Tsubahara et al., 1990; Izumi et al., 1999; Jiang et al., 2000; Chuang et al., 2002; Heaton and Kobler, 2005; Burns et al., 2007) to maintain body temperature between 36–37.5°C (Salafsky et al., 1968; Heckmann and Ludin, 1982). Catecholamine, isoprenaline and adrenalin increase the Fib firing rate (Smith and Thesleff, 1976; Izumi et al., 1999) while ouabain and tetrodotoxin block it (Smith and Thesleff, 1976).
Blood supply and partial pressure of oxygen (PaO2)
Fib firing rate positively correlates with PaO2. Decreased perfusion to the denervated EDL in a rat prompts an initial increase and then rapid disappearance of Fibs (Izumi et al., 1999). Subsequent reperfusion restores Fibs (Izumi et al., 1999).
Muscle fiber type
Two weeks after a sciatic nerve excision, EDL and soleus fibers demonstrated Fib frequencies of 60.3 and 3.0 respectively (Izumi et al., 1999), suggesting that Fib frequency is greater in fast twitch muscle fibers.
Electrolytes
The depolarization of a denervated muscle fiber membrane is affected by its surrounding electrolyte milieu. Decreases in calcium chloride concentration increase Fib activity (Thesleff and Ward, 1975; Smith and Thesleff, 1976). Hypokalemic and hyperosmotic solutions decrease Fibs (Smith and Thesleff, 1976).
Time course and extent of nerve injury
Fib magnitude is proportional to the number of denervated muscle fibers (Izumi et al., 1999). As compared to partial denervation, the Fib in complete denervation appears earlier, has larger amplitude, and has a shorter latency period (Desmedt and Borenstein, 1975; Arancio et al., 1989). Notably, Fib amplitude increases during early denervation (Salafsky et al., 1968) and then decreases with time (Kraft, 1990; Jiang et al., 2000). This change likely reflects the time course of reinnervation, indicating that Fib magnitude is closely tied to the interplay of injury severity and the extent of healing. Recent findings (Heaton et al., 2014) indicated that cholinergic, autonomic reinnervation of rat whisker pad muscles would cause the disappearance of Fibs, and that subsequent disruption of the autonomic input would cause the re-appearance of Fibs. Therefore, the disappearance of Fibs might stem from autonomic input in the case of head & neck muscles, where parasympathetic (cholinergic) axons innervated blood vessels within muscles. It is not known whether this happens in other body locations or in animals aside from rats.
Nerve injury level and recording position
Location of the injury affects Fib amplitude. Injuries located more proximally along a nerve will create a longer distal nerve stump and are associated with smaller maximal Fib amplitudes (Salafsky et al., 1968). The cause is unclear, but the different onset time of fibrillation potentials and loss time of neuromuscular transmission following denervation in different nerve injury locations could probably partially explain this observation (Luco and Eyzaguirre, 1955; Salafsky et al., 1968; Willmott et al., 2012). As previously established, Fib amplitude is maximal when recorded near the end-plate area (Thesleff and Ward, 1975).
Clinical significance of Fibs
Fib appears about 1 week after denervation (Desmedt and Borenstein, 1975; Thesleff and Ward, 1975; Heckmann and Ludin, 1982; Arancio et al., 1989; Kraft, 1990; Dumitru and King, 1998; Izumi et al., 1999; Heaton and Kobler, 2005), thereby providing a sensitive indication of early stage muscle denervation. This makes Fibs a likely gold standard for electrophysiological diagnosis (Burns et al., 2007). Both Fib amplitude and muscle fiber diameter reflect the severity of and length of denervation (Kraft, 1990; Jiang et al., 2000). Longer delays in repair and more extensive injuries are associated with poorer nerve recovery (Brown et al., 2002; Aydin et al., 2004; Ma et al., 2007). Fibs may therefore help predict the success of a delayed nerve repair.
Fib decreases over the course of reinnervation and fully disappears upon complete healing. Fib onset and gradual dissipation provides a qualitative index of how reinnervation is progressing. This information, in turn, can help evaluate the effectiveness of treatment modalities. One should also keep in mind that, aside from successful somatic reinnervation, Fibs can also disappear due to muscle atrophy and autonomic axon sprouting.
Muscle fiber conduction velocity (MFCV)
MFCV is primarily used to gauge muscle fatigue after bouts of exercise (Sakamoto and Mito, 2000). MFCV can also be used to reflect the extent of denervation (Troni et al., 1983; Van der Hoeven et al., 1993; Cruz-Martinez and Arpa, 1999; Ruegg et al., 2003; Blijham et al., 2006).
Parameters and changes of MFCV after denervation
MFCV encompasses the fastest, slowest, and mean values. Additionally, the ratio of fastest/slowest MFCV (F/S) is used to evaluate conduction variability.
The fastest and slowest MFCV correspond with the largest and smallest muscle fiber diameters, respectively, in normal, myopathic and neuropathic muscles (Blijham et al., 2006). Post-denervation atrophy decreases the fastest MFCV. Likewise, reinnervation generates hypertrophy enabling maximal MFCV to rebound (Van der Hoeven et al., 1993; Cruz-Martinez and Arpa, 1999). Denervation can reduce the slowest MFCV to values as low as 0.5 m/s (Van der Hoeven et al., 1993; Cruz-Martinez and Arpa, 1999). The slowest MFCV does not recover with reinnervation (Van der Hoeven et al., 1993), suggesting that severely atrophied smaller fibers are rendered unreceptive to regenerating axons.
Mean MFCV in normal muscle follows a Gaussian distribution (Arendt-Nielsen and Zwarts, 1989; Cruz-Martinez and Arpa, 1999) with reported ranges of 3.4–4.0 m/s (Troni et al., 1983; Arendt-Nielsen and Zwarts, 1989). Mean MFCV begins to decrease exponentially soon after denervation. Therefore, MFCV distribution shifts to the left after denervation, but loses its normal Gaussian distribution (Van der Hoeven et al., 1993). Reinnervation generates hypertrophy enabling maximal MFCV to rebound and MFCV distribution shifts back to the right (Van der Hoeven et al., 1993; Cruz-Martinez and Arpa, 1999).
F/S is small in a normal muscle (Andersen et al., 1996). There is a positive correlation between F/S and the time course of amyotrophic lateral sclerosis (ALS) (Van der Hoeven et al., 1993), reflecting the coexistence of atrophy and hyperplasia. Likewise, F/S increases after a brachial plexus injury (Van der Hoeven et al., 1993; Cruz-Martinez and Arpa, 1999) indicating variability in atrophy of various muscle fibers. An increase in F/S persists after reinnevation as larger fibers continue to grow while atrophic smaller fibers are unable to recover or become even more atrophic (Van der Hoeven et al., 1993; Cruz-Martinez and Arpa, 1999).
Detection of MFCV
MFCV can be detected with needle and surface electrodes. Troni and colleaguesintroduced the measurement of MFCV by needle electrodes in human biceps brachii (Troni et al., 1983). The anode of thestimulatingelectrode is placed far from the end-plate to avoid inadvertently stimulating adjacent nerves. Then the cathode of the stimulating electrode is placed 3.0 mm proximal to the anode. This bipolar configuration minimizes the inadvertent spreading of stimulation current. Initial stimulation uses pulses 0.2 ms in duration, of 1 Hz frequency, and at an intensity of 1–2 mA. The intensity is gradually increased until a small, localizedmuscle twitch is palpable. After careful palpation of the muscle twitch, a single fiber recording electrode is inserted proximally and at a constant distance (50 mm) from the stimulating cathode. Once a multiphase potential is recorded, indicating the electrode is properly located within the activated muscle bundle, the stimulating intensity is gradually decreased until a variable number of individual fiber potentials, known as MFCV of a single muscle fiber, can be recorded (Troni et al., 1983; Van der Hoeven et al., 1993; Andersen et al., 1996; Blijham et al., 2006). Then, 20 MFCV from three to five sites are collected (Van der Hoeven et al., 1993; Blijham et al., 2006).
The multichannel surface electrode, composed of more than three small electrodes, has also been used to measure MFCV (Rainoldi et al., 2004; Gallinaet al., 2013; Butugan et al., 2014). These three electrodes are configured to yield two sets of EMG signals. Cross-correlation method is used to calculate the time lag between these two EMG recordings along the muscle. Combined with the distance between the electrodes, MFCV is calculated (Arendt-Nielsen and Zwarts, 1989; Zwarts, 1989; McIntosh and Gabriel, 2012). Once again, accurate data collection hinges on electrode placement. This is guided by three landmarks along the muscle fiber's length: the tendon and end plates, at either end of the fiber, and the innervation zone (IZ). MFCV measurements near the tendons are the most variable (Rainoldi et al., 2004; Nielsen et al., 2008). Data collected near the muscle belly are less variable, but the MFCV phase reverses as electrodes cross the IZ (Rainoldi et al., 2004). To ensure optimal measurements, surface electrodes are placed between the IZ and the tendon, in-line with the muscle fiber. Theanatomic position of IZ in several muscles has been described in details (Rainoldi et al., 2004; Beretta et al., 2014), which helps to guide the placement of surface electrodes. The local skin is shaved, abraded, and cleansed with alcohol to reduce the impedance at the skin-electrode interface. The stimulation could be electrical (McIntosh and Gabriel, 2012) or voluntary contraction of the muscle (Gallinaet al., 2013; Butugan et al., 2014). Surface electrodes offer a non-invasive means of measuring MFCV. It should be noted that while MFCV measured by surface electrodes positively correlates with needle electrode measurements, they are around 1.0 m/s higher (Aquilonius et al., 1984; Zwarts, 1989). The reason for this difference is unclear. One possible explanation is that surface EMG measurements are the average of a large amount of most normal conducting fibers, because surface electrodes are unable to record very slow MFCV (Arendt-Nielsen and Zwarts, 1989).
MFCV determinants
Patient variables
Many factors influence MFCV acquisition. Men demonstrate larger MFCV than women (Andersen et al., 1996). MFCV is temperature sensitive, so it is important to control body temperature when measuring MFCV (Arendt-Nielsen and Zwarts, 1989). Mean MFCV in human brachioradialis decreases from 3.9 to 3.2 m/s when local skin temperature drops from 30 to 18°C after the application of ice packs around the forearm and elbow (Blijham et al., 2008). MFCV decreases as muscle fibers are stretched (Trontelj, 1993; Van der Hoeven et al., 1993). As the elbow joint angle progressed through 90, 120 and 150 degrees with biceps brachii lengths of 12.8, 15.4 and 16.8 cm, the MFCV was 4.29, 4.02 and 3.96 m/s respectively (Arendt-Nielsen and Zwarts, 1989).
Recording electrode diameter and position
Decreasing electrode diameter increases the noise in recordings. Electrodes that are 30–50 μm in diameter are considered to optimally balance accuracy and noise reduction (Hakansson, 1956). MFCV magnitude is proportional to the recording electrode's proximity to either the end-plate or tendon (Troni et al., 1983; Nielsen et al., 2008). MFCV recorded near either site by cross-correlation method of surface electrodes approaches 10 m/s, and reduces to 4.5 m/s after moving 20–45 mm away from the end-plate (Li and Sakamoto, 1996; Sakamoto and Li, 1997).
Inserting direction of electrodes
Accurate MFCV measurements require that the stimulating and recording needle electrodes be placed perpendicular to the muscle fiber. Improper placement can result in errors; in fact, deviation of as little as five degrees can produce errors of 5–8% (Andersen et al., 1996). Alternatively, it is important that surface electrodes be aligned parallel to muscle fibers.
Clinical significance of MFCV
MFCV acquired by surface electrodes provides a non-invasive means of studying muscle in health, injury and recovery. A positive relationship has been reported between MFCV and human arm size (Troni et al., 1983). MFCV and target muscle fiber diameter both decrease after denervation (Troni et al., 1983; Van der Hoeven et al., 1993; Cruz-Martinez and Arpa, 1999; Ruegg et al., 2003; Blijham et al., 2006). The linear relationship between MFCV and quadriceps femoris diameter has been used to non-invasively track atrophy in patients with knee degeneration (Gechev et al., 2004; Blijham et al., 2006). Interestingly, MFCV offers clues of diagnosing and following myopathy in Duchenne's muscular dystrophy (DMD). In DMD atrophy and hyperplasia coexist. As these changes progress they decrease MFCV frequency and elicit a multi-peak frequency distribution (Al-Ani et al., 2001). F/S and slowest MFCV values can be used to monitor the health of motor axons in diabetic patients who suffer from motor neuropathy (Meijer et al., 2008). Specifically, the linear relationship between slowest MFCV and F/S and peroneal nerve conduction velocity facilitates a measurement of the extent of lower extremity motor axon denervation. This is of particular interest because the extent of damage is closely correlated with the success of nerve repairs. MFCV can help predict the success of delayed nerve repairs and, thereby, offer evidence for or against pursing surgical intervention.
Muscle wet weight
Changes of muscle wet weight after denervation and reinnervation
After denervation, rat muscle wet weight decreases dramatically initially, and then stabilizes at 10–20% the weight of healthy, contralateral muscle by 3–12 months (Xu and Gu, 1999; Wang et al., 2001; Ma et al., 2007). Wet weight recovers well with short-term and intermediate-delayed repairs, and poorly with long-term delays (Bain et al., 2001; Brown et al., 2002; Aydin et al., 2004). Changes in rat muscle wet weight associated with denervation and repairs at different time points are shown in Table 2.
Table 2.
Changes of muscle wet weight after denervation and reinnervation in rats
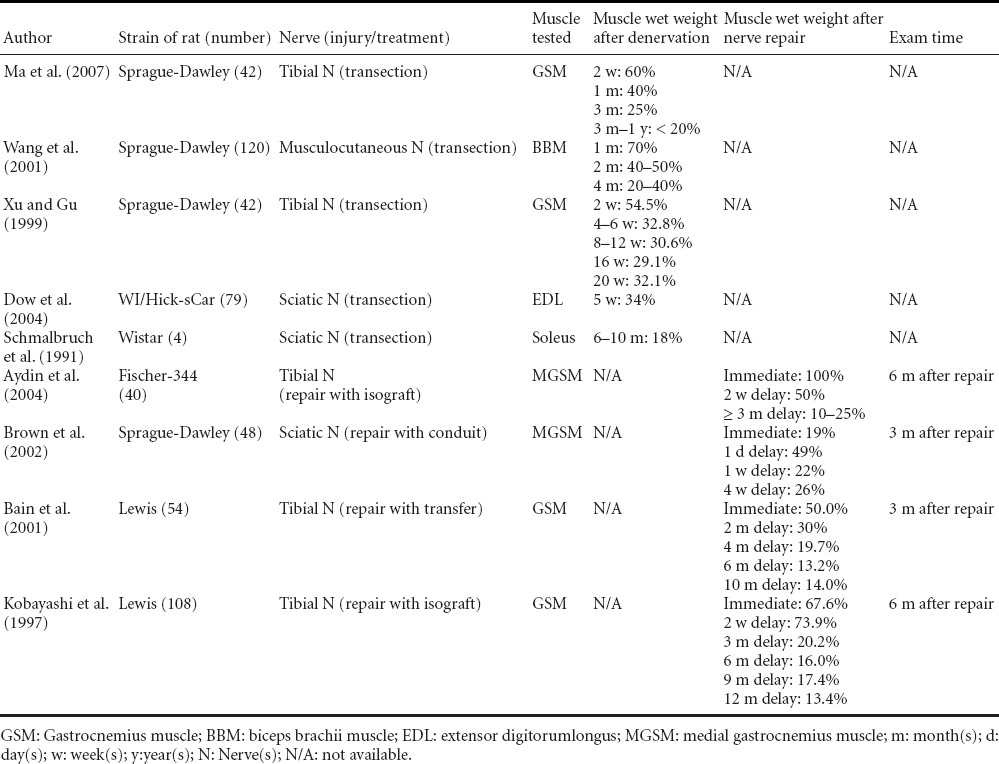
Muscle wet weight of rat gastrocnemius muscles was 55–60%, 33–40%, 25–32% and 18–20% of that of the contralateral side at 2 weeks, 1 month, 3–5 months and 1 year after complete denervation (Schmalbruch et al., 1991; Ma et al., 2007). After prolonged denervation, muscle wet weight stabilizes at 10–20% of that of the contralateral side, partially due to connective tissue proliferation (Al-Amood and Lewis, 1989; Xu and Gu, 1999; Wang et al., 2001; Ma et al., 2007).
Muscle wet weight recovery rate ranges from 19% to 100% after immediate repair of its innervating nerve (Table 2). Longer follow-up time, lower injury level and more motor axons will bring better recovery after immediate repair (Kobayashi et al., 1997; Bain et al., 2001; Brown et al., 2002; Aydin et al., 2004). When nerve repair is delayed, muscle wet weight recovery is good when the delay is within 1 month, and is much poorer after more than 3 months of delay, being only 10 % to 20% of the contralateral healthy side (Kobayashi et al., 1997; Bain et al., 2001; Aydin et al., 2004). This nearly equals the rate of 20% that is left after prolonged denervation (Wang et al., 2001; Dow et al., 2004; Ma et al., 2007).
Clinical significance of muscle wet weight in denervation
The aforementioned changes in muscle weight associated with both denervation and delayed repair could theoretically be used to evaluate the extent of muscle atrophy and predict the success of nerve repair. However, these applications have limited clinical value as it is impossible to obtain muscle wet weight in patients.
Muscle fiber types and diameter
Classification of muscle fiber types
Several approaches can be used to classify muscle fiber types. Broadly, muscle fibers can be categorized as type I and type II. Metabolically, the former favors oxidative enzymes to phosphorylase and produces small sustained forces (slow twitch) and the latter favors phosphorylase and rapidly generates large forces (fast twitch). Type II fibers can be further sub-categorized by their ATPases or myosin heavy chain isoforms. Differentiation by ATPases yields subtypes IIA, IIB, and IIC (Brooke and Kaiser, 1970; Manetti et al., 2007) and by myosin heavy chain isoform renders MHCIIA, MHCIIB, and MHCIIX/MHCIID (Schiaffino et al., 1989; Bortolotto et al., 2000; Manetti et al., 2007; Naderi et al., 2009). Some fibers are a hybrid of two other fiber types (Bortolotto et al., 2000) such as type IIC (Manetti et al., 2007; Naderi et al., 2009). Types I and IIA have the smallest diameters while IIX and IIB have intermediate and the largest diameters, respectively (Westgaard and Lomo, 1988). Individual muscles are composed of a mixture of different fiber types; the predominance of a certain fiber type determines the muscle's metabolic profile and force generating capacity. Normal rat soleus primarily contains type I fibers, so it is considered slow twitch. By comparison, the tibialis anterior predominantly contains type II fibers and is considered fast twitch (Brooke and Kaiser, 1970; Dow et al., 2006).
Changes of muscle fiber types and diameter after denervation
Changes of muscle fiber diameter after denervation
Naturally, as muscle atrophies after denervation, individual muscle fiber's diameter decreases (Rowan et al., 2012). By 4 months post-musculocutaneous nerve injury, the diameter of rat biceps brachii muscle fibers has been measured at 20% that of fibers in the contralateral muscle (Wang et al., 2001). While debate exists over how atrophy rates vary by muscle fiber types, the consensus is that type II fibers atrophy more rapidly than type I (Niederle and Mayr, 1978; Kraft, 1990; Zealear et al., 1994; Lu et al., 1997; Prakash et al., 1999; Jiang et al., 2000; Kostrominova et al., 2005). However, the exact time course and rate of type I fiber atrophy remain unknown. Proposed timelines include: an initial augmentation of type I fiber (Prakash et al., 1999), a decrease in fiber size that follows type II fiber atrophy (Lu et al., 1997; Kostrominova et al., 2005; Blijham et al., 2006), and finally, the idea that type I fiber does not atrophy until several months after denervation (Ashley et al., 2007). While this issue remains unresolved, there is agreement that both type I and II fiber atrophy after denervation and that, in the short term, these changes are more evident in type II fibers (Zealear et al., 1994; Lu et al., 1997; Jiang et al., 2000; Kostrominova et al., 2005).
Changes of muscle fiber type distributions after denervation
After denervation, the total number of muscle fibers does not change within the first 51 weeks (Ashley et al., 2007), rather the individual fibers shrink and change types, altering the distribution and proportion of fiber types within the muscle (Ashley et al., 2007). As an example, after denervation type IIB and IIC fast fibers in rat EDL and tibialis anterior muscles convert to type I and IIA slow fibers (Pette and Vrbova, 1985; Windisch et al., 1998; Kostrominova et al., 2005). If reinnervation is achieved, the signal generated by the motor cortex will determine the type of fibers, their proportion, and distribution in the muscle (Hughes et al., 1993).
Clinical significance of muscle fiber diameter change
Muscle fiber diameter is closely related to multiple electrical and structural parameters that are affected by denervation. Fiber diameter is negatively correlated with time since denervation and it is positively correlated with mean MFCV and maximal Fib amplitude. In this way, muscle fiber diameter provides a reliable index by which to evaluate the extent of denervation and disuse (Cruz-Martinez and Arpa, 1999) and predict the degree of recovery achievable by nerve repair.
Isometric force and torque
Types and important parameters of muscle fiber contraction
Isometric and isotonicare are two main types of muscle contraction. During isometric contractions, the joint angle does not change, while during an isotonic contraction the muscle shortens producing a constant force. Isometric force is composed of individual contractions called twitches and is referred to as tetanic contractions. Isometric tetanic contraction force hinges on stimulating intensity, frequency, duration, and muscle fiber preload (Dow et al., 2004; Shin et al., 2008).
Isometric force can be described in terms of twitch force, maximal isometric twitch force (Pt), time-to-peak tension (TPT), half-relaxation time (HRT), tetanic force, maximal isometric tetanic force (Po), the ratio of Pt/Po, and specific force (SPo). SPo is defined as the ratio of Po and muscle fiber cross sectional area (Po/CSA). Isometric force, in particular Pt, Po, and SPo, are used to evaluate the extent of denervation (Dow et al., 2004).
Changes of isometric force after denervation and reinnervation
Changes of isometric force after denervation
Upon denervation isometric twitch and tetanic force both decrease. Po undergoes an initial rapid decrease followed by a sustained gradual decrease (Spector, 1985; Carlson et al., 1996; Kalliainen et al., 2002; Dow et al., 2004). Changes in Po associated with denervation and subsequent reinnervation are detailed in Table 3, which illustrates the exponential nature of Po's decrease. At 2 weeks, 4–5 weeks, 8 weeks, and 4–12 months post-denervation, the Po in rat lower extremity is measured as 25.5–26% (Spector, 1985; Kalliainen et al., 2002) of contralateral value, then 6–13% (Spector, 1985; Carlson et al., 1996; Dow et al., 2004), 2% (Carlson et al., 1996), and finally 0–0.3% (Al-Amood et al., 1991; Carlson et al., 1996).
Table 3.
Changes of maximal isometric force (Po) after denervation and reinnervation in rats
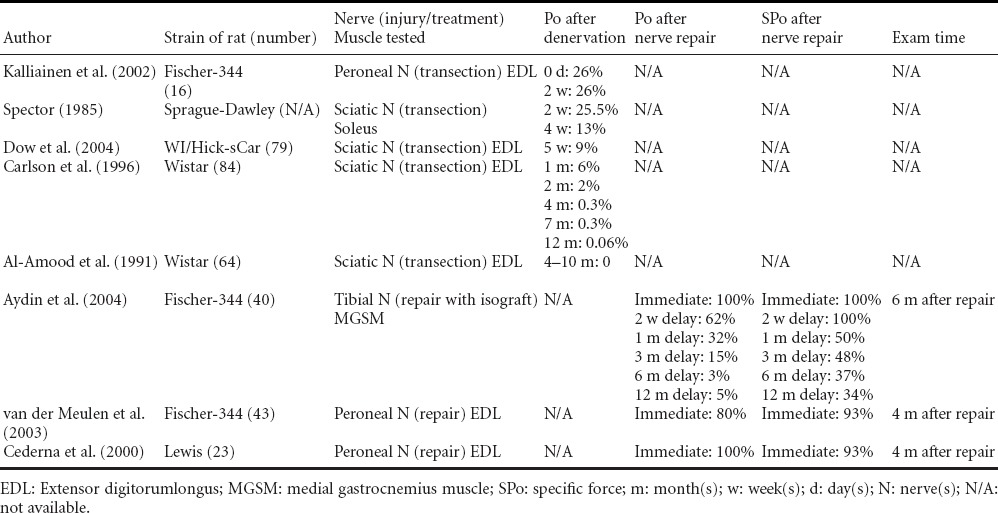
SPo also decreases after denervation (Carlson et al., 1996; Dennis, 1998; Kalliainen et al., 2002; Ashley et al., 2007). In the rat lower extremity, SPo is 37% (Kalliainen et al., 2002), 5–15% (Carlson et al., 1996; Dennis, 1998) and 0.2–1.6% (Carlson et al., 1996) at 2 weeks, 4–8 weeks and 4–12 months after denervation, respectively. Muscle fiber atrophy and decreased contractility account for these changes (Kalliainen et al., 2002). The rate at which Pt decreases is less than that at which Po drops off, so in the wake of denervation, Pt/Po increases (Dow et al., 2004; Ashley et al., 2007). TPT and HRT increase after denervation (Al-Amood and Lewis, 1989; Buffelli et al., 1997; Leong et al., 1999), doubling and tripling the contralateral values by 5 weeks post injury (Dow et al., 2004).
Changes of isometric force after reinnervation
Electric stimulation can impact Po and SPo recovery, maintain Po, and prevent it from decreasing (Dow et al., 2004, 2006) after denervation. Additionally, reinnervation gradually restores Po (Cederna et al., 2000; Brown et al., 2002; Aydin et al., 2004). SPo undergoes a similar, but slower recovery with reinnervation (Cederna et al., 2000; van der Meulen et al., 2003; Aydin et al., 2004). This difference in recovery may be attributed to one of many factors including: decreased contractility among recovered fibers, failure of some fibers to regain contractility, and impaired force conduction at the muscle tendon interface (Aydin et al., 2004). Regardless, Po and SPo recovery depends on the interval between injury and intervention. Longer delays are associated with poorer outcomes (Brown et al., 2002; Aydin et al., 2004). However, it has been shown that delays of 20 days are associated with better Po and SPo recovery than repairs performed immediately or with shorter delay (Brunetti et al., 1985). This relationship may imply that mild to moderate delays improve nerve regeneration. This coincides with the phenomena of nerve pre-degeneration which allows Wallerian degeneration and prepares the distal nerve for regenerating axons (Brunetti et al., 1985; Brown et al., 2002; Wu et al., 2013). The advantage may have subsequently benefited the reinnervation of the target muscle and hence its contractile function.
Measurements of maximal isometric titanic force and torque
Measurements of Po
When measuring Po in vitro, the target muscle is dissected out and placed in a ringer solution maintained at 26°C and oxygenated with a mixture of 95% O2 and 5% CO2. Muscle tendons are fixed in a clamp and force transducer, respectively. Muscle preload is adjusted and electrodes are used to directly stimulate the tissue and obtain the Po (Buffelli et al., 1997; Van Balkom et al., 1997; Johns et al., 2001). For in vivo measurement, the target muscle and its innervating branches are surgically exposed. The distal tendon is cut at its insertion and clamped to a force transducer. Tissue is moistened with saline and maintained between 35–37°C (Cederna et al., 2000; van der Meulen et al., 2003; Shin et al., 2008). Adjacent muscles are dissected so that their respective nerves and tendons may be cut. Doing so eliminates extraneous muscle activity during electric stimulation (Cederna et al., 2000; van der Meulen et al., 2003). Bipolar electrodes are used to stimulate the nerve for up to 15 seconds at a time with 5 minute rest intervals. Preload is adjusted and various stimulation frequencies and intensities are employed until Po is elicited (Shin et al., 2008).
A non-invasive method of measuring Po was introduced by transcutaneously stimulating the sternocleidomastoid. This approach requires the head and chest to be fixed at a set angle. A transducer that is positioned over the tendon of the sternal head measures force (Moxham et al., 1980; Lewis et al., 1986). These measurements can longitudinally track muscle force recovery after nerve repair. The limitation of this method is that it can be affected by synergistic muscle activity. This method demonstrates a 4–6% variation in force measurement compared to the 3% seen with in vitro techniques (Warren et al., 1999).
Measurement of maximal isometric torque
Grill and Mortimer devised a method of three dimensionally capturing maximal isometric torque in an animal model using cats (Grill and Mortimer, 1996). They utilized a polyimide cuff containing 12 electrodes arranged into four tripolar configurations. Once placed around the nerve, this cuff allowed selective stimulation of different nerve branches. The cat's knee and ankle angles were fixed and the ankle joint was connected to a force transducer. Their measurements were reproducible and demonstrated a mere 0.3% systemic error with a 2.7% deviation.
Clinical significance of Po
After denervation, Po exponentially decreases over time (Al-Amood and Lewis, 1989; Al-Amood et al., 1991; Carlson et al., 1996; Dow et al., 2004). Po and SPo will recover after reinnervation (Brunetti et al., 1985; Cederna et al., 2000; van der Meulen et al., 2003; Aydin et al., 2004). Moreover, Po correlates with muscle wet weight and time course/extent of muscle atrophy. Po can be used as an index of post denervation atrophy and as a predictor of nerve repair success. Clinically, these measurements could also be used for testing muscle fatigue of quadriceps femoris (Deley et al., 2014), evaluating thigh muscle force for stroke patients (Wen et al., 2014), and detecting the effect of muscle training after trauma (Jayaraman et al., 2013).
Myogenesis related genes
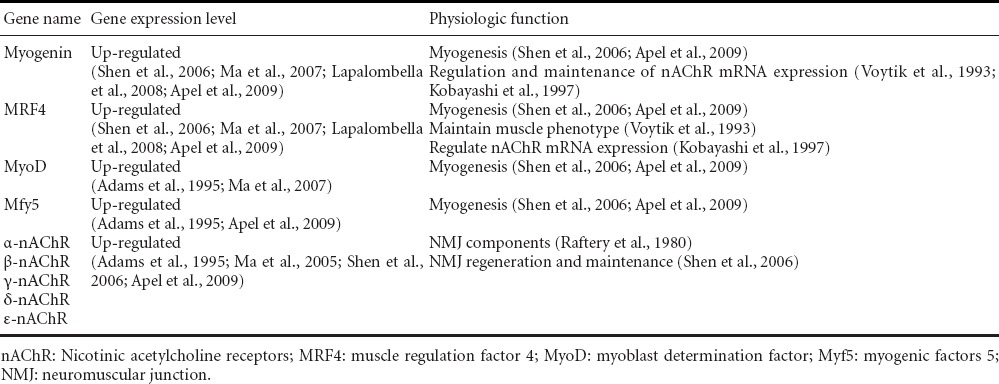
Many molecules play an important role in muscle regeneration. Some of these molecules along with their physiological functions are listed in Table 4. Nicotinic acetylcholine receptor (nAChR) subtypes (α, β, γ, δ or ε) constitute and maintain the neuromuscular junction (NMJ) (Raftery et al., 1980; Shen et al., 2006) and facilitate synaptic transmission. Muscle regulation factors (MRFs) regulate nAChR gene expression, promote muscle regeneration and maintain muscle fiber phenotypes (Apel et al., 2009). The mRNA expression levels of some of these genes have close relationships with the time course and extent of muscle denervation, which may reflect the microenvironment changes within the muscle.
Table 4.
Myogenesis related gene expression in rat denervated muscles and their physiological functions
Changes of myogenesis related genes in denervated muscles of rats
The time course of MRFs gene expression changes in rat denervated muscles is shown in Table 5. MRFs include MyoD (myoblast determination factor), Myf5 (myogenic factors 5), MRF4 and Myogenin which all belong to basic helix-loop-helix family members (Charbonnier et al., 2003). Myogenin is an important promoter and an essential factor for nAChR gene expression. In a myogenin gene knock-out mouse, α- and γ-nAChR are not expressed in target muscles (Brunetti and Goldfine, 1990; Eftimie et al., 1991; Voytik et al., 1993). MyoD and Myf5 are expressed in neonatal muscles, while MRF4 is expressed in both neonatal and adult muscles (Lapalombella et al., 2008). Myf5 and MRF4 have a fair expression level in all muscle fiber types, (Voytik et al., 1993; Weis et al., 2000), while Myogenin and MyoD are evidently expressed in slow (Voytik et al., 1993; Adams et al., 1995) and fast muscle fibers (Voytik et al., 1993; Weis et al., 2000; Shen et al., 2006), respectively. Upon denervation skeletal muscle MRFs gene expression levels increase initially and then decrease (Table 5). Most reports show that these gene expression levels start to increase at 8 hours to 3 days (Eftimie et al., 1991; Voytik et al., 1993; Weis et al., 2000; Shen et al., 2006; Ma et al., 2007), peak at 1-2 weeks (Voytik et al., 1993; Adams et al., 1995; Ma et al., 2007), and drop to normal level at 1-3 months after denervation (Voytik et al., 1993; Adams et al., 1995; Shen et al., 2006; Ma et al., 2007). Myogenin and MRF4 gene expression levels in rat gastrocnemius muscle are lower than normal after prolonged denervation, a phenomenon that can possibly be attributed to the fact that sources for genes expression in these severely atrophic muscles are exhausted (Ma et al., 2007).
Table 5.
Time course of MRFs gene expression changes in denervated muscles of rats
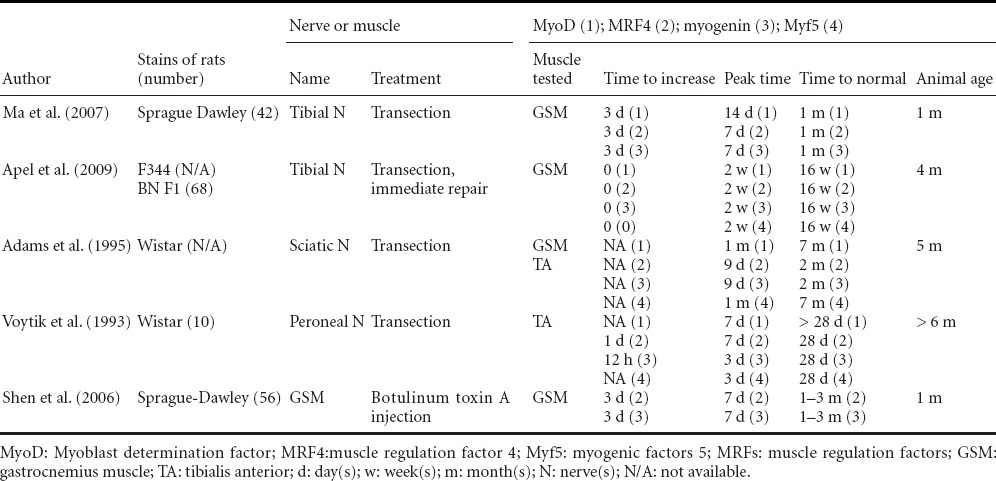
Myogenin and MRF4 gene expression levels are higher than MyoD and Myf5 (Witzemann and Sakmann, 1991; Voytik et al., 1993; Adams et al., 1995), and are maintained at a high level for shorter time (Voytik et al., 1993; Adams et al., 1995). Ten days after denervation, myogenin and MRF4 mRNA expression levels are 100 and 40 times higher than that of the control muscle, while MyoD and Myf5 mRNA expression levels are 7 and 17 times higher than control, respectively (Adams et al., 1995). The high expression of myogenin and MRF4 lasts less than one month, while MyoD and Myf5 gene expression is maintained at a high level for up to 7 months (Adams et al., 1995). Initial up-regulation of myogenin mRNA expression in the muscle is earlier (starts to up-regulate at 8–16 hours post-denervation) than the initial up-regulation of MyoD (starts at 16–24 hours after denervation) (Eftimie et al., 1991).
nAChR is an important component of NMJ and helps synaptic transmission in NMJ (Raftery et al., 1980). Embryonic nAChR is composed of α, β, δ and γ subtypes, distributing extensively in the whole muscle fiber, while adult nAChR is composed of α, β, δ and ε subtypes, distributing only in end-plate area of the muscle (Adams et al., 1995; Ma et al., 2007). NMJ is unstable and post-synaptic nAChR turnover is faster after denervation (Adams et al., 1995; Ma et al., 2007) with a decrease of turnover half-time from ten days to 1–2 days (Loring and Salpeter, 1980). Embryonic nAChR increases initially after denervation and then returns to normal level. Adult ε-nAChR will replace embryonic γ-nAChR and is expressed in end-plate area of the muscle for a long time after reinnervation (Adams et al., 1995). Electric stimulation prevents embryonic nAChR gene expression, elongates half-time of turnover of nAChR and stabilizes NMJ structure (Adams et al., 1995; Bezakova et al., 2001).
Levels of mRNA expression of nAChR subtypes in the denervated muscle increase initially before returning to normal (Adams et al., 1995; Ma et al., 2005; Shen et al., 2006; Ma et al., 2007), or even lower than normal after 3–12 months denervation (Ma et al., 2007) (Table 6). Up-regulation of nAChR subtype gene expression starts from 0-3 days (Adams et al., 1995; Li and Sakamoto, 1996; Ma et al., 2005; Shen et al., 2006; Ma et al., 2007), peaks at 1–2 weeks (Ma et al., 2005, 2007; Shen et al., 2006; Ma et al., 2007), drops to normal level, ranges in the time frame of 2 weeks (Ma et al., 2005; Shen et al., 2006) to 1 month (Ma et al., 2007), and even to 7–12 months after denervation (Adams et al., 1995).
Table 6.
Time course of nAChR gene expression in denervated muscles of rats
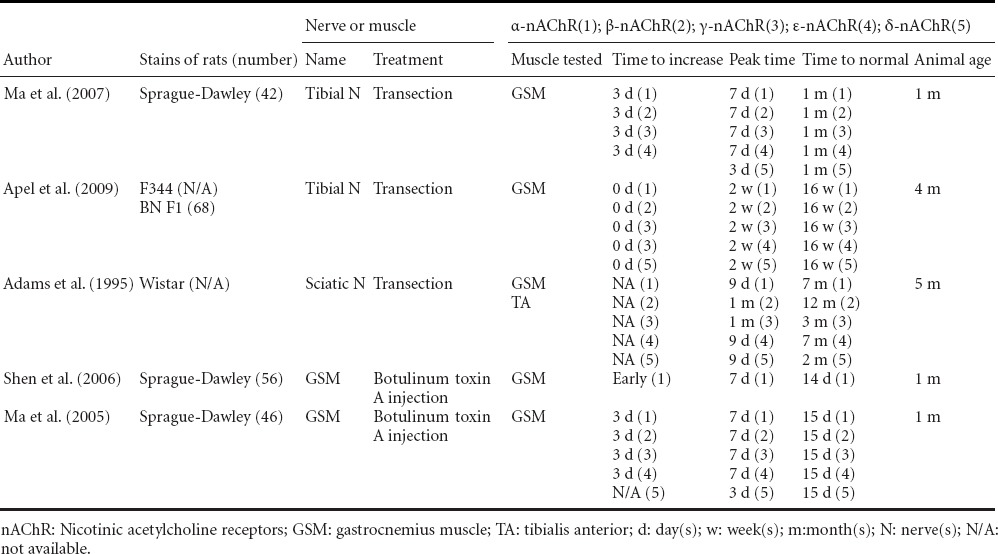
Various nAChR subtypes have different gene expression timelines: embryonic γ-nAChR drops to normal level whereas adult ε-nAChR is still maintained at a high level for a long time after sciatic nerve transection in rat (Adams et al., 1995). It is hard to detect embryonic γ-nAChR mRNA in normal rat muscles, while in denervated muscles, γ-nAChR gene expression increases significantly (Ma et al., 2005), making γ-nAChR gene expression level a hallmark of acute reaction after denervation.
Clinical significance of myogenesis related genes
Expression of myogenesis related genes is initially up-regulated and then down-regulated in the course of muscle denervation, a characteristic that may be used to help determine the presence of denervation atrophy (Kostrominova et al., 2000). More importantly, the timeline of changes in mRNA levels of these molecules provides clues to as when nerve repair should be done to ensure good outcomes. MRFs regulate nAChR gene expressions, and both play an important role in NMJ stability (Ma et al., 2007), so nerve repair should be done before the NMJ becomes unstable. Schwann cell number peaks at 1weekafter denervation (Zhang et al., 2008). In this very early phase of denervation the microenvironment in the denervated muscle is conducive for nerve regeneration making it an optimal time for nerve repair (Ma et al., 2007). One to 2 weeks after denervation is also when the highest gene expression is observed in denervated muscles, indicating gene expression level can be used as a clue to optimal timing of nerve repair. Sunderland has demonstrated that the denervated muscle would gain best recovery if repair was done with high gene expression levels. Otherwise the recovery possibility of denervated muscles would largely decrease if the optimal time had passed (Sunderland, 1978). Comparing to immediate repair, moderate delayed repair (1–2 weeks) is found to have at least equal, if not even better, recovery (Jergovic et al., 2001; Kline, 2009). On the other hand, prolonged delay will result in poor recovery when the regenerating nerve axons are more likely to fail to enter atrophic muscle fibers, and fail to make contact with NMJs, and when the target muscles have lost the receptiveness to regenerating axons (Brunetti et al., 1985; Fu and Gordon, 1995; Kobayashi et al., 1997). However, good functional recovery was reported when there was a two months’ delay in nerve repair due to elevated embryonic nAChR gene expression in sarcolemma. This underscores the importance of finding indexes other than time for judgment of muscle receptiveness. Embryonic nAChR may be an acceptable marker (Adams et al., 1995). MRFs and nAChR gene expression levels can potentially reflect the receptiveness of denervated muscles to regenerating axons, offering important clues for surgery choices and predicting surgical effects.
Neuromuscular junction (NMJ)
Number and density of regenerating axons do not differ between immediate and delayed nerve repairs (Brunetti et al., 1985; Fu and Gordon, 1995). Over time intramuscular nerve axon sheath degenerates (Kobayashi et al., 1997), which hinders axon infiltration into muscle fibers, and in turn decreases the number of motor units (Fu and Gordon, 1995). These changes induce a decrease in NMJ receptiveness to reinnervation.
Changes of NMJ after denervation
In normal rat muscle NMJ structure is very stable (Santos and Caroni, 2003). Specifically, in NMJ with nAChR, turnover half-time is ten days. NMJ's extensive remodeling ability helps hasten recovery after a nerve repair (Santos and Caroni, 2003). After denervation NMJ structure becomes unstable with turnover half-time of 1 day (Bezakova et al., 2001). At the same time, NMJ fragmentation and plantar area increases (Ma et al., 2005; Apel et al., 2009) as well as gutter depth (Ma et al., 2005). Post injury pre-synaptic changes are more evident than post synaptic ones. Pre-synaptic NMJ structures, like nerve terminal axons disappear within one week of injury, as part of Wallerian degeneration (Ma et al., 2007).
Clinical significance of NMJ in denervated muscle
NMJ fragments and plantar area increase initially and then decrease, following trends in nAChR and MRFs gene expression, which affect NMJ morphology (Ma et al., 2005). So, NMJ fragmentation and plantar area in denervated muscles can be used to evaluate the time course and extent of denervation and to assess the potential success of a nerve repair.
Discussion
Relationships among key changes in denervated muscle
Changes in the aforementioned parameters are interrelated. MFD positively correlates with maximal Fib amplitude and MFCV (Troni et al., 1983; Van der Hoeven et al., 1993; Cruz-Martinez and Arpa, 1999; Jiang et al., 2000; Ruegg et al., 2003; Blijham et al., 2006). Po has a positive correlation with muscle wet weight (Zealear et al., 1994). MRFs regulate nAChR gene expression, muscle wet weight and muscle fiber conversion (Aguiar et al., 2013). nAChR affects NMJ stability and regeneration after denervation (Ma et al., 2005; Shen et al., 2006).
Relationships between key changes and time course of denervation
Post denervation events can be divided into early and late stage changes (Ma et al., 2007). Early stage events create a microenvironment that is conducive to reinnervation (Ma et al., 2007). These acute changes include up-regulated expression of MRFs, nAChR (Kobayashi et al., 1997; Weis et al., 2000; Zhao et al., 2004; Ma et al., 2005; Shen et al., 2006; Ma et al., 2007; Lapalombella et al., 2008), and Schwann cell proliferation. On the other hand, late stage denervation sets into motion changes that render the NMJ and target muscle unreceptive to regenerating axons. Schwann cell number normalizes, target muscles atrophy severely, muscle wet weight reduces to 15% by 12 months of denervation (Ma et al., 2007), and connective tissues proliferate between muscle fibers preventing regenerating axons from entering into intramuscular nerve sheaths (Kobayashi et al., 1997). Due to these late stage changes, nerve repairs are less likely to succeed. Intervention past the optimal window almost guarantees poor recovery. Naturally, any measurement sensitive enough to detect residual muscle receptivity to reinnervation would be invaluable in planning patient care. As we have discussed, Fib, MFD, MRFs and nAChR gene expression can be used in exactly this capacity. The information that these parameters yield would enable physicians to individualize their approach to each patient and to ensure the best outcome feasible on a case by case basis.
Conclusion
Fib amplitude, MFCV, MFD, and mRNA expression levels of MRFs and nAChR could well reflect the severity and length of denervation as well as the receptiveness of denervated muscle to regenerating axons. They could possibly offer an important clue for surgical choices and predict the outcomes of delayed nerve repair.
Acknowledgements:
This study was sponsored by the Armed Forces Institute of Regenerative Medicine award number W81XWH-08-2-0034. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. The content of the manuscript does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred. Peng Wu, MD, was supported by the Sundt Fellowship fund, Department of Neurologic Surgery, Mayo Clinic, USA.
Footnotes
Conflicts of interest: None declared.
References
- Adams L, Carlson BM, Henderson L, Goldman D. Adaptation of nicotinic acetylcholine receptor, myogenin, and MRF4 gene expression to long-term muscle denervation. J Cell Biol. 1995;131:1341–1349. doi: 10.1083/jcb.131.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar AF, Vechetti-Junior IJ, Alves DSR, Castan EP, Milanezi-Aguiar RC, Padovani CR, Carvalho RF, Silva MD. Myogenin, MyoD and IGF-I regulate muscle mass but not fiber-type conversion during resistance training in rats. Int J Sports Med. 2013;34:293–301. doi: 10.1055/s-0032-1321895. [DOI] [PubMed] [Google Scholar]
- Al-Amood WS, Lewis DM. A comparison of the effects of denervation on the mechanical properties of rat and guinea-pig skeletal muscle. J Physiol. 1989;414:1–16. doi: 10.1113/jphysiol.1989.sp017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Amood WS, Lewis DM, Schmalbruch H. Effects of chronic electrical stimulation on contractile properties of long-term denervated rat skeletal muscle. J Physiol. 1991;441:243–256. doi: 10.1113/jphysiol.1991.sp018749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ani FS, Hamdan FB, Shaikhly KI. In situ measurements of muscle fiber conduction velocity in Duchenne muscular dystrophy. Saudi Med J. 2001;22:259–261. [PubMed] [Google Scholar]
- Andersen H, Poulsen PL, Mogensen CE, Jakobsen J. Isokinetic muscle strength in long-term IDDM patients in relation to diabetic complications. Diabetes. 1996;45:440–445. doi: 10.2337/diab.45.4.440. [DOI] [PubMed] [Google Scholar]
- Apel PJ, Alton T, Northam C, Ma J, Callahan M, Sonntag WE, Li Z. How age impairs the response of the neuromuscular junction to nerve transection and repair: An experimental study in rats. J Orthop Res. 2009;27:385–393. doi: 10.1002/jor.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquilonius SM, Askmark H, Gillberg PG, Nandedkar S, Olsson Y, Stalberg E. Topographical localization of motor endplates in cryosections of whole human muscles. Muscle Nerve. 1984;7:287–293. doi: 10.1002/mus.880070406. [DOI] [PubMed] [Google Scholar]
- Arancio O, Cangiano A, De Grandis D. Fibrillatory activity and other membrane changes in partially denervated muscles. Muscle Nerve. 1989;12:149–153. doi: 10.1002/mus.880120210. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Zwarts M. Measurement of muscle fiber conduction velocity in humans: techniques and applications. J Clin Neurophysiol. 1989;6:173–190. doi: 10.1097/00004691-198904000-00004. [DOI] [PubMed] [Google Scholar]
- Ashley Z, Sutherland H, Lanmuller H, Russold MF, Unger E, Bijak M, Mayr W, Boncompagni S, Protasi F, Salmons S, Jarvis JC. Atrophy, but not necrosis, in rabbit skeletal muscle denervated for periods up to one year. Am J Physiol Cell Physiol. 2007;292:C440–C451. doi: 10.1152/ajpcell.00085.2006. [DOI] [PubMed] [Google Scholar]
- Aydin MA, Mackinnon SE, Gu XM, Kobayashi J, Kuzon WJ. Force deficits in skeletal muscle after delayed reinnervation. Plast Reconstr Surg. 2004;113:1712–1718. doi: 10.1097/01.prs.0000118049.93654.ca. [DOI] [PubMed] [Google Scholar]
- Bain JR, Veltri KL, Chamberlain D, Fahnestock M. Improved functional recovery of denervated skeletal muscle after temporary sensory nerve innervation. Neuroscience. 2001;103:503–510. doi: 10.1016/s0306-4522(00)00577-7. [DOI] [PubMed] [Google Scholar]
- Beretta Piccoli M, Rainoldi A, Heitz C, Wuthrich M, Boccia G, Tomasoni E, Spirolazzi C, Egloff M, Barbero M. Innervation zone locations in 43 superficial muscles: toward a standardization of electrode positioning. Muscle Nerve. 2014;49:413–421. doi: 10.1002/mus.23934. [DOI] [PubMed] [Google Scholar]
- Bezakova G, Rabben I, Sefland I, Fumagalli G, Lomo T. Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers. Proc Natl Acad Sci U S A. 2001;98:9924–9929. doi: 10.1073/pnas.171539698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blijham PJ, ter Laak HJ, Schelhaas HJ, van Engelen BG, Stegeman DF, Zwarts MJ. Relation between muscle fiber conduction velocity and fiber size in neuromuscular disorders. J Appl Physiol. 2006;100:1837–1841. doi: 10.1152/japplphysiol.01009.2005. [DOI] [PubMed] [Google Scholar]
- Blijham PJ, Drost G, Stegeman DF, Zwarts MJ. Reduced muscle-fiber conduction but normal slowing after cold exposure in paramyotonia congenita. Muscle Nerve. 2008;37:23–26. doi: 10.1002/mus.20885. [DOI] [PubMed] [Google Scholar]
- Bortolotto SK, Cellini M, Stephenson DG, Stephenson GM. MHC isoform composition and Ca(2+)- or Sr(2+)-activation properties of rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2000;279:C1564–1577. doi: 10.1152/ajpcell.2000.279.5.C1564. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brown DL, Bennett TM, Dowsing BJ, Hayes A, Abate M, Morrison WA. Immediate and delayed nerve repair: improved muscle mass and function with leukemia inhibitory factor. J Hand Surg Am. 2002;27:1048–1055. doi: 10.1053/jhsu.2002.36518. [DOI] [PubMed] [Google Scholar]
- Brunetti A, Goldfine ID. Role of myogenin in myoblast differentiation and its regulation by fibroblast growth factor. J Biol Chem. 1990;265:5960–5963. [PubMed] [Google Scholar]
- Brunetti O, Carretta M, Magni F, Pazzaglia U. Role of the interval between axotomy and nerve suture on the success of muscle reinnervation: an experimental study in the rabbit. Exp Neurol. 1985;90:308–321. doi: 10.1016/0014-4886(85)90021-4. [DOI] [PubMed] [Google Scholar]
- Buffelli M, Pasino E, Cangiano A. Paralysis of rat skeletal muscle equally affects contractile properties as does permanent denervation. J Muscle Res Cell Motil. 1997;18:683–695. doi: 10.1023/a:1018687923929. [DOI] [PubMed] [Google Scholar]
- Burns AS, Lemay MA, Tessler A. Abnormal spontaneous potentials in distal muscles in animal models of spinal cord injury. Muscle Nerve. 2005;31:46–51. doi: 10.1002/mus.20229. [DOI] [PubMed] [Google Scholar]
- Burns AS, Boyce VS, Tessler A, Lemay MA. Fibrillation potentials following spinal cord injury: improvement with neurotrophins and exercise. Muscle Nerve. 2007;35:607–613. doi: 10.1002/mus.20738. [DOI] [PubMed] [Google Scholar]
- Butugan MK, Sartor CD, Watari R, Martins MC, Ortega NR, Vigneron VA, Sacco IC. Multichannel EMG-based estimation of fiber conduction velocity during isometric contraction of patients with different stages of diabetic neuropathy. J Electromyogr Kinesiol. 2014;24:465–472. doi: 10.1016/j.jelekin.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Carlson BM, Billington L, Faulkner J. Studies on the regenerative recovery of long-term denervated muscle in rats. Restor Neurol Neurosci. 1996;10:77–84. doi: 10.3233/RNN-1996-10203. [DOI] [PubMed] [Google Scholar]
- Cederna PS, Youssef MK, Asato H, Urbanchek MG, Kuzon WJ. Skeletal muscle reinnervation by reduced axonal numbers results in whole muscle force deficits. (2010-2011).Plast Reconstr Surg. 2000;105:2003–2009. doi: 10.1097/00006534-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Charbonnier F, Della GB, Armand AS, Lecolle S, Launay T, Gallien CL, Chanoine C. Specific activation of the acetylcholine receptor subunit genes by MyoD family proteins. J Biol Chem. 2003;278:33169–33174. doi: 10.1074/jbc.M304744200. [DOI] [PubMed] [Google Scholar]
- Chuang TY, Huang MC, Chen KC, Chang YC, Yen YS, Lee LS, Cheng H. Forelimb muscle activity following nerve graft repair of ventral roots in the rat cervical spinal cord. Life Sci. 2002;71:487–496. doi: 10.1016/s0024-3205(02)01623-5. [DOI] [PubMed] [Google Scholar]
- Cruz-Martinez A, Arpa J. Muscle fiber conduction velocity in situ (MFCV) in denervation, reinnervation and disuse atrophy. Acta Neurol Scand. 1999;100:337–340. doi: 10.1111/j.1600-0404.1999.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Deley G, Laroche D, Babault N. Effects of electrical stimulation pattern on quadriceps force production and fatigue. Muscle Nerve. 2014;49:760–763. doi: 10.1002/mus.24210. [DOI] [PubMed] [Google Scholar]
- Dennis RG. Bipolar implantable stimulator for long-term denervated-muscle experiments. Med Biol Eng Comput. 1998;36:225–228. doi: 10.1007/BF02510747. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Borenstein S. Relationship of spontaneous fibrillation potentials to muscle fibre segmentation in human muscular dystrophy. Nature. 1975;258:531–534. doi: 10.1038/258531a0. [DOI] [PubMed] [Google Scholar]
- Dow DE, Cederna PS, Hassett CA, Kostrominova TY, Faulkner JA, Dennis RG. Number of contractions to maintain mass and force of a denervated rat muscle. Muscle Nerve. 2004;30:77–86. doi: 10.1002/mus.20054. [DOI] [PubMed] [Google Scholar]
- Dow DE, Carlson BM, Hassett CA, Dennis RG, Faulkner JA. Electrical stimulation of denervated muscles of rats maintains mass and force, but not recovery following grafting. Restor Neurol Neurosci. 2006;24:41–54. [PubMed] [Google Scholar]
- Dumitru D, King JC. Fibrillation potential amplitude after denervation. Am J Phys Med Rehabil. 1998;77:483–489. doi: 10.1097/00002060-199811000-00005. [DOI] [PubMed] [Google Scholar]
- Eftimie R, Brenner HR, Buonanno A. Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proc Natl Acad Sci U S A. 1991;88:1349–1353. doi: 10.1073/pnas.88.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Merletti R. Estimation of average muscle fiber conduction velocity from two-dimensional surface EMG recordings. J Neurosci Methods. 2004;134:199–208. doi: 10.1016/j.jneumeth.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina A, Ritzel CH, Merletti R, Vieira TM. Do surface electromyograms provide physiological estimates of conduction velocity from the medial gastrocnemius muscle? J Electromyogr Kinesiol. 2013;23:319–325. doi: 10.1016/j.jelekin.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Gechev AG, Ilieva EM, Marinkev MD, Hadjigeorgiev GH. Diagnostic value of the muscle fiber conduction velocity for evaluation of muscle hypotrophy. Folia Med (Plovdiv) 2004;46:41–46. [PubMed] [Google Scholar]
- Gregory J, Cowey A, Jones M, Pickard S, Ford D. The anatomy, investigations and management of adult brachial plexus injuries. Orthop Trauma. 2009;23:6. [Google Scholar]
- Grill WM, Mortimer JT. Non-invasive measurement of the input-output properties of peripheral nerve stimulating electrodes. J Neurosci Methods. 1996;65:43–50. doi: 10.1016/0165-0270(95)00143-3. [DOI] [PubMed] [Google Scholar]
- Hadlock TA, Heaton J, Cheney M, Mackinnon SE. Functional recovery after facial and sciatic nerve crush injury in the rat. Arch Facial Plast Surg. 2005;7:17–20. doi: 10.1001/archfaci.7.1.17. [DOI] [PubMed] [Google Scholar]
- Haig AJ, Gelblum JB, Rechtien JJ, Gitter AJ. Technology assessment: the use of surface EMG in the diagnosis and treatment of nerve and muscle disorders. Muscle Nerve. 1996;19:392–395. doi: 10.1002/(SICI)1097-4598(199603)19:3<392::AID-MUS21>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Hakansson CH. Conduction velocity and amplitude of the action potential as related to circumference in the isolated fibre of frog muscle. Acta Physiol Scand. 1956;37:14–34. doi: 10.1111/j.1748-1716.1956.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Heaton JT, Kobler JB. Use of muscle fibrillation for tracking nerve regeneration. Muscle Nerve. 2005;31:235–241. doi: 10.1002/mus.20257. [DOI] [PubMed] [Google Scholar]
- Heaton JT, Sheu SH, Hohman MH, Knox CJ, Weinberg JS, Kleiss IJ, Hadlock TA. Ratwhisker movement after facial nerve lesion: evidence for autonomic contraction of skeletal muscle. Neuroscience. 2014;265:9–21. doi: 10.1016/j.neuroscience.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann R, Ludin HP. Differentiation of spontaneous activity from normal and denervated skeletal muscle. J Neurol Neurosurg Psychiatry. 1982;45:331–336. doi: 10.1136/jnnp.45.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SM, Taylor JM, Tapscott SJ, Gurley CM, Carter WJ, Peterson CA. Selective accumulation of MyoD and myogenin mRNAs in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development. 1993;118:1137–1147. doi: 10.1242/dev.118.4.1137. [DOI] [PubMed] [Google Scholar]
- Izumi SI, Tsubahara A, Chino N. Relationship between hypoxemia and fibrillation potential firing rate in denervated muscle. Muscle Nerve. 1999;22:933–936. doi: 10.1002/(sici)1097-4598(199907)22:7<933::aid-mus18>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Jayaraman A, Thompson CK, Rymer WZ, Hornby TG. Short-term maximal- intensity resistance training increases volitional function and strength in chronic incomplete spinal cord injury: a pilot study. J Neurol Phys Ther. 2013;37:112–117. doi: 10.1097/NPT.0b013e31828390a1. [DOI] [PubMed] [Google Scholar]
- Jergovic D, Stal P, Lidman D, Lindvall B, Hildebrand C. Changes in a rat facial muscle after facial nerve injury and repair. Muscle Nerve. 2001;24:1202–1212. doi: 10.1002/mus.1133. [DOI] [PubMed] [Google Scholar]
- Jiang GL, Zhang LY, Shen LY, Xu JG, Gu YD. Fibrillation potential amplitude to quantitatively assess denervation muscle atrophy. Neuromuscul Disord. 2000;10:85–91. doi: 10.1016/s0960-8966(99)00075-9. [DOI] [PubMed] [Google Scholar]
- Johns MM, Urbanchek M, Chepeha DB, Kuzon WJ, Hogikyan ND. Thyroarytenoid muscle maintains normal contractile force in chronic vocal fold immobility. Laryngoscope. 2001;111:2152–2156. doi: 10.1097/00005537-200112000-00014. [DOI] [PubMed] [Google Scholar]
- Kalliainen LK, Jejurikar SS, Liang LW, Urbanchek MG, Kuzon WJ. A specific force deficit exists in skeletal muscle after partial denervation. Muscle Nerve. 2002;25:31–38. doi: 10.1002/mus.1216. [DOI] [PubMed] [Google Scholar]
- Keller SP, Sandrock AW, Gozani SN. Noninvasive detection of fibrillation potentials in skeletal muscle. IEEE Trans Biomed Eng. 2002;49:788–795. doi: 10.1109/TBME.2002.800756. [DOI] [PubMed] [Google Scholar]
- Kerns JM, Shott S, Brubaker L, Sakamoto K, Benson JT, Fleischer AE, Coleman ME. Effects of IGF-I gene therapy on the injured rat pudendal nerve. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:2–7,8. doi: 10.1007/s00192-002-0995-2. [DOI] [PubMed] [Google Scholar]
- Kline DG. Timing for brachial plexus injury: a personal experience. Neurosurg Clin N Am. 2009;20:24–26. doi: 10.1016/j.nec.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Mackinnon SE, Watanabe O, Ball DJ, Gu XM, Hunter DA, Kuzon WJ. The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve. 1997;20:858–866. doi: 10.1002/(sici)1097-4598(199707)20:7<858::aid-mus10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kostrominova TY, Macpherson PC, Carlson BM, Goldman D. Regulation of myogenin protein expression in denervated muscles from young and old rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R179–188. doi: 10.1152/ajpregu.2000.279.1.R179. [DOI] [PubMed] [Google Scholar]
- Kostrominova TY, Dow DE, Dennis RG, Miller RA, Faulkner JA. Comparison of gene expression of 2-mo denervated, 2-mo stimulated-denervated, and control rat skeletal muscles. Physiol Genomics. 2005;22:227–243. doi: 10.1152/physiolgenomics.00210.2004. [DOI] [PubMed] [Google Scholar]
- Kraft GH. Fibrillation potential amplitude and muscle atrophy following peripheral nerve injury. Muscle Nerve. 1990;13:814–821. doi: 10.1002/mus.880130907. [DOI] [PubMed] [Google Scholar]
- Lapalombella R, Kern H, Adami N, Biral D, Zampieri S, Scordari A, di Tullio S, Marini M. Persistence of regenerative myogenesis in spite of down-regulation of activity-dependent genes in long-term denervated rat muscle. Neurol Res. 2008;30:197–206. doi: 10.1179/174313208X281091. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kwon HK. Semiquantification of fibrillation potentials with intramuscular temperature drop using an animal model. J Korean Med Sci. 1997;12:550–552. doi: 10.3346/jkms.1997.12.6.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J, Hayes A, Austin L, Morrison W. Muscle protection following motor nerve repair in combination with leukemia inhibitory factor. J Hand Surg Am. 1999;24:37–45. doi: 10.1053/jhsu.1999.jhsy24a0037. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Belman MJ, Sieck GC. Aminophylline and fatigue of the sternomastoid muscle. Am Rev Respir Dis. 1986;133:672–675. doi: 10.1164/arrd.1986.133.4.672. [DOI] [PubMed] [Google Scholar]
- Li W, Sakamoto K. The influence of location of electrode on muscle fiber conduction velocity and EMG power spectrum during voluntary isometric contraction measured with surface array electrodes. Appl Human Sci. 1996;15:25–32. doi: 10.2114/jpa.15.25. [DOI] [PubMed] [Google Scholar]
- Loring RH, Salpeter MM. Denervation increases turnover rate of junctional acetylcholine receptors. Proc Natl Acad Sci U S A. 1980;77:2293–2297. doi: 10.1073/pnas.77.4.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DX, Huang SK, Carlson BM. Electron microscopic study of long-term denervated rat skeletal muscle. Anat Rec. 1997;248:355–365. doi: 10.1002/(SICI)1097-0185(199707)248:3<355::AID-AR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Luco JV, Eyzaguirre C. Fibrillation and hypersensitivity to ACh in denervated muscle: effect of length of degenerating nerve fibers. J Neurophysiol. 1955;18:65–73. doi: 10.1152/jn.1955.18.1.65. [DOI] [PubMed] [Google Scholar]
- Ma J, Shen J, Lee CA, Elsaidi GA, Smith TL, Walker FO, Rushing JT, Tan KH, Koman LA, Smith BP. Gene expression of nAChR, SNAP-25 and GAP-43 in skeletal muscles following botulinum toxin A injection: a study in rats. J Orthop Res. 2005;23:302–309. doi: 10.1016/j.orthres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Ma J, Shen J, Garrett JP, Lee CA, Li Z, Elsaidi GA, Ritting A, Hick J, Tan KH, Smith TL, Smith BP, Koman LA. Gene expression of myogenic regulatory factors, nicotinic acetylcholine receptor subunits, and GAP-43 in skeletal muscle following denervation in a rat model. J Orthop Res. 2007;25:1498–1505. doi: 10.1002/jor.20414. [DOI] [PubMed] [Google Scholar]
- Manetti M, Neumann E, Milia AF, Tarner IH, Bechi P, Matucci-Cerinic M, Ibba-Manneschi L, Muller-Ladner U. Severe fibrosis and increased expression of fibrogenic cytokines in the gastric wall of systemic sclerosis patients. Arthritis Rheum. 2007;56:3442–3447. doi: 10.1002/art.22940. [DOI] [PubMed] [Google Scholar]
- McIntosh KC, Gabriel DA. Reliability of a simple method for determining muscle fiber conduction velocity. Muscle Nerve. 2012;45:257–265. doi: 10.1002/mus.22268. [DOI] [PubMed] [Google Scholar]
- Meijer JW, Lange F, Links TP, van der Hoeven JH. Muscle fiber conduction abnormalities in early diabetic polyneuropathy. Clin Neurophysiol. 2008;119:1379–1384. doi: 10.1016/j.clinph.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Merletti R, Holobar A, Farina D. Analysis of motor units with high-density surface electromyography. J Electromyogr Kinesiol. 2008;18:879–890. doi: 10.1016/j.jelekin.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Moxham J, Wiles CM, Newham D, Edwards RH. Sternomastoid muscle function and fatigue in man. Clin Sci (Lond) 1980;59:463–468. doi: 10.1042/cs0590463. [DOI] [PubMed] [Google Scholar]
- Naderi J, Bernreuther C, Grabinski N, Putman CT, Henkel B, Bell G, Glatzel M, Sultan KR. Plasminogen activator inhibitor type 1 up-regulation is associated with skeletal muscle atrophy and associated fibrosis. Am J Pathol. 2009;175:763–771. doi: 10.2353/ajpath.2009.081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederle B, Mayr R. Course of denervation atrophy in type I and type II fibres of rat extensor digitorum longus muscle. Anat Embryol (Berl) 1978;153:9–21. doi: 10.1007/BF00569846. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Graven-Nielsen T, Farina D. Effect of innervation-zone distribution on estimates of average muscle-fiber conduction velocity. Muscle Nerve. 2008;37:68–78. doi: 10.1002/mus.20895. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbova G. Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve. 1985;8:676–689. doi: 10.1002/mus.880080810. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22:307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Raftery MA, Hunkapiller MW, Strader CD, Hood LE. Acetylcholine receptor: complex of homologous subunits. Science. 1980;208:1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Rainoldi A, Melchiorri G, Caruso I. A method for positioning electrodes during surface EMG recordings in lower limb muscles. J Neurosci Methods. 2004;134:37–43. doi: 10.1016/j.jneumeth.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One. 2012;7:e29082. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg DG, Kakebeeke TH, Gabriel JP, Bennefeld M. Conduction velocity of nerve and muscle fiber action potentials after a space mission or a bed rest. Clin Neurophysiol. 2003;114:86–93. doi: 10.1016/s1388-2457(02)00329-2. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Li W. Effect of muscle length on distribution of muscle fiber conduction velocity for M. biceps brachii. Appl Human Sci. 1997;16:1–7. doi: 10.2114/jpa.16.1. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Mito K. Muscle fiber conduction velocity during isometric contraction and the recovery period. Electromyogr Clin Neurophysiol. 2000;40:151–161. [PubMed] [Google Scholar]
- Salafsky B, Bell J, Prewitt MA. Development of fibrillation potentials in denervated fast and slow skeletal muscle. Am J Physiol. 1968;215:637–643. doi: 10.1152/ajplegacy.1968.215.3.637. [DOI] [PubMed] [Google Scholar]
- Santos AF, Caroni P. Assembly, plasticity and selective vulnerability to disease of mouse neuromuscular junctions. J Neurocytol. 2003;32:849–862. doi: 10.1023/B:NEUR.0000020628.36013.88. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H, Al-Amood WS, Lewis DM. Morphology of long-term denervated rat soleus muscle and the effect of chronic electrical stimulation. J Physiol. 1991;441:233–241. doi: 10.1113/jphysiol.1991.sp018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Ma J, Lee C, Smith BP, Smith TL, Tan KH, Koman LA. How muscles recover from paresis and atrophy after intramuscular injection of botulinum toxin A: Study in juvenile rats. J Orthop Res. 2006;24:1128–1135. doi: 10.1002/jor.20131. [DOI] [PubMed] [Google Scholar]
- Shin RH, Vathana T, Giessler GA, Friedrich PF, Bishop AT, Shin AY. Isometric tetanic force measurement method of the tibialis anterior in the rat. Microsurgery. 2008;28:452–457. doi: 10.1002/micr.20520. [DOI] [PubMed] [Google Scholar]
- Smith JW, Thesleff S. Spontaneous activity in denervated mouse diaphragm muscle. J Physiol. 1976;257:171–186. doi: 10.1113/jphysiol.1976.sp011362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector SA. Trophic effects on the contractile and histochemical properties of rat soleus muscle. J Neurosci. 1985;5:2189–2196. doi: 10.1523/JNEUROSCI.05-08-02189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland S. Edinburgh. 2nd edition. Churchill-livingston; 1978. Nerve and nerve injuies. [Google Scholar]
- Thesleff S, Ward MR. Studies on the mechanism of fibrillation potentials in denervated muscle. J Physiol. 1975;244:313–323. doi: 10.1113/jphysiol.1975.sp010800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totosy DZJ, Zung HV, Erdebil S, Gordon T. Innervation ratio is an important determinant of force in normal and reinnervated rat tibialis anterior muscles. J Neurophysiol. 1992;67:1385–1403. doi: 10.1152/jn.1992.67.5.1385. [DOI] [PubMed] [Google Scholar]
- Trail IA. Delayed repair of the ulnar nerve. J Hand Surg Br. 1985;10:345–346. doi: 10.1016/s0266-7681(85)80058-9. [DOI] [PubMed] [Google Scholar]
- Troni W, Cantello R, Rainero I. Conduction velocity along human muscle fibers in situ. Neurology. 1983;33:1453–1459. doi: 10.1212/wnl.33.11.1453. [DOI] [PubMed] [Google Scholar]
- Trontelj JV. Muscle fiber conduction velocity changes with length. Muscle Nerve. 1993;16:506–512. doi: 10.1002/mus.880160512. [DOI] [PubMed] [Google Scholar]
- Tsubahara A, Chino N, Mineo K. Fibrillation potentials and muscle fiber types. Muscle Nerve. 1990;13:983. doi: 10.1002/mus.880131015. [DOI] [PubMed] [Google Scholar]
- Van Balkom RH, Zhan WZ, Prakash YS, Dekhuijzen PN, Sieck GC. Corticosteroid effects on isotonic contractile properties of rat diaphragm muscle. J Appl Physiol. 1997;83:1062–1067. doi: 10.1152/jappl.1997.83.4.1062. [DOI] [PubMed] [Google Scholar]
- Van der Hoeven JH, Zwarts MJ, Van Weerden TW. Muscle fiber conduction velocity in amyotrophic lateral sclerosis and traumatic lesions of the plexus brachialis. Electroencephalogr Clin Neurophysiol. 1993;89:304–310. doi: 10.1016/0168-5597(93)90069-2. [DOI] [PubMed] [Google Scholar]
- van der Meulen JH, Urbanchek MG, Cederna PS, Eguchi T, Kuzon WJ. Denervated muscle fibers explain the deficit in specific force following reinnervation of the rat extensor digitorum longus muscle. Plast Reconstr Surg. 2003;112:1336–1346. doi: 10.1097/01.PRS.0000081464.98718.E3. [DOI] [PubMed] [Google Scholar]
- Voytik SL, Przyborski M, Badylak SF, Konieczny SF. Differential expression of muscle regulatory factor genes in normal and denervated adult rat hindlimb muscles. Dev Dyn. 1993;198:214–224. doi: 10.1002/aja.1001980307. [DOI] [PubMed] [Google Scholar]
- Wang H, Gu Y, Xu J, Shen L, Li J. Comparative study of different surgical procedures using sensory nerves or neurons for delaying atrophy of denervated skeletal muscle. J Hand Surg Am. 2001;26:326–331. doi: 10.1053/jhsu.2001.22522. [DOI] [PubMed] [Google Scholar]
- Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27:43–59. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]
- Weis J, Kaussen M, Calvo S, Buonanno A. Denervation induces a rapid nuclear accumulation of MRF4 in mature myofibers. Dev Dyn. 2000;218:438–451. doi: 10.1002/1097-0177(200007)218:3<438::AID-DVDY1001>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Wen H, Dou Z, Cheng S, Qiu W, Xie L, Yang H. Activity of thigh muscles during static and dynamic stances in stroke patients: a pilot case-control study. Top Stroke Rehabil. 2014;21:163–172. doi: 10.1310/tsr2102-163. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, Lomo T. Control of contractile properties within adaptive ranges by patterns of impulse activity in the rat. J Neurosci. 1988;8:4415–4426. doi: 10.1523/JNEUROSCI.08-12-04415.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechers DO. Mechanically provoked insertional activity before and after nerve section in rats. Arch Phys Med Rehabil. 1977;58:402–405. [PubMed] [Google Scholar]
- Willmott AD, White C, Dukelow SP. Fibrillation potential onset in peripheralnerve injury. Muscle Nerve. 2012;46:332–340. doi: 10.1002/mus.23310. [DOI] [PubMed] [Google Scholar]
- Windisch A, Gundersen K, Szabolcs MJ, Gruber H, Lomo T. Fast to slow transformation of denervated and electrically stimulated rat muscle. J Physiol. 1998;510:623–632. doi: 10.1111/j.1469-7793.1998.623bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzemann V, Sakmann B. Differential regulation of MyoD and myogenin mRNA levels by nerve induced muscle activity. FEBS Lett. 1991;282:259–264. doi: 10.1016/0014-5793(91)80490-t. [DOI] [PubMed] [Google Scholar]
- Wu P, Spinner RJ, Gu Y, Yaszemski MJ, Windebank AJ, Wang H. Delayed repair of the peripheral nerve: a novel model in the rat sciatic nerve. J Neurosci Methods. 2013;214:37–44. doi: 10.1016/j.jneumeth.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Xu J, Gu Y. Experimental study of morphology and electrophysiology on denervated skeletal muscle. Zhongguo Xiufu Chongjian Waike Zazhi. 1999;13:202–205. [PubMed] [Google Scholar]
- Zealear DL, Hamdan AL, Rainey CL. Effects of denervation on posterior cricoarytenoid muscle physiology and histochemistry. Ann Otol Rhinol Laryngol. 1994;103:780–788. doi: 10.1177/000348949410301007. [DOI] [PubMed] [Google Scholar]
- Zhao C, Veltri K, Li S, Bain JR, Fahnestock M. NGF, BDNF, NT-3, and GDNF mRNA expression in rat skeletal muscle following denervation and sensory protection. J Neurotrauma. 2004;21:1468–1478. doi: 10.1089/neu.2004.21.1468. [DOI] [PubMed] [Google Scholar]
- Zhang PX, Xue F, Zhao FQ, Lu H, Zhang HB, Jiang BG. The immunohistological observation of proliferation rule of Schwann cell after sciatic nerve injury in rats. Artif Cells Blood Substit Immobil Biotechnol. 2008;36:150–155. doi: 10.1080/10731190801932132. [DOI] [PubMed] [Google Scholar]
- Zwarts MJ. Evaluation of the estimation of muscle fiber conduction velocity. Surface versus needle method. Electroencephalogr Clin Neurophysiol. 1989;73:544–548. doi: 10.1016/0013-4694(89)90263-0. [DOI] [PubMed] [Google Scholar]


