Abstract
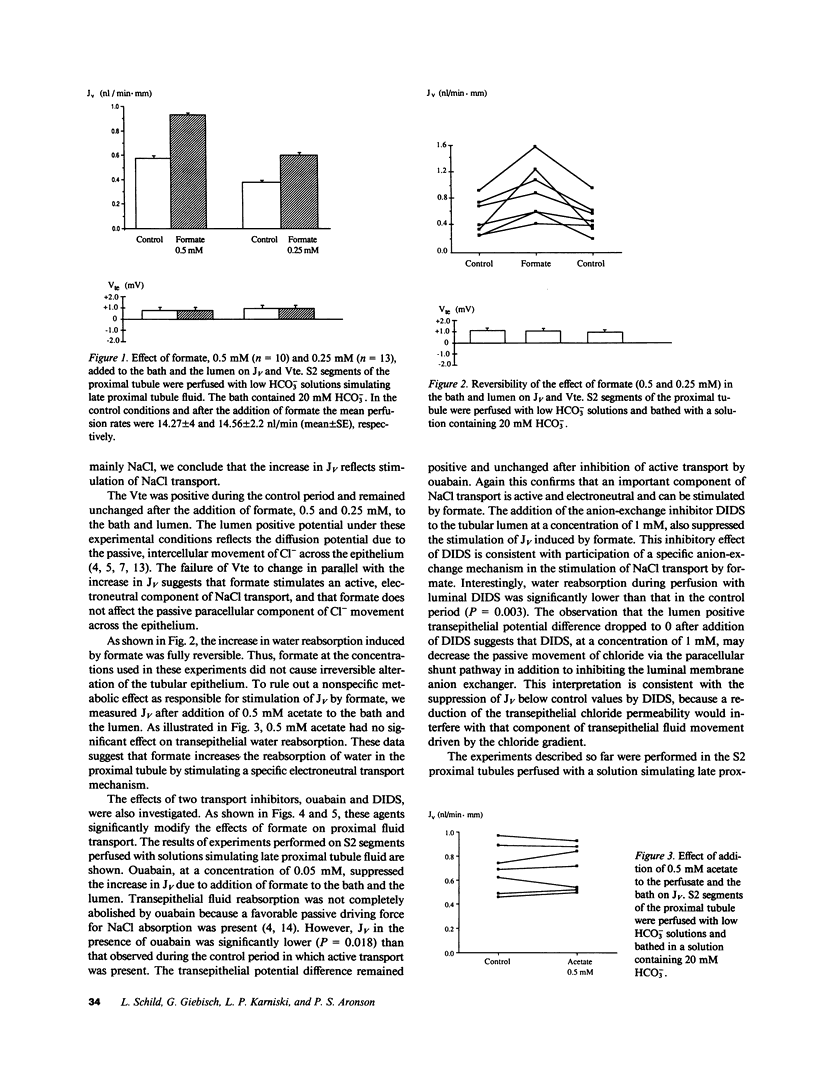
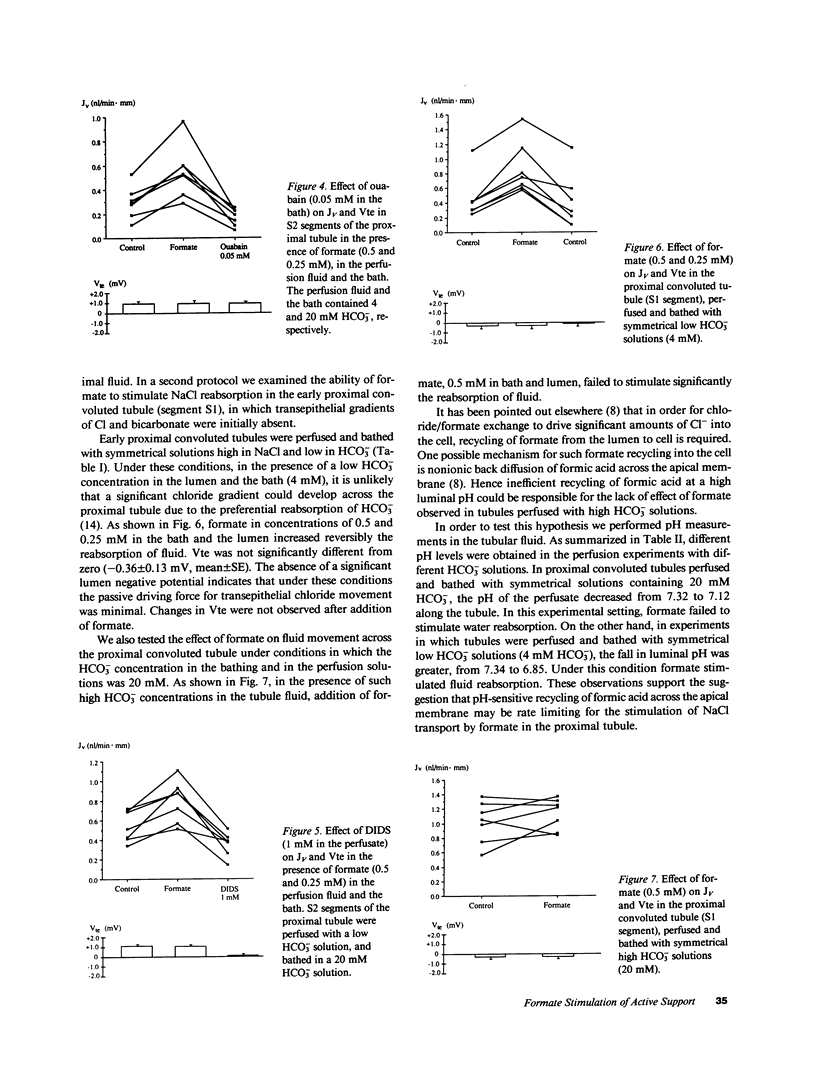
Studies on microvillus membrane from rabbit kidney cortex suggest that chloride absorption may occur by chloride/formate exchange with recycling of formic acid by nonionic diffusion. We tested whether this transport mechanism participates in active NaCl reabsorption in the rabbit proximal tubule. In proximal tubule S2 segments perfused with low HCO-3 solutions, the addition of formate (0.25-0.5 mM) to the lumen and the bath increased volume reabsorption (JV) by 60%; the transepithelial potential difference remained unchanged. The effect of formate on JV was completely reversible and was inhibited both by ouabain and by luminal 4,4'-diisothiocyanostilbene-2,2'-disulfonate. Formate (0.5 mM) failed to stimulate JV in early proximal convoluted tubules perfused with high HCO-3 solutions. As measured by miniature glass pH microelectrodes, this lack of formate effect on JV was related to a less extensive acidification of the tubule fluid when high HCO-3 solutions were used as perfusate. These data suggest that chloride/formate exchange with recycling of formic acid by nonionic diffusion represents a mechanism for active, electroneutral NaCl reabsorption in the proximal tubule.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F. Studies on the volatile fatty acids of sheep blood with special reference to formic acid. Biochem J. 1954 Dec;58(4):670–680. doi: 10.1042/bj0580670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J., Howlin K. J., Preisig P. A. Active and passive components of chloride transport in the rat proximal convoluted tubule. J Clin Invest. 1985 Oct;76(4):1360–1366. doi: 10.1172/JCI112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M., Berry C. A. Evidence for neutral transcellular NaCl transport and neutral basolateral chloride exit in the rabbit proximal convoluted tubule. J Clin Invest. 1984 Jul;74(1):205–211. doi: 10.1172/JCI111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi B. A., Giebisch G. Temperature dependence of transepithelial potential in isolated perfused rabbit proximal tubules. Am J Physiol. 1979 Mar;236(3):F302–F310. doi: 10.1152/ajprenal.1979.236.3.F302. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Cardinal J., Lutz M. D., Burg M. B., Orloff J. Lack of relationship of potential difference to fluid absorption in the proximal renal tubule. Kidney Int. 1975 Feb;7(2):94–102. doi: 10.1038/ki.1975.14. [DOI] [PubMed] [Google Scholar]
- Cassola A. C., Mollenhauer M., Frömter E. The intracellular chloride activity of rat kidney proximal tubular cells. Pflugers Arch. 1983 Dec;399(4):259–265. doi: 10.1007/BF00652749. [DOI] [PubMed] [Google Scholar]
- Chantrelle B. M., Cogan M. G., Rector F. C., Jr Active and passive components of NaCl absorption in the proximal convoluted tubule of the rat kidney. Miner Electrolyte Metab. 1985;11(4):209–214. [PubMed] [Google Scholar]
- Green R., Bishop J. H., Giebisch G. Ionic requirements of proximal tubular sodium transport. III. Selective luminal anion substitution. Am J Physiol. 1979 Mar;236(3):F268–F277. doi: 10.1152/ajprenal.1979.236.3.F268. [DOI] [PubMed] [Google Scholar]
- Green R., Moriarty R. J., Giebisch G. Ionic requirements of proximal tubular fluid reabsorption flow dependence of fluid transport. Kidney Int. 1981 Nov;20(5):580–587. doi: 10.1038/ki.1981.180. [DOI] [PubMed] [Google Scholar]
- Karniski L. P., Aronson P. S. Chloride/formate exchange with formic acid recycling: a mechanism of active chloride transport across epithelial membranes. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6362–6365. doi: 10.1073/pnas.82.18.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen B. M., Helman S. I. Acidification of luminal fluid by the rabbit cortical collecting tubule perfused in vitro. Am J Physiol. 1982 May;242(5):F521–F531. doi: 10.1152/ajprenal.1982.242.5.F521. [DOI] [PubMed] [Google Scholar]
- Lapointe J. Y., Laprade R., Cardinal J. Characterization of the apical membrane ionic permeability of the rabbit proximal convoluted tubule. Am J Physiol. 1986 Feb;250(2 Pt 2):F339–F347. doi: 10.1152/ajprenal.1986.250.2.F339. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Patlak C. S., Andreoli T. E. A component of fluid absorption linked to passive ion flows in the superficial pars recta. J Gen Physiol. 1975 Oct;66(4):445–471. doi: 10.1085/jgp.66.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L., Andreoli T. E. Volume reabsorption, transepithelial potential differences, and ionic permeability properties in mammalian superficial proximal straight tubules. J Gen Physiol. 1974 Nov;64(5):582–607. doi: 10.1085/jgp.64.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J. Absence of Cl- -OH- or Cl- -HCO3- exchange in the rabbit renal proximal tubule. Am J Physiol. 1983 Oct;245(4):F462–F469. doi: 10.1152/ajprenal.1983.245.4.F462. [DOI] [PubMed] [Google Scholar]
- Seifter J. L., Aronson P. S. Cl- transport via anion exchange in Necturus renal microvillus membranes. Am J Physiol. 1984 Dec;247(6 Pt 2):F888–F895. doi: 10.1152/ajprenal.1984.247.6.F888. [DOI] [PubMed] [Google Scholar]
- Seifter J. L., Knickelbein R., Aronson P. S. Absence of Cl-OH exchange and NaCl cotransport in rabbit renal microvillus membrane vesicles. Am J Physiol. 1984 Nov;247(5 Pt 2):F753–F759. doi: 10.1152/ajprenal.1984.247.5.F753. [DOI] [PubMed] [Google Scholar]
- Walter A., Gutknecht J. Monocarboxylic acid permeation through lipid bilayer membranes. J Membr Biol. 1984;77(3):255–264. doi: 10.1007/BF01870573. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Yee V. J. Chloride uptake by brush border membrane vesicles isolated from rabbit renal cortex. Coupling to proton gradients and K+ diffusion potentials. J Clin Invest. 1981 Jan;67(1):103–115. doi: 10.1172/JCI110002. [DOI] [PMC free article] [PubMed] [Google Scholar]



