Abstract
OBJECTIVE:
To evaluate the effects of olfactory ensheathing cell transplantation on functional recovery of rats with complete spinal cord transection.
DATA SOURCES:
A computer-based online search of Medline (1989–2013), Embase (1989–2013), Cochrane library (1989–2013), Chinese Biomedical Literature Database (1989–2013), China National Knowledge Infrastructure (1989–2013), VIP (1989–2013), Wanfang databases (1989–2013) and Chinese Clinical Trial Register was conducted to collect randomized controlled trial data regarding olfactory ensheathing cell transplantation for the treatment of complete spinal cord transection in rats.
SELECTION CRITERIA:
Randomized controlled trials investigating olfactory ensheathing cell transplantation and other transplantation methods for promoting neurological functional recovery of rats with complete spinal cord transection were included in the analysis. Meta analysis was conducted using RevMan 4.2.2 software.
MAIN OUTCOME MEASURES:
Basso, Beattie and Bresnahan scores of rats with complete spinal cord transection were evaluated in this study.
RESULTS:
Six randomized controlled trials with high quality methodology were included. Meta analysis showed that Basso, Beattie and Bresnahan scores were significantly higher in the olfactory ensheathing cell transplantation group compared with the control group (WMD = 3.16, 95% CI (1.68, 4.65); P < 0.00001).
CONCLUSION:
Experimental studies have shown that olfactory ensheathing cell transplantation can promote the functional recovery of motor nerves in rats with complete spinal cord transection.
Keywords: nerve regeneration, olfactory ensheathing cells, cell transplantation, spinal cord injury, complete transection, BBB scores, meta analysis
Introduction
Spinal cord injury (SCI) is one of the most devastating forms of trauma affecting humans. Approximately half of patients with SCI have complete transection, with no preservation of voluntary motor or sensory function below the corresponding level of injury (Tator et al., 1990). The development of powerful strategies to treat SCI has always been a major clinical challenge, although with recent dramatic progresses in cellular transplantation, gene therapy and molecular treatment has increased optimism for a future cure for SCI (Rapalino et al., 1998; Jones et al., 2001; Blits et al., 2002; Schwab, 2002).
Olfactory ensheathing cells (OECs) promote the axonal growth of neurons in the olfactory mucosa of the nasal cavity to innervate the olfactory bulb of the brain and form synapses with second-order neurons (Doucette, 1984). Recent studies have shown that implantation of rodent and human OECs appears to be one of the most promising strategies to promote long-distance regeneration in the injured spinal cord (Doucette, 1984; Li et al., 1998; Barnett et al., 2000; Kato et al., 2000).
Animal models are invaluable for research on SCI because they allow for precise manipulations and measurements, as well as a means to evaluate potential therapeutic interventions. However, SCI models can vary between different studies. The induction of injury to the spinal cord can be done by several techniques, including aspiration, resection, hemisection, transection, contusion, compression, ischemia, and neurotoxin injection. Complete spinal cord transection is a frequently-used model in SCI studies. After complete spinal cord transection, axonal regeneration across the lesion for functional recovery remains a difficult challenge (Imaizumi et al., 1998; Ramón-Cueto et al., 2000; Fouad et al., 2005). OEC transplantation reportedly promotes tissue sparing, axon remyelination, and improvements in motor performance in incomplete and complete adult SCI models (Ramón-Cueto et al., 2000; García-Alías et al., 2003; Li et al., 2003, 2007; Kubasak et al., 2008). This model exhibits huge advantages in studying nerve injury repair and regeneration (Dobkin et al., 1995; Yates et al., 2008; Muñoz-Quiles et al., 2009; Tillakaratne et al., 2010). To exclude interference and bias induced by incomplete transection during model establishment and to clarify more clearly the advantages and effects of OEC transplantation in SCI, this review included only the studies of complete spinal cord transection and conducted a meta analysis.
Data and Methods
Data retrieval
Boolean logic operation was used to link retrieval words and Cochrane Collaboration Handbook RCT was used for advanced searches. SCI, complete transection, and olfactory ensheathing cells were used as key words in Chinese and English to retrieve Medline, Embase, Cochrane library, Chinese Biomedical Literature Database, China National Knowledge Infrastructure, VIP, Wanfang databases and Chinese Clinical Trial Register, to search for basic studies published between January 1989 and December 2013. Chinese articles were only selected from Chinese journals with statistical sources.
Inclusion and exclusion criteria
Randomized controlled trials on OEC transplantation for promoting neurological functional recovery in complete spinal cord transection were included, in which the animal models were rats. Trials without complete quantitative data and repeat publications were excluded.
Data extraction and quality evaluation
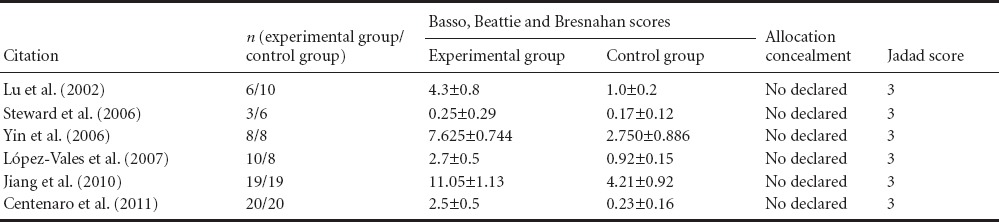
The Jadad scale was used to evaluate the quality of included studies (Jadad et al., 1996), which contained three items relating directly to the reduction of bias and whose frequency of endorsement was between 15% and 85%. The three items were the following questions: 1. Was the study described as double blind? 2. Was the study described as randomized (including the application of the words randomly, random, or randomization)? 3. Was there a description of dropouts and withdrawals? The items were raised as questions to draw the yes or no answers. Points awarded for items 1 and 2 relied on the quality of the introduction of the research methods to generate the sequence of randomization and/or the quality of the introduction of the methods of double-blinding. If the trial had been described as randomized and/or double-blind, but there was no introduction of the methods applied to generate the sequence of randomization or the double-blind conditions, one point was awarded in each case. If the methods of generating the sequence of randomization and/or blinding had been introduced, one additional point was given to each item, if the method was appropriate. A method to generate randomization of sequences was considered as sufficient if it allowed each study participant to have the same chance of receiving each intervention, and if the investigators could not predict which intervention was next. Double-blinding was considered appropriate if it was described or hinted that neither the person doing the assessment nor the study participant could identify the intervention being assessed. Oppositely, the relevant item was given the point of zero, if the method of generating the sequence of randomization and/or blinding was introduced but inappropriate. The third item, dropouts and withdrawals, was awarded zero points for a negative answer and one point for a positive. For a positive answer, the number of dropouts, withdrawals, and the reasons for either had to be described in each of the comparison groups. It should have been demonstrated in the report if there were no withdrawals. The quality of studies was graded as 0 to 5 points. Studies with 3 points or more were regarded as high quality, with more than 1 point as moderate, but with 1 point or less as low quality.
Extraction content included baseline data (sample size, randomization and blinding method); and original data of statistical analysis: Basso, Beattie and Bresnahan (BBB) scores.
Data analysis
Meta analysis was conducted using RevMan 4.2.2 software (offered by the Cochrane Collaboration). BBB scores of hindlimb were used as the evaluation index (Basso et al., 1996). Included studies were subjected to heterogeneity testing. RevMan boundary value α = 0.10. P < 0.01 indicates heterogeneity of included studies, which should be further analyzed with heterogeneity. If P > 0.01, the studies were analyzed using fixed effect model. I2 > 50% does not represent homogeneity. Measurement data were represented by weighted mean difference (95% confidence interval (CI)).
Results
Data retrieval results
A total of 95 articles were retrieved, including 49 Chinese and 46 English, published between January 1989 and December 2013. After reading the titles and abstracts, nine (two Chinese and seven English) were selected for further analysis. After reading the full text of each article, six were included (Lu et al., 2002; Steward et al., 2006; Yin et al., 2006; López-Vales et al., 2007; Jiang et al., 2010; Centenaro et al., 2011) and three were excluded because of incomplete quantitative data (Figure 1).
Figure 1.

Data retrieval and inclusion processing of the meta-analysis of olfactory ensheathing cell transplantation for the promotion of neurological functional recovery of rats with complete spinal cord transection.
Quality estimation and data extraction
All included studies were randomized controlled trials and BBB scores were used as the main evaluation index. A total of 137 rats were involved in the six included studies, with definite inclusion and exclusion criteria. The characteristics and Jadad scores of included studies are listed in Tables 1 and 2.
Table 1.
Characteristics of studies included in the meta-analysis of olfactory ensheathing cell (OEC) transplantation for the promotion of neurological functional recovery of rats with complete spinal cord transection
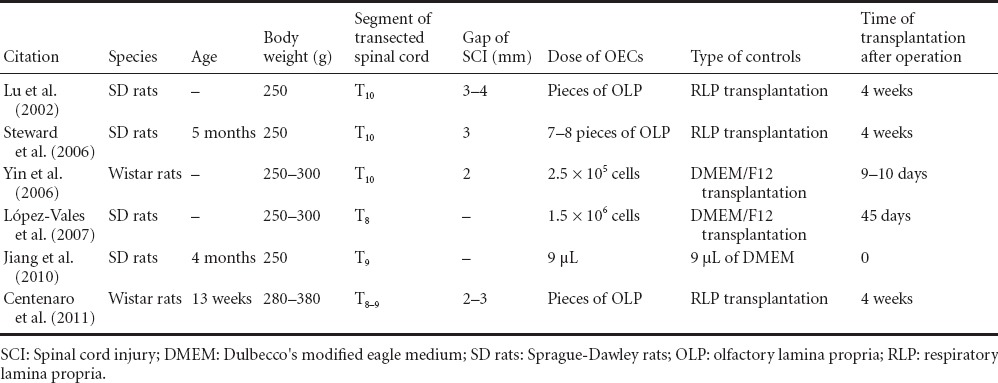
Table 2.
Baseline data and scores of included studies
Meta analysis
A total of 137 rats were included. Due to heterogeneity in studies (I2 = 98.7%, P < 0.00001), the random effect model was used. Results showed that the BBB scores between OEC transplantation and control groups at the final follow-up were significantly different (WMD = 3.16, 95% CI (1.68, 4.65), P < 0.00001). This evidence indicates that OEC transplantation promotes increased motor functional recovery of rat hindlimbs compared with the control group (Figure 2).
Figure 2.
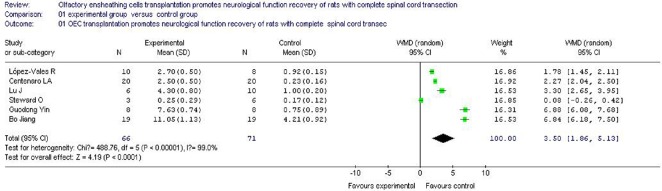
Meta analysis of the effects of olfactory ensheathing cells transplantation on neurological function recovery of rats with complete spinal cord transection.
The meta analysis shows that olfactory ensheathing cells transplantation promotes increased motor functional recovery of rat hindlimbs compared with the control group.
Sensitivity analysis
To eliminate the influence of heterogeneity on the results, the sensitivity of BBB scores of included studies was analyzed. Results showed statistical significance after excluding one study due to a very small size of samples (Tillakaratne et al., 2010). This indicates that BBB scores in the group of OEC transplantation were still higher than the control group (P < 0.05), i.e., the influence of heterogeneity is limited to the meta analysis.
Information of included studies
Lu et al. (2002) transplanted OECs from the olfactory mucosa into the T10 thoracic site of a SCI model in adult rats at 4 weeks after transection. There was a significant recovery of locomotor behavior and restoration of descending inhibition of spinal cord reflexes, 10 weeks after transection and transplantation. There was also growth of axons across the transection site, including serotonergic axons arising from the brainstem raphe nuclei. Four weeks later, the scar tissue and cavities at the transection site were removed to develop a 3–4 mm gap. Then, pieces of olfactory lamina propria (OLP) were placed into this gap, between the cut surfaces of the spinal cord. Ten weeks later, the locomotor activity of these animals was significantly improved compared with control animals, which received implants of either pieces of nasal respiratory lamina propria (RLP) or collagen. The behavioral recovery was still improving 10 weeks after transplantation. Regrowth of brainstem raphe axons across the transplant site was shown by the presence of serotonergic axons in the spinal cord caudal to the transection site, and by retrograde labeling of cells in the nucleus raphe magnus after injections of fluorogold into the caudal spinal cord. Neither serotonergic axons nor labeled brainstem cells were observed in the control animals. These results show that OECs from the nasal OLP have the ability to promote axonal regeneration of spinal cord, when transplanted 4 weeks after complete transection. Their study further supports clinical application of OECs for the repair of human SCI through autologous transplantation, as these cells are accessible and available in nose.
Steward et al. (2006) found that, after OEC-rich OLP and OEC-free RLP were implanted into the spinal cord injury site, 30 days after spinal cord transection at the T10 segment in adult female rats, BBB rating results showed that hindlimb motor function showed no obvious recovery after OLP transplantation, and the motor function was similar after OLP and RLP transplantation. No flourogold was detected in the spinal cord injury site after transplantation of OLP or RLP. Immunostaining results showed that a small amount of 5-hydroxytryptamine-labeled axons grew into the transplantation site. Their results suggest that OLP transplantation is not an efficient treatment for SCI, although the transplantation could promote axonal regeneration under certain conditions.
Yin et al. (2006) investigated the transplantation of human embryonic OECs in adult rats with complete spinal cord transection. Separated, cultured human embryonic OECs from abortus were obtained. All rats were transected at the T10 segment of spinal cord. Nine to ten days later, 5 μL (2.5 × 105) human embryonic OECs (labeled by Hoechst 33342) were injected in the contusion area of the lesion site. Transplanted OECs could survive for at least 10 weeks and even migrated from the injured area in the spinal cord. From 4 to 10 weeks, the BBB locomotor scores of the experimental group were improved significantly compared with the control group (P < 0.05). Immunohistochemical staining showed an increased number of p75NTR-positive nerve fibers and synapses in the lesion site of the experimental group compared with the control group. Finally, they found that transplantation of human embryonic OECs could enhance the recovery of locomotor function for adult rats with SCI.
López-Valeset et al. (2007) examined whether OECs could stimulate the regeneration of axons and functional recovery when transplanted 45 days after complete transection of thoracic spinal cord in adult rats. OECs partially improved restitution of supraspinal pathways, as evaluated by motor-evoked potentials and modest recovery of hindlimb movements. Furthermore, OEC transplantations decreased lumbar reflex hyperexcitability from 1 month after transplantation. Histological examinations showed that OECs facilitated corticospinal and raphespinal axonal regrowth through the transection site and into the caudal spinal cord segments. It is interesting that raphespinal but not corticospinal fibers regenerated long distances through the gray matter and reached the lower lumbar segments (L5) of the spinal cord. However, delayed OEC transplantations failed to decrease posttraumatic astrogliosis. Thus, the beneficial results shown in their study further support the application of OECs for the treatment of chronic SCI.
Jiang et al. (2010) examined the survival and reparative effects of OECs, following transplantation in a complete spinal cord transection model at the T9 segment, immediately after injury. The locomotor behavior improved 4 weeks after surgery. By immunohistochemical staining, OECs could be observed around the spinal injury area, and had migrated 1.0 cm away from the injury area. There was a positive correlation between the number of regenerating axons and the recovery of locomotor function. Thus, they found that OECs can promote axonal regeneration and the recovery of locomotor function after spinal cord transection.
Centenaro et al. (2011) examined the effects of OLP or RLP transplantation and the appropriate timing for their application after SCI. Adult male rats received spinal cord transection, followed by acute, 2- or 4-week post-injury transplantation with pieces of OLP or RLP (control). Animals, after grafting with OLP and RLP, showed discrete and similar motor improvement of the hindlimb, with comparable spinal cord tissue sparing and sprouting in the transection site. Acute transplantation of OLP and RLP appeared to foster limited supraspinal axonal regeneration, as shown by the presence of neurons stained by retrograde tracing in the brainstem nuclei. A larger number of 5-hydroxytryptamine-positive fibers were found in the cranial stump of the OLP and RLP groups compared with the lesion and caudal regions. Calcitonin gene-related peptide fibers were present in considerable numbers at the SCI area in both transplantation groups. Their results failed to show differences between acute, 2- and 4-week delayed transplantations of OLP and RLP, implying that the limited functional and axonal reparative effects observed could not be exclusively related to OECs. A further understanding of the effects of these tissue transplantations is necessary to emphasize the rationale for use of this therapeutic method in humans.
Discussion
Difficulties in axonal regeneration post-SCI are a main reason for extensive patient rehabilitation (Ung et al., 2010; Min et al., 2011). The following factors hinder axonal regeneration after injury in the central nervous system (Zhang et al., 2011): inhibitory factors in the myelin sheath, such as Nogo and related ligands; the glial scar; and nutrient deficiency. Increasing studies have focused on the elimination of these factors to promote axonal regeneration. Some have proposed the application of methods to bridge the injury site, including embryonic tissue (Kawaguchi et al., 2004; Steward et al., 2006), peripheral nerve tissue (David and Aguayo, 1981; Kunkel-Bagden and Bregman, 1990; Cheng et al., 1996), and artificial stents (Richardson et al., 1984; Bakshi et al., 2004; Patist et al., 2004). Other investigations have focused on a variety of potential therapeutic cell grafts, such as Schwann cells (Meijs et al., 2004; Tsai et al., 2004; Kamada et al., 2005), OECs (Paino and Bunge, 1991; Cao et al., 2004; Chuah et al., 2004; Ramer et al., 2004), macrophages (Shen et al., 2004), various sources of stem cells (Bomstein et al., 2003), as well as the combination of growth factors and graft co-transplantations (Kwon et al., 2002; Dolbeare and Houle, 2003; Iannotti et al., 2003; Shumsky et al., 2003; Zhou and Shine, 2003; Nikulina et al., 2004; Pearse et al., 2004; Iwanami et al., 2005). Some studies have addressed the elimination of axonal regeneration suppressors, such as Nogo and its ligand (Lu et al., 2004), the glial scar (Xu et al., 2004) or cell adhesion molecules (Chau et al., 2003), inflammatory mediators (Roonprapunt et al., 2003), and other molecules (Demjen et al., 2004). Although growing evidence has highlighted the contribution of transplantation on promoting nerve regeneration or restoration, the majority of related studies have low reproducibility. OECs in the OLP and olfactory bulb appear to play a key role in providing an enabling environment for axonal growth. OECs are a type of glial cell that are functionally situated between Schwann cells and oligodendrocytes, exhibiting numerous properties, including neurotrophic, anti-gliotic effects, glial scar inhibitory, and pro-sheath formation effects. OECs may provide a preferred microenvironment for axonal growth and migration, making them an ideal candidate cell for the promotion of nerve regeneration in the central nervous system (Tanaka et al., 2004).
New evidence indicates that OECs have properties of cells in both the central and peripheral nervous systems (Au and Roskams, 2003; Su and He, 2010; King-Robson, 2011). They can express and secrete a variety of cell surface adhesion molecules and neurotrophic factors and play a critical support role in axonal regeneration of central neurons (Sasaki et al., 2004; Richter et al., 2005; Radtke et al., 2010). A large number of studies have demonstrated that OEC transplantation can promote neurological functional recovery following SCI (Boyd et al., 2005; Aoki et al., 2010; Tharion et al., 2011).
The present study found that there is sufficient evidence showing that olfactory nasal cell grafts can promote functional recovery after complete transection of the spinal cord in the adult rat, both when transplanted as whole pieces of OLP or as 50% pure cultures of OECs. Animals receiving olfactory grafts have recovered hindlimb movement and spinal reflex inhibition. Histological investigations have shown the obvious growth of axons across the transection site, through the graft and into the distal cord stump and regrowth of brainstem serotonergic fibers into the distal cord. Re-section of the cord after 10 weeks confirmed that locomotor recovery was dependent on regeneration through the transplant site.
OECs can fuse well with host spinal cord and promote myelination and axonal regeneration. OECs are considered the most promising candidate cells for SCI repair (Sobani et al., 2010; Chuah et al., 2011; Mackay-Sim and St John, 2011; Tetzlaff et al., 2011). However, related animal experiments and clinical studies have been limited by the small size of samples and short-term follow-up periods (Fouad et al., 2005; Li et al., 2011).
Several different animal models for SCI have been developed, each with their own advantages and disadvantages. The animals of choice for most studies are rats and mice; larger species, such as canines, felines, and nonhuman primates, are used infrequently. Typically, most characteristics of the nervous system, including architecture, biochemistry, and physiology, are conserved across species, allowing for reliable and valid comparisons. However, it should be noted that some differences may exist that can produce different results, such as a reduced sensitivity to excitotoxic damage in some strains of mice, and the atypical absence of gross cavitation following SCI in mice. The differences in animals may not affect the damage, but can influence the behavioral recovery (Yui et al., 2011). This review chose only the studies with rats to avoid the adverse effects associated with mice.
The location of the damage to the spinal cord can also vary, but in most animal models, the site of injury is usually in the lower thoracic region, as this reduces the demands of animal care, while still providing a good model for SCI. This can produce significant motor impairment in the hindlimbs, but avoids drastic impairments of higher-level damage, such as difficulty with respiration. The locations of SCI in the studies included in this review were all in the lower thoracic region.
A variety of methods have been used to establish SCI models in animals. Different models reproduce different types of anatomical structural injury and neurological functional effects. Currently available animal models of SCI are characterized by the animal species employed and the mechanism by which the spinal trauma is induced (weight drop, contusion, calibrated forceps compression, or laceration). The power of any model in determining the clinical relevance of a therapy is determined by the following: (1) how similar the model is to the clinical and histopathology of human SCI, (2) the reproducibility of neurological dysfunction across individual experimental subjects exposed to an identical injury, (3) the degree and the time course of spontaneous motor/sensory recovery, (4) tolerability to chronic neurological dysfunction and related post-injury animal care, (5) the capability of generating different degrees of functional outcome after different severities of injury, and (6) the ability to qualitatively and quantitatively describe the spinal histopathological changes (Mills et al., 2001).
However, there is no model that can completely simulate clinical SCI (Navarro et al., 2012). Contusion, semi-transection and complete transection are commonly utilized, all of which have advantages and disadvantages (Pu et al., 2007; Li and Dai, 2009; Bazley et al., 2012). Contusion and semi-transection models cannot confirm well whether the newly generated axons in the injury region result from the regeneration of injured nerves or compensatory lateral branch sprouting of residual healthy nerve tissues. Complete transection also has disadvantages, such as low association with clinical SCI, high animal mortality, high postoperative complications, and laborious postoperative nursing. However, the complete transection model can exactly identify regenerated axons and effective treatment strategies (Talac et al., 2004), therefore confirming association between regenerated axons and limb functional recovery. It is commonly used for studies investigating possible cell transplantation therapies for SCI. Neural and non-neuronal transplants are used to bridge severed axons or provide a site for neuronal grafts that can promote regeneration or establish functional connections. It is much easier to localize and track the transplants in these models than other methods for SCI, where the lesion is not as well defined. Thus, complete spinal cord transection has been frequently used in studies of SCI repair and regeneration, as well as transplantation therapies (Lee et al., 2008; Li and Zhao, 2008; Fan et al., 2011; Kang et al., 2011).
A key point after treatments to repair the injured spinal cord is whether functional recovery is due to axonal pathways that regenerate across the lesion or to changes in spinal circuits caudal to the lesion. In rats with OEC transplants, many studies (López-Vales et al., 2006) have shown that descending fibers from the motor cortex and brainstem grew through the lesion and projected caudally to lumbar segments. After spinal cord transection, 70–80% of OEC-transplanted rats showed recovery of motor-evoked potentials (MEPs), but none of the control RLP-injected rats. MEPs are induced by electrical stimulation of cortical and brainstem motor neurons, which propagate impulses along the spinal cord to excite lumbar motor neurons. After complete spinal cord transection, MEPs disappear because all descending tracts are disrupted, and they do not return with time because spontaneous axonal regeneration fails within the non-transplanted spinal cord. Therefore, recovery of electrophysiological responses observed after OEC transplantation is attributable to regrowth and caudal functional reconnection of a number of supraspinal axons that have crossed the lesion site. OEC-grafted animals exhibit improved locomotor behavior only when MEPs reappear, suggesting that recovery of motor skills is due to a return of descending control of hindlimb movements. Ramón-Cueto et al. (2000) reported recovery of hindlimb function in the climbing test, following acute OEC transplantation after complete spinal cord transection.
The present review included studies of SCI animal models of complete spinal cord transection to investigate the effects of OEC transplantation on motor functional recovery. The ability to move in an open space is a good index of functional ability in response to SCI and can provide reliable quantitative information. The most basic measure of locomotor activity is exploratory activity in an open field. Rodents have a natural tendency to explore a new environment, with most of the activity being around the periphery of the open field. Rats are placed in the center of a square box that is marked with a grid of equal-sized squares or a grid of photoelectric beams. The primary measures taken to determine open-field activity are time to reach the perimeter of the open field and number of lines crossed. Other types of behaviors that can be monitored include grooming and stereotypic movements, such as rotation and vertical movements. Testing can be performed for single or multiple trials; however, multiple testing can lead to acclimation and reduced activity. Activity levels are important to assess hyperactivity or hypoactivity, but may not provide specific information on the quality of movement. In addition to general activity levels, automated tracking systems commonly used for these measures can also assess the movement according to small or large and vertical or horizontal movements. The addition of type and direction of movement adds another dimension to the data, to grossly assess the quality and quantity of movement.
An overall assessment of the quantity and quality of movement can be obtained using ordinal scales that rate locomotor ability, most notably the BBB locomotor scale, which is a modification of an earlier scale. The BBB scale is quickly becoming the standard for overground locomotor testing, because it assesses multiple parameters and is widely affordable due to the fact that it requires no specialized equipment. The BBB scale rates motor ability on a 21-point scale designed to assess recovery of hindlimb function in an open field after thoracic SCI. Scores range from a score of 0 for total paralysis to a score of 21 for normal locomotion, characterized by coordinated and consistent gait, correct hindlimb paw placement, and trunk stability.
After injury, there may be a reliable pattern of recovery that is documented by the scale. Scores of 0 through 7 are characterized by isolated joint movement (hip, knee, and ankle); scores of 8 through 13 describe paw placement, stepping, and coordination of the forelimbs and hindlimbs; and scores of 14 through 21 assess toe clearance during stepping, paw position, tail position, and trunk stability. Left and right hindlimbs can be scored separately to assess asymmetric damage and recovery, but the final rank is a composite score of both sides.
The present study utilized meta-analysis and BBB scores to analyze motor functional recovery of the hindlimbs in rats following OEC transplantation. Results showed that OEC transplantation had higher BBB scores than control animals, indicating that OECs can promote motor functional recovery of rat hindlimbs.
Limitations
Notably, this review included a small number of studies, so the size of samples was not sufficient. Further study of a larger sample size is needed to verify the results. Moreover, heterogeneity in studies may result from several factors as follows: BBB scores were subjective, directly leading to heterogeneity; rat strain, gender, number, age and nursing technique were different; laboratory conditions were not standardized, and data processing was different; and the time of OEC transplantation was not the same. Additionally, the inclusion criteria of the present review were limited, as we only selected studies published in Chinese and English, and none mentioned allocation concealment, possibly resulting in selection bias.
Therefore, future reviews should select studies with sufficient sample sizes, utilizing a blind method to reduce bias. Moreover, objective, unified motor function evaluation criteria should be established to reduce experimental errors.
To assess the reproducibility and reliability of OLP transplantation on promoting motor functional recovery after complete transection injury in thoracic spinal cord, Steward et al. (2006) repeated the experiments previously done by Lu et al. (2002). The results showed no functional recovery following OLP transplantation, with the improvements in hindlimb motor function being similar to that after RLP transplantation. This discrepancy can be explained by (1) insufficient/immature experimental techniques; (2) variation of animals, such as genetic diversity and different graft rejections; (3) different directional controlling of the grafts.
Conclusion
Previous studies have shown that OEC transplantation promoted hindlimb motor functional recovery in rats with complete spinal cord transection injury. However, the clinical application of OEC transplantation requires further evidence from large-sample or multi-center randomized controlled trials.
Footnotes
Conflicts of interest: None declared.
Copyedited by Wallace M, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- Aoki M, Kishima H, Yoshimura K, Ishihara M, Ueno M, Hata K, Yamashita T, Iwatsuki K, Yoshimine T. Limited functional recovery in rats with complete spinal cord injury after transplantation of whole-layer olfactory mucosa. J Neurosurg Spine. 2010;12:122–130. doi: 10.3171/2009.9.SPINE09233. [DOI] [PubMed] [Google Scholar]
- Au E, Roskams AJ. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia. 2003;41:224–236. doi: 10.1002/glia.10160. [DOI] [PubMed] [Google Scholar]
- Bakshi A, Fisher O, Dagci T, Himes BT, Fischer I, Lowman A. Mechanically engineered hydrogel scaffolds for axonal growth and angiogenesis after transplantation in spinal cord injury. J Neurosurg Spine. 2004;1:322–329. doi: 10.3171/spi.2004.1.3.0322. [DOI] [PubMed] [Google Scholar]
- Barnett SC, Alexander CL, Iwashita Y, Gilson JM, Crowther J, Clark L, Dunn LT, Papanastassiou V, Kennedy PG, Franklin RJ. Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons. Brain. 2000;123:1581–1588. doi: 10.1093/brain/123.8.1581. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Bazley FA, Hu C, Maybhate A, Pourmorteza A, Pashai N, Thakor NV, Kerr CL, All AH. Electrophysiological evaluation of sensory and motor pathways after incomplete unilateral spinal cord contusion. J Neurosurg Spine. 2012;16:414–423. doi: 10.3171/2012.1.SPINE11684. [DOI] [PubMed] [Google Scholar]
- Blits B, Boer GJ, Verhaagen J. Pharmacological, cell, and gene therapy strategies to promote spinal cord regeneration. Cell Transplant. 2002;11:593–613. [PubMed] [Google Scholar]
- Bomstein Y, Marder JB, Vitner K, Smirnov I, Lisaey G, Butovsky O, Fulga V, Yoles E. Features of skin-coincubated macrophages that promote recovery from spinal cord injury. J Neuroimmunol. 2003;142:10–16. doi: 10.1016/s0165-5728(03)00260-1. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Doucette R, Kawaja MD. Defining the role of olfactory ensheathing cells in facilitating axon remyelination following damage to the spinal cord. FASEB J. 2005;19:694–703. doi: 10.1096/fj.04-2833rev. [DOI] [PubMed] [Google Scholar]
- Cao L, Liu L, Chen ZY, Wang LM, Ye JL, Qiu HY, Lu CL, He C. Olfactory ensheathing cells genetically modified to secrete GDNF to promote spinal cord repair. Brain. 2004;127:535–549. doi: 10.1093/brain/awh072. [DOI] [PubMed] [Google Scholar]
- Centenaro LA, Jaeger MdC, Ilha J, de Souza MA, Kalil-Gaspar PI, Cunha NB, Marcuzzo S, Achaval M. Olfactory and respiratory lamina propria transplantation after spinal cord transection in rats: Effects on functional recovery and axonal regeneration. Brain Res. 2011;1426:54–72. doi: 10.1016/j.brainres.2011.09.054. [DOI] [PubMed] [Google Scholar]
- Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, Chan YS, Xu XM. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J. 2003;18:194–196. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- Chuah MI, Hale DM, West AK. Interaction of olfactory ensheathing cells with other cell types in vitro and after transplantation: glial scars and inflammation. Exp Neurol. 2011;229:46–53. doi: 10.1016/j.expneurol.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Chuah MI, Choi-Lundberg D, Weston S, Vincent AJ, Chung RS, Vickers JC, West AK. Olfactory ensheathing cells promote collateral axonal branching in the injured adult rat spinal cord. Exp Neurol. 2004;185:15–25. doi: 10.1016/j.expneurol.2003.09.008. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Demjen D, Klussmann S, Kleber S, Zuliani C, Stieltjes B, Metzger C, Hirt UA, Walczak H, Falk W, Essig M, Edler L, Krammer PH, Martin-Villalba A. Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat Med. 2004;10:389–395. doi: 10.1038/nm1007. [DOI] [PubMed] [Google Scholar]
- Dobkin BH, Harkema S, Requejo P, Edgerton VR. Modulation of locomotor-like EMG activity in subjects with complete and incomplete spinal cord injury. J Neurol Rehabil. 1995;9:183–190. [PubMed] [Google Scholar]
- Dolbeare D, Houle JD. Restriction of axonal retraction and promotion of axonal regeneration by chronically injured neurons after intraspinal treatment with glial cell line-derived neurotrophic factor (GDNF) J Neurotrauma. 2003;20:1251–1261. doi: 10.1089/089771503770802916. [DOI] [PubMed] [Google Scholar]
- Doucette JR. The glial cells in the nerve fiber layer of the rat olfactory bulb. Anat Rec. 1984;210:385–391. doi: 10.1002/ar.1092100214. [DOI] [PubMed] [Google Scholar]
- Fan J, Zhang H, He J, Xiao Z, Chen B, Xiaodan J, Dai J, Xu R. Neural regrowth induced by PLGA nerve conduits and neurotrophin-3 in rats with complete spinal cord transection. J Biomed Mater Res B Appl Biomater. 2011;97:271–277. doi: 10.1002/jbm.b.31810. [DOI] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Alías G, Verdú E, Forés J, López-Vales R, Navarro X. Functional and electrophysiological characterization of photochemical graded spinal cord injury in the rat. J Neurotrauma. 2003;20:501–510. doi: 10.1089/089771503765355568. [DOI] [PubMed] [Google Scholar]
- Iannotti C, Li H, Yan P, Lu X, Wirthlin L, Xu XM. Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Exp Neurol. 2003;183:379–393. doi: 10.1016/s0014-4886(03)00188-2. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Waxman SG, Greer CA, Kocsis JD. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. J Neurosci. 1998;18:6176–6185. doi: 10.1523/JNEUROSCI.18-16-06176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanami A, Kaneko S, Nakamura M, Kanemura Y, Mori H, Kobayashi S, Yamasaki M, Momoshima S, Ishii H, Ando K, Tanioka Y, Tamaoki N, Nomura T, Toyama Y, Okano H. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res. 2005;80:182–190. doi: 10.1002/jnr.20436. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Jiang B, Shen YX, Wang PJ, Lu ZF, Fan ZH. Repairing effect of OECs transplantation for spinal cord injury. Suzhou Daxue Xuebao: Yixue Ban. 2010;30:34–37. [Google Scholar]
- Jones LL, Oudega M, Bunge MB, Tuszynski MH. Neurotrophic factors, cellular bridges and gene therapy for spinal cord injury. J Physiol. 2001;533:83–89. doi: 10.1111/j.1469-7793.2001.0083b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada T, Koda M, Dezawa M, Yoshinaga K, Hashimoto M, Koshizuka S, Nishio Y, Moriya H, Yamazaki M. Transplantation of bone marrow stromal cell-derived Schwann cells promotes axonal regeneration and functional recovery after complete transection of adult rat spinal cord. J Neuropathol Exp Neurol. 2005;64:37–45. doi: 10.1093/jnen/64.1.37. [DOI] [PubMed] [Google Scholar]
- Kang KN, Lee JY, Kim da Y, Lee BN, Ahn HH, Lee B, Khang G, Park SR, Min BH, Kim JH, Lee HB, Kim MS. Regeneration of completely transected spinal cord using scaffold of poly(D,L-lactide-co-glycolide)/small intestinal submucosa seeded with rat bone marrow stem cells. Tissue Eng Part A. 2011;17:2143–2152. doi: 10.1089/ten.TEA.2011.0122. [DOI] [PubMed] [Google Scholar]
- Kato T, Honmou O, Uede T, Hashi K, Kocsis JD. Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia. 2000;30:209–218. doi: 10.1002/(sici)1098-1136(200005)30:3<209::aid-glia1>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi S, Iseda T, Nishio T. Effects of an embryonic repair graft on recovery from spinal cord injury. Prog Brain Res. 2004;143:155–162. doi: 10.1016/S0079-6123(03)43015-X. [DOI] [PubMed] [Google Scholar]
- King-Robson J. Encouraging regeneration in the central nervous system: is there a role for olfactory ensheathing cells? Neurosci Res. 2011;69:263–275. doi: 10.1016/j.neures.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Kubasak MD, Jindrich DL, Zhong H, Takeoka A, McFarland KC, Muñoz-Quiles C, Roy RR, Edgerton VR, Ramón-Cueto A, Phelps PE. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain. 2008;131:264–276. doi: 10.1093/brain/awm267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel-Bagden E, Bregman BS. Spinal cord transplants enhance the recovery of locomotor function after spinal cord injury at birth. Exp Brain Res. 1990;81:25–34. doi: 10.1007/BF00230097. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Borisoff JF, Tetzlaff W. Molecular targets for therapeutic intervention after spinal cord injury. Mol Interv. 2002;2:244–258. doi: 10.1124/mi.2.4.244. [DOI] [PubMed] [Google Scholar]
- López-Vales R, Forés J, Verdú E, Navarro X. Acute and delayed transplantation of olfactory ensheathing cells promote partial recovery after complete transection of the spinal cord. Neurobiol Dis. 2006;21:57–68. doi: 10.1016/j.nbd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- López-Vales R, Forés J, Navarro X, Verdú E. Chronic transplantation of olfactory ensheathing cells promotes partial recovery after complete spinal cord transection in the rat. Glia. 2007;55:303–311. doi: 10.1002/glia.20457. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Chen CJ, Cheng CH, Huang WC, Kuo HS, Wu JC, Tsai MJ, Huang MC, Chang WC, Cheng H. Combined treatment using peripheral nerve graft and FGF-1: Changes to the glial environment and differential macrophage reaction in a complete transected spinal cord. Neurosci Lett. 2008;433:163–169. doi: 10.1016/j.neulet.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Li BC, Li Y, Chen LF, Chang JY, Duan ZX. Olfactory ensheathing cells can reduce the tissue loss but not the cavity formation in contused spinal cord of rats. J Neurol Sci. 2011;303:67–74. doi: 10.1016/j.jns.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Li XF, Dai LY. Three-dimensional finite element model of the cervical spinal cord: preliminary results of injury mechanism analysis. Spine (Phila Pa 1976) 2009;34:1140–1147. doi: 10.1097/BRS.0b013e31819e2af1. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhao Q. The effects of fibrin glue on acute complete transection spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22:828–831. [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J Neurosci. 1998;18:10514–10524. doi: 10.1523/JNEUROSCI.18-24-10514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sauvé Y, Li D, Lund RD, Raisman G. Transplanted olfactory ensheathing cells promote regeneration of cut adult rat optic nerve axons. J Neurosci. 2003;23:7783–7788. doi: 10.1523/JNEUROSCI.23-21-07783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yamamoto M, Raisman G, Choi D, Carlstedt T. An experimental model of ventral root repair showing the beneficial effect of transplanting olfactory ensheathing cells. Neurosurgery. 2007;60:734–741. doi: 10.1227/01.NEU.0000255406.76645.EA. [DOI] [PubMed] [Google Scholar]
- Lu J, Féron F, Mackay-Sim A, Waite PM. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain. 2002;125:14–21. doi: 10.1093/brain/awf014. [DOI] [PubMed] [Google Scholar]
- Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A, St John JA. Olfactory ensheathing cells from the nose: Clinical application in human spinal cord injuries. Exp Neurol. 2011;229:174–180. doi: 10.1016/j.expneurol.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Meijs MF, Timmers L, Pearse DD, Tresco PA, Bates ML, Joosten EA, Bunge MB, Oudega M. Basic fibroblast growth factor promotes neuronal survival but not behavioral recovery in the transected and Schwann cell implanted rat thoracic spinal cord. J Neurotrauma. 2004;21:1415–1430. doi: 10.1089/neu.2004.21.1415. [DOI] [PubMed] [Google Scholar]
- Mills CD, Hains BC, Johnson KM, Hulsebosch CE. Strain and model differences in behavioral outcomes after spinal cord injury in rat. J Neurotrauma. 2001;18:743–756. doi: 10.1089/089771501316919111. [DOI] [PubMed] [Google Scholar]
- Min SH, Lee SH, Shim H, Park JS, Lee YI, Kim HW, Hyun JK. Development of complete thoracic spinal cord transection model in rats for delayed transplantation of stem cells. Spine (Phila Pa 1976) 2011;36:E155–163. doi: 10.1097/BRS.0b013e3181d8b92a. [DOI] [PubMed] [Google Scholar]
- Muñoz-Quiles C, Santos-Benito FF, Llamusí MB, Ramón-Cueto A. Chronic spinal injury repair by olfactory bulb ensheathing glia and feasibility for autologous therapy. J Neuropathol Exp Neurol. 2009;68:1294–1308. doi: 10.1097/NEN.0b013e3181c34bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro R, Juhas S, Keshavarzi S, Juhasova J, Motlik J, Johe K, Marsala S, Scadeng M, Lazar P, Tomori Z, Schulteis G, Beattie M, Ciacci JD, Marsala M. Chronic spinal compression model in minipigs: a systematic behavioral, qualitative, and quantitative neuropathological study. J Neurotrauma. 2012;29:499–513. doi: 10.1089/neu.2011.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paino CL, Bunge MB. Induction of axon growth into schwann cell implants grafted into lesioned adult rat spinal cord. Exp Neurol. 1991;114:254–257. doi: 10.1016/0014-4886(91)90043-c. [DOI] [PubMed] [Google Scholar]
- Patist CM, Mulder MB, Gautier SE, Maquet V, Jérôme R, Oudega M. Freeze-dried poly(d,l-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials. 2004;25:1569–1582. doi: 10.1016/s0142-9612(03)00503-9. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Pu Y, Guo QS, Wang AM, Wu SY, Xing SX, Zhang ZR. Repair of acutely injured spinal cord through constructing tissue-engineered neural complex in adult rats. Chin J Traumatol. 2007;10:171–176. [PubMed] [Google Scholar]
- Radtke C, Lankford KL, Wewetzer K, Imaizumi T, Fodor WL, Kocsis JD. Impaired spinal cord remyelination by long-term cultured adult porcine olfactory ensheathing cells correlates with altered in vitro phenotypic properties. Xenotransplantation. 2010;17:71–80. doi: 10.1111/j.1399-3089.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- Ramer LM, Au E, Richter MW, Liu J, Tetzlaff W, Roskams AJ. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J Comp Neurol. 2004;473:1–15. doi: 10.1002/cne.20049. [DOI] [PubMed] [Google Scholar]
- Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM, Aguayo AJ. Regeneration of long spinal axons in the rat. J Neurocytol. 1984;13:165–182. doi: 10.1007/BF01148324. [DOI] [PubMed] [Google Scholar]
- Richter MW, Fletcher PA, Liu J, Tetzlaff W, Roskams AJ. Lamina propria and olfactory bulb ensheathing cells exhibit differential integration and migration and promote differential axon sprouting in the lesioned spinal cord. J Neurosci. 2005;25:10700–10711. doi: 10.1523/JNEUROSCI.3632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roonprapunt C, Huang W, Grill R, Friedlander D, Grumet M, Chen S, Schachner M, Young W. Soluble cell adhesion molecule L1-Fc promotes locomotor recovery in rats after spinal cord injury. J Neurotrauma. 2003;20:871–882. doi: 10.1089/089771503322385809. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Lankford KL, Zemedkun M, Kocsis JD. Identified olfactory ensheathing cells transplanted into the transected dorsal funiculus bridge the lesion and form myelin. J Neurosci. 2004;24:8485–8493. doi: 10.1523/JNEUROSCI.1998-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME. Repairing the injured spinal cord. Science. 2002;295:1029–1031. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- Shen HY, Yin DZ, Tang Y, Wu YF, Cheng ZA, Yang R, Huang L. Influence of cryopreserved olfactory ensheathing cells transplantation on axonal regeneration in spinal cord of adult rats. Chin J Traumatol. 2004;7:179–183. [PubMed] [Google Scholar]
- Shumsky JS, Tobias CA, Tumolo M, Long WD, Giszter SF, Murray M. Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT-3 into a spinal cord injury site is associated with limited recovery of function. Exp Neurol. 2003;184:114–130. doi: 10.1016/s0014-4886(03)00398-4. [DOI] [PubMed] [Google Scholar]
- Sobani ZA, Quadri SA, Enam SA. Stem cells for spinal cord regeneration: Current status. Surg Neurol Int. 2010;1:93. doi: 10.4103/2152-7806.74240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Sharp K, Selvan G, Hadden A, Hofstadter M, Au E, Roskams J. A re-assessment of the consequences of delayed transplantation of olfactory lamina propria following complete spinal cord transection in rats. Exp Neurol. 2006;198:483–499. doi: 10.1016/j.expneurol.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Su Z, He C. Olfactory ensheathing cells: Biology in neural development and regeneration. Prog Neurobiol. 2010;92:517–532. doi: 10.1016/j.pneurobio.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Talac R, Friedman JA, Moore MJ, Lu L, Jabbari E, Windebank AJ, Currier BL, Yaszemski MJ. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials. 2004;25:1505–1510. doi: 10.1016/s0142-9612(03)00497-6. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yamashita T, Yachi K, Fujiwara T, Yoshikawa H, Tohyama M. Cytoplasmic p21Cip1/WAF1 enhances axonal regeneration and functional recovery after spinal cord injury in rats. Neuroscience. 2004;127:155–164. doi: 10.1016/j.neuroscience.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Tator CH, Duncan EG, Charles D. Comparisons of the clinical and radiological features and surgical management of posterior fossa meningiomas and acoustic neuromas. Can J Neurol Sci. 1990;17:170–176. doi: 10.1017/s0317167100030407. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharion G, Indirani K, Durai M, Meenakshi M, Devasahayam SR, Prabhav NR, Solomons C, Bhattacharji S. Motor recovery following olfactory ensheathing cell transplantation in rats with spinal cord injury. Neurol India. 2011;59:566–572. doi: 10.4103/0028-3886.84339. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, Guu JJ, de Leon RD, Bigbee AJ, London NJ, Zhong H, Ziegler MD, Joynes RL, Roy RR, Edgerton VR. Functional recovery of stepping in rats after a complete neonatal spinal cord transection is not due to regrowth across the lesion site. Neuroscience. 2010;166:23–33. doi: 10.1016/j.neuroscience.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai EC, Dalton PD, Shoichet MS, Tator CH. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. J Neurotrauma. 2004;21:789–804. doi: 10.1089/0897715041269687. [DOI] [PubMed] [Google Scholar]
- Ung RV, Lapointe NP, Rouleau P, Guertin PA. Non-assisted treadmill training does not improve motor recovery and body composition in spinal cord-transected mice. Spinal Cord. 2010;48:750–755. doi: 10.1038/sc.2010.19. [DOI] [PubMed] [Google Scholar]
- Xu G, Nie DY, Chen JT, Wang CY, Yu FG, Sun L, Luo XG, Ahmed S, David S, Xiao ZC. Recombinant DNA vaccine encoding multiple domains related to inhibition of neurite outgrowth: a potential strategy for axonal regeneration. J Neurochem. 2004;91:1018–1023. doi: 10.1111/j.1471-4159.2004.02803.x. [DOI] [PubMed] [Google Scholar]
- Yates C, Charlesworth A, Allen SR, Reese NB, Skinner RD, Garcia-Rill E. The onset of hyperreflexia in the rat following complete spinal cord transection. Spinal Cord. 2008;46:798–803. doi: 10.1038/sc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin GD, Tang X, Lin YQ, Xu YQ, Zhou TH. Experimental study of transplantion human embryonic OECs on transected spinal cord of rat. Zhongguo Jiaoxing Waike Zazhi. 2006;14:1093–1095. [Google Scholar]
- Yui S, Ito D, Fujita N, Nishimura R. Effects of fibroblasts derived from the olfactory bulb and nasal olfactory mucosa on proliferation of olfactory ensheathing cells harvested from the olfactory bulb. J Vet Med Sci. 2011;73:133–137. doi: 10.1292/jvms.10-0344. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Huang F, Gates M, White J, Holmberg EG. Histological repair of damaged spinal cord tissue from chronic contusion injury of rat: a LM observation. Histol Histopathol. 2011;26:45–58. doi: 10.14670/HH-26.45. [DOI] [PubMed] [Google Scholar]
- Zhou L, Shine HD. Neurotrophic factors expressed in both cortex and spinal cord induce axonal plasticity after spinal cord injury. J Neurosci Res. 2003;74:221–226. doi: 10.1002/jnr.10718. [DOI] [PubMed] [Google Scholar]


