Abstract
Magnesium-protoporphyrin chelatase lies at the branch point of the heme and (bacterio)chlorophyll biosynthetic pathways. In this work, the photosynthetic bacterium Rhodobacter sphaeroides has been used as a model system for the study of this reaction. The bchH and the bchI and -D genes from R. sphaeroides were expressed in Escherichia coli. When cell-free extracts from strains expressing BchH, BchI, and BchD were combined, the mixture was able to catalyze the insertion of Mg into protoporphyrin IX in an ATP-dependent manner. This was possible only when all three genes were expressed. The bchH, -I, and -D gene products are therefore assigned to the Mg chelatase step in bacteriochlorophyll biosynthesis. The mechanism of the Mg chelation reaction and the implications for chlorophyll biosynthesis in plants are discussed.
Full text
PDF
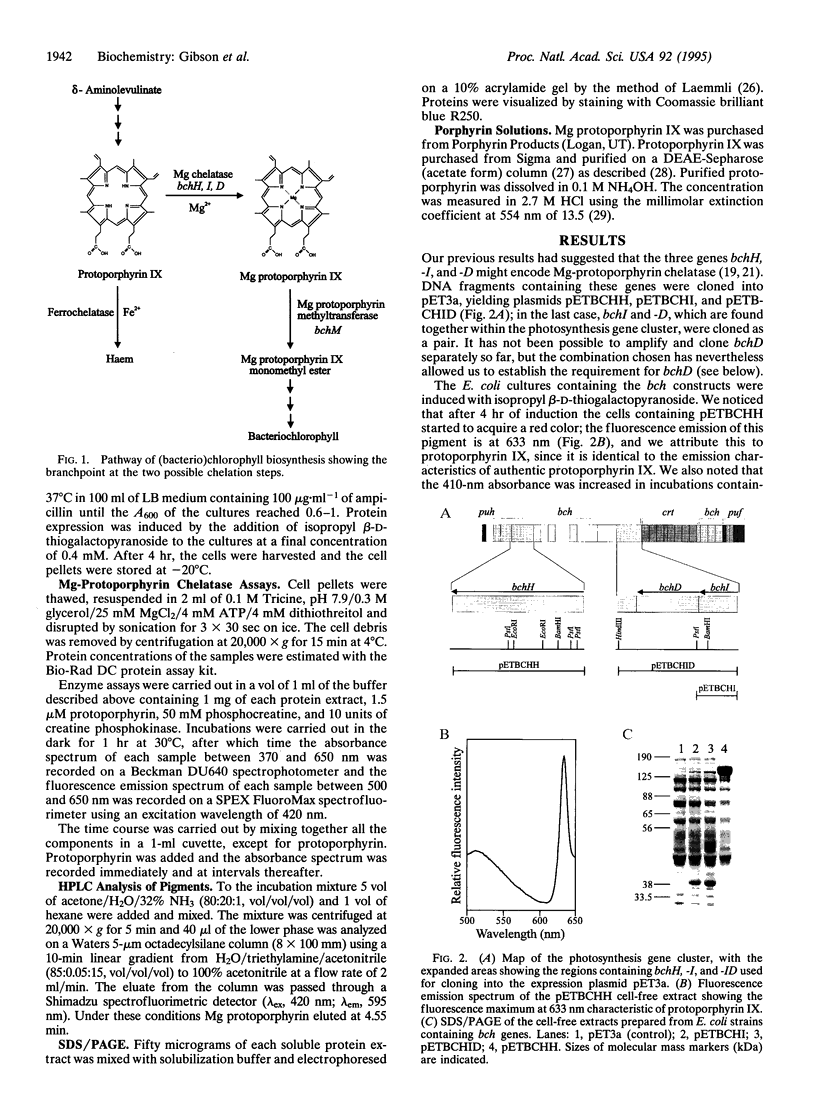
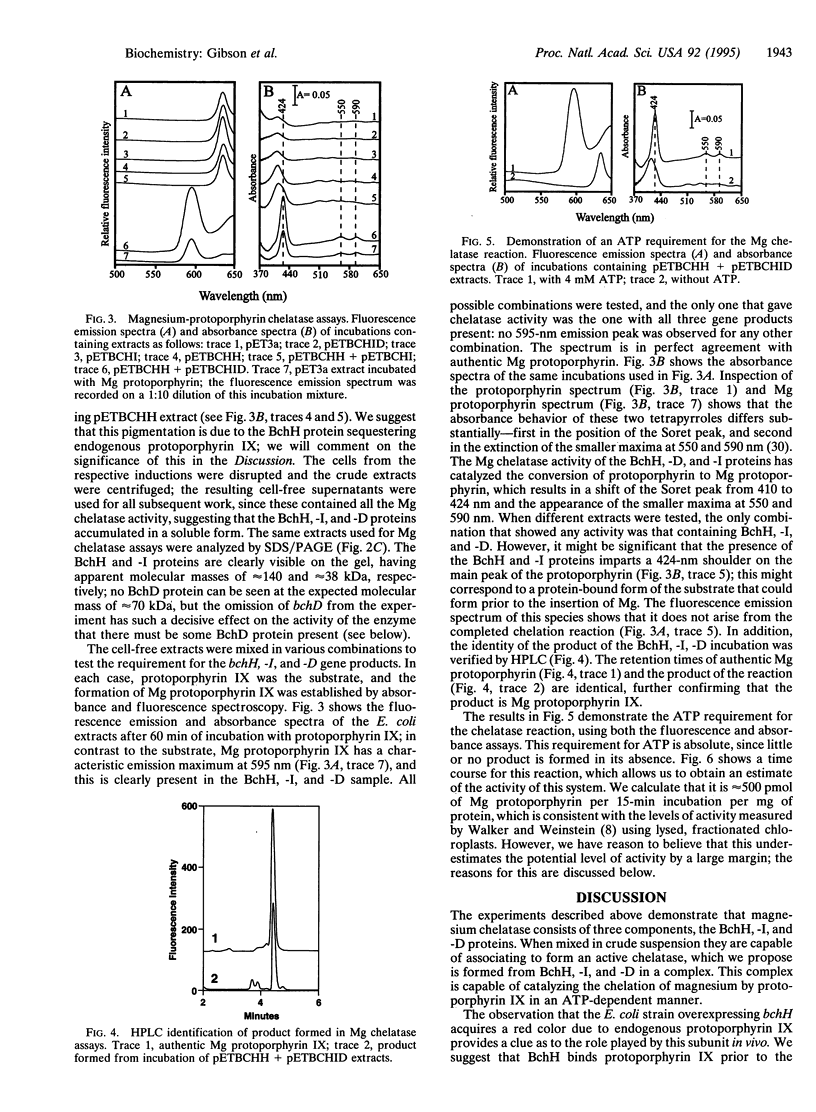

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer C. E., Bollivar D. W., Suzuki J. Y. Genetic analyses of photopigment biosynthesis in eubacteria: a guiding light for algae and plants. J Bacteriol. 1993 Jul;175(13):3919–3925. doi: 10.1128/jb.175.13.3919-3925.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollivar D. W., Jiang Z. Y., Bauer C. E., Beale S. I. Heterologous expression of the bchM gene product from Rhodobacter capsulatus and demonstration that it encodes S-adenosyl-L-methionine:Mg-protoporphyrin IX methyltransferase. J Bacteriol. 1994 Sep;176(17):5290–5296. doi: 10.1128/jb.176.17.5290-5296.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollivar D. W., Suzuki J. Y., Beatty J. T., Dobrowolski J. M., Bauer C. E. Directed mutational analysis of bacteriochlorophyll a biosynthesis in Rhodobacter capsulatus. J Mol Biol. 1994 Apr 15;237(5):622–640. doi: 10.1006/jmbi.1994.1260. [DOI] [PubMed] [Google Scholar]
- Castelfranco P. A., Weinstein J. D., Schwarcz S., Pardo A. D., Wezelman B. E. The Mg insertion step in chlorophyll biosynthesis. Arch Biochem Biophys. 1979 Feb;192(2):592–598. doi: 10.1016/0003-9861(79)90130-9. [DOI] [PubMed] [Google Scholar]
- Coomber S. A., Chaudhri M., Connor A., Britton G., Hunter C. N. Localized transposon Tn5 mutagenesis of the photosynthetic gene cluster of Rhodobacter sphaeroides. Mol Microbiol. 1990 Jun;4(6):977–989. doi: 10.1111/j.1365-2958.1990.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Darie S., Gunsalus R. P. Effect of heme and oxygen availability on hemA gene expression in Escherichia coli: role of the fnr, arcA, and himA gene products. J Bacteriol. 1994 Sep;176(17):5270–5276. doi: 10.1128/jb.176.17.5270-5276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debussche L., Couder M., Thibaut D., Cameron B., Crouzet J., Blanche F. Assay, purification, and characterization of cobaltochelatase, a unique complex enzyme catalyzing cobalt insertion in hydrogenobyrinic acid a,c-diamide during coenzyme B12 biosynthesis in Pseudomonas denitrificans. J Bacteriol. 1992 Nov;174(22):7445–7451. doi: 10.1128/jb.174.22.7445-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuesler T. P., Wright L. A., Castelfranco P. A. Properties of Magnesium Chelatase in Greening Etioplasts: METAL ION SPECIFICITY AND EFFECT OF SUBSTRATE CONCENTRATIONS. Plant Physiol. 1981 Feb;67(2):246–249. doi: 10.1104/pp.67.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson L. C., Hunter C. N. The bacteriochlorophyll biosynthesis gene, bchM, of Rhodobacter sphaeroides encodes S-adenosyl-L-methionine: Mg protoporphyrin IX methyltransferase. FEBS Lett. 1994 Sep 26;352(2):127–130. doi: 10.1016/0014-5793(94)00934-1. [DOI] [PubMed] [Google Scholar]
- Gorchein A. Control of magnesium-protoporphyrin chelatase activity in Rhodopseudomonas spheroides. Role of light, oxygen, and electron and energy transfer. Biochem J. 1973 Aug;134(4):833–845. doi: 10.1042/bj1340833d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A. Magnesium protoporphyrin chelatase activity in Rhodopseudomonas spheroides. Studies with whole cells. Biochem J. 1972 Mar;127(1):97–106. doi: 10.1042/bj1270097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A. Uptake of protoporphyrin and continuous spectrophotometric assay for magnesium chelatase in Rhodobacter spheroides. Biochem J. 1994 May 1;299(Pt 3):869–874. doi: 10.1042/bj2990869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A., Carpenter R., Doyle S., Coen E. S. Olive: a key gene required for chlorophyll biosynthesis in Antirrhinum majus. EMBO J. 1993 Oct;12(10):3711–3719. doi: 10.1002/j.1460-2075.1993.tb06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C., Mayerhofer R., Koncz-Kalman Z., Nawrath C., Reiss B., Redei G. P., Schell J. Isolation of a gene encoding a novel chloroplast protein by T-DNA tagging in Arabidopsis thaliana. EMBO J. 1990 May;9(5):1337–1346. doi: 10.1002/j.1460-2075.1990.tb08248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Ball M. D., Parham R., Rebeiz C. A. Chloroplast Biogenesis 65 : Enzymic Conversion of Protoporphyrin IX to Mg-Protoporphyrin IX in a Subplastidic Membrane Fraction of Cucumber Etiochloroplasts. Plant Physiol. 1992 Jul;99(3):1134–1140. doi: 10.1104/pp.99.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. The energy-state of mitochondria during the transport of Ca2+. Eur J Biochem. 1980 Sep;110(1):211–216. doi: 10.1111/j.1432-1033.1980.tb04857.x. [DOI] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A. D., Chereskin B. M., Castelfranco P. A., Franceschi V. R., Wezelman B. E. ATP requirement for mg chelatase in developing chloroplasts. Plant Physiol. 1980 May;65(5):956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Walker C. J., Weinstein J. D. Further characterization of the magnesium chelatase in isolated developing cucumber chloroplasts : substrate specificity, regulation, intactness, and ATP requirements. Plant Physiol. 1991 Apr;95(4):1189–1196. doi: 10.1104/pp.95.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. J., Weinstein J. D. In vitro assay of the chlorophyll biosynthetic enzyme Mg-chelatase: resolution of the activity into soluble and membrane-bound fractions. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5789–5793. doi: 10.1073/pnas.88.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. J., Weinstein J. D. The magnesium-insertion step of chlorophyll biosynthesis is a two-stage reaction. Biochem J. 1994 Apr 1;299(Pt 1):277–284. doi: 10.1042/bj2990277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsebo K. M., Hearst J. E. Genetic-physical mapping of a photosynthetic gene cluster from R. capsulata. Cell. 1984 Jul;37(3):937–947. doi: 10.1016/0092-8674(84)90428-8. [DOI] [PubMed] [Google Scholar]