Abstract
Thyroid cancer (TC) is the most common type of endocrine malignancy and accounts for nearly 3 % of all malignancies. The incidence of TC in Spain was 5/100,000 in women and 1.9/100,000 in men in 2013. The diagnosis of TC usually follows the identification of a thyroid nodule on physical examination or as an incidental finding on diagnostic imaging performed for other reasons. In most of the cases, the prognosis is excellent but despite low mortality rates, local recurrence occurs in up to 20 %, and distant metastases can occur in approximately 10 % at 10 years. The better knowledge of molecular biology of TC has allowed to the development of new targeted agents directed to the main pathways involved in TC pathogenesis. Knowing all these new strategies will help us face the therapeutic management of TC more effectively.
Keywords: Endocrine malignancy, Thyroid carcinoma, Thyroid carcinoma treatment, Thyroid carcinoma antitarget therapies
Method–methodology
To identify the main topics published in medical literature, a search in “PubMed” and “isiknowledge” (that includes both full papers and abstracts) has been performed. Sentences used were “thyroid carcinoma”, “thyroid carcinoma treatment”, and “thyroid carcinoma antitarget therapies”. Main reviews on the topics: Clinical Guidelines of the European Society of Endocrinology, ESMO clinical guides, NCCN guides and American Thyroid Association guidelines for TC, have been consulted.
Introduction
Thyroid cancer is the most common type of endocrine malignancy and accounts for nearly 3 % of all malignancies [1]. The incidence of thyroid cancer has increased nearly threefold from 1975 to 2009. The age-adjusted incidence rate of thyroid cancer in women is nearly three times that of men (17.3/100,000 for women, 5.9/100,000 for men) [2]. The cause of this disparity is unknown, but generally the less aggressive types of thyroid cancer are more common in women, whereas the more aggressive types have similar sex distributions [3]. The incidence of thyroid cancer in Spain was 5/100,000 in women and 1.9/100,000 in men in 2013. Thyroid cancer is diagnosed at a higher rate among Caucasians than other ethnic groups. The age-adjusted mortality rate from thyroid cancer is the same in men and women, and is roughly the same across ethnic groups [2].
The only established environmental risk factor for thyroid carcinoma is exposure to ionizing radiation, and the risk, particularly of papillary carcinoma, is greater in subjects of younger age at exposure [4].
Thyroid cancer can be divided into three general subtypes based on pathology: differentiated (papillary, follicular, and Hürthle cell), medullary, and anaplastic thyroid cancers. Differentiated thyroid cancer, arising from thyroid follicular epithelial cells, accounts for the vast majority (90 %) of thyroid cancers. Of the differentiated cancers, papillary cancer comprises about 85 % of cases compared to about 10 % that have follicular histology, and 3 % that are Hürthle cell or oxyphil tumors. In general, stage for stage, the prognosis of papillary and follicular cancer is similar. Certain histologic subtypes of papillary cancer have a worse prognosis (tall cell variant, columnar cell variant, and diffuse sclerosing variant), as do more highly invasive variants of follicular cancer.
The prognosis is excellent for most patients with thyroid cancer; the overall 5-year relative survival for 2002–2008 from Surveillance, Epidemiology, and End Results program geographic areas was 97.5 % [2]. Despite low mortality rates, local recurrence occurs in up to 20 % of patients, and distant metastases occur in approximately 10 % at 10 years [5].
Diagnosis
As in all thyroid cancers, the diagnosis of TC usually follows the identification of a thyroid nodule on physical examination or as an incidental finding on diagnostic imaging performed for other reasons. Occasionally, discomfort in the neck or a hoarse voice or a change in collar size can be presenting complaints.
The standard of care for the workup of a thyroid nodule includes identification and characterization with ultrasonography. Using this modality, nodules and lymph nodes can be identified as potentially malignant based on their size and vascular properties. Nodules that are worrisome due to recent growth or sonographic characteristics are biopsied with fine-needle aspiration (FNA).
Patients with carcinoma for whom curative treatment with surgery is planned should undergo neck ultrasonography to identify lymph nodes that are likely to be involved, so that a neck dissection can be performed to remove these nodes where indicated. Routine preoperative measurement of serum thyroglobulin (Tg) is not recommended.
Recommendation: Patients with carcinoma for whom curative treatment with surgery is planned should undergo neck ultrasonography.
Surgical treatment
For patients with thyroid cancer >1 cm, the initial surgical procedure should be a near-total or total thyroidectomy unless there are contraindications to this surgery. Thyroid lobectomy alone may be sufficient treatment for small (<1 cm), low-risk, unifocal, intrathyroidal papillary carcinomas in the absence of prior head and neck irradiation or radiologically or clinically involved cervical nodal metastases.
The benefit of prophylactic central node dissection in the absence of evidence of nodal disease is controversial. There is no evidence that it improves recurrence or mortality rate, but it permits an accurate staging of the disease that may guide subsequent treatment and follow-up [6]. Compartment-oriented microdissection of lymph nodes should be performed in cases of preoperatively suspected and/or intraoperatively proven lymph node metastases in follicular thyroid cancer.
Following thyroidectomy, neck ultrasound, and high-dose radioactive iodine (RAI) can help identify and treat any residual disease, respectively. MRI, non-enhanced CT, and serum Tg levels are used to identify extrathyroidal disease. Common sites of metastatic spread include neck, chest, bone, and brain.
Recommendation: following thyroidectomy, neck ultrasound, and high-dose radioactive iodine (RAI) can help identify and treat any residual disease, respectively.
Staging
Staging takes into consideration tumor characteristics, metastasis, and patient age (Table 1). Although all staging systems are able to predict high or low risk of cancer mortality, they fail to predict the risk of recurrence. To overcome this limitation, both the American Thyroid Association (ATA) and the European Thyroid Association (ETA) have recently published practical guidelines [6] in which they graded the risk of recurrence in three categories of increasing risk on the basis of tumor-related parameters (pTNM and histological variant) integrated with other clinical features, including the result of the post-ablative whole-body scan (WBS) and serum Tg measurement (Table 2).
Table 1.
TNM classification system for differentiated thyroid carcinoma
| T1 | Tumor diameter 2 cm or smaller | |
| T2 | Primary tumor diameter >2–4 cm | |
| T3 | Primary tumor diameter >4 cm limited to the thyroid or with minimal extrathyroidal extension | |
| T4a | Tumor of any size extending beyond the thyroid capsule to invade subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve | |
| T4b | Tumor invades prevertebral fascia or encases carotid artery or mediastinal vessels | |
| TX | Primary tumor size unknown, but without extrathyroidal invasion | |
| N0 | No metastatic nodes | |
| N1a | Metastases to level VI (pretracheal, paratracheal, and prelaryngeal/Delphian lymph nodes) | |
| N1b | Metastasis to unilateral, bilateral, contralateral cervical or superior mediastinal nodes | |
| NX | Nodes not assessed at surgery | |
| M0 | No distant metastases | |
| M1 | Distant metastases | |
| MX | Distant metastases not assessed | |
| Stages | ||
| Patient age <45 years | Patient age 45 years or older | |
| Stage I | Any T, any N, M0 | T1, N0, M0 |
| Stage II | Any T, any N, M1 | T2, N0, M0 |
| Stage III | T3, N0, M0 | |
| T1, N1a, M0 | ||
| T2, N1a, M0 | ||
| T3, N1a, M0 | ||
| Stage IVA | T4a, N0, M0 | |
| T4a, N1a, M0 | ||
| T1, N1b, M0 | ||
| T2, N1b, M0 | ||
| T3, N1b, N0 | ||
| T4a, N1b, M0 | ||
| Stage IVB | T4b, Any N, M0 | |
| Stage IVC | Any T, Any N, M1 | |
Table 2.
Risk stratification according to the ATA and ETA guidelines
| ATA risk stratification low risk | Intermediate risk | High risk |
|---|---|---|
| No local or distant metastases All macroscopic tumor has been resected No tumor invasion of locoregional tissues or structures No aggressive histology or vascular invasion If 131I was given, no 131I uptake outside the thyroid bed on the post-therapeutic WBS |
Microscopic invasion of tumor into the perithyroidal soft tissues at initial surgery Cervical lymph node metastases or 131I uptake outside the thyroid bed on the post-therapeutic WBS or Tumor with aggressive histology or vascular invasion |
Macroscopic tumor invasion Incomplete tumor resection Distant metastases Thyroglobulinemia out of proportion to what is seen on the post-ablative scan |
| ETA risk stratification very low risk | Low risk | High risk |
|---|---|---|
| Complete surgery Patients with unifocal microcarcinoma (<1 cm) with no extension beyond the thyroid capsule and without lymph node metastases |
No local or distant metastases No tumor invasion of locoregional tissues or structures No aggressive histology or vascular invasion |
Less than total thyroidectomy Tumor invasion of locoregional tissues or structures Cervical lymph node metastases Distant metastases Aggressive histology or vascular invasion |
Postoperative adjuvant therapy in differentiated thyroid cancer (DTC)
Two main strategies are recommended after radical surgery of primary DTC: postoperative radioactive iodine (RAI) therapy to eliminate the postsurgical thyroid remnant and TSH suppression therapy using high doses of levothyroxine (LT4) to reduce the risk of recurrence.
RAI ablation is recommended in all patients with high-risk DTC defined as tumor size >4 cm and extrathyroidal invasion. RAI ablation is also recommended in selected patients with intermediate risk, with 1–4 cm tumors limited to the thyroid and additional poor prognostic factors, such as lymph node metastases, age >45 years old, aggressive histological features or multifocal disease. To increase the efficacy of RAI, treatment with RAI should be applied with high levels of TSH that could be achieved by elevation of endogenous TSH removing intake of LT4 or with recombinant TSH (rhTHS) previous RAI administration [7]. Doses of RAI between 30 and 100 mCi are usually enough in adjuvant setting to remove uptake in iodine scan and to obtain undetectable stimulated thyroglobulin levels after treatment. The current trend is to use lower doses, mainly in low-risk patients, showing in randomized clinical trials equivalence between high and low doses [8].
Accurate follow-up during the first year after RAI adjuvant therapy with stimulated thyroglobulin levels, thyroglobulin antibodies, neck ultrasound, and RAI whole-body scans will classify patients unlikely to experience tumor relapse or higher risk patients, and proceed with long-term or closer follow-up strategies [9].
Recommendations: to increase the efficacy of RAI, treatment with RAI should be applied with high levels of TSH that could be achieved by elevation of endogenous TSH removing intake of LT4 or with recombinant TSH (rhTHS) previous RAI administration (category 1). The current trend is to use lower doses, mainly in low-risk patients, showing in randomized clinical trials equivalence between high and low doses. Accurate follow-up during the first year after RAI adjuvant therapy with stimulated thyroglobulin levels, thyroglobulin antibodies, neck ultrasound, and RAI whole-body scans will classify patients unlikely to experience tumor relapse or higher risk patients, and proceed with long-term or closer follow-up strategies.
Treatment of tumor relapse/metastatic DTC
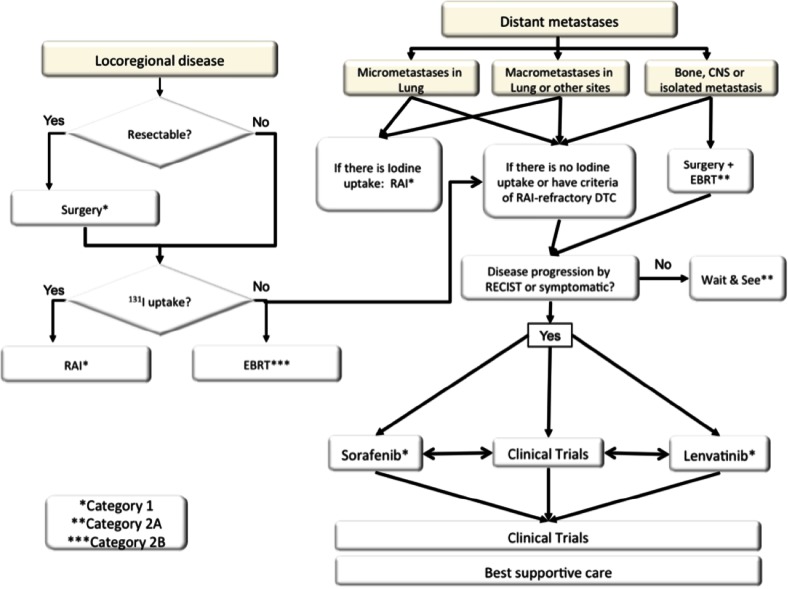
Tumor relapse in patients with DTC is not infrequent (5–20 % of locoregional relapse and 10–15 % of distant metastases). Treatment algorithm should be guided by the following criteria, in order of preference: surgical excision of locoregional or isolated distant metastasis in potentially curable patients; RAI therapy for patients with uptake in iodine scans; wait-and-see policy in patients with low tumor burden, asymptomatic and with no evidence of disease progression; new targeted agents for RAI-refractory DTC; and external beam radiotherapy for unresectable locoregional disease, symptomatic bone metastases, and central nervous system spread (Fig. 1).
Fig. 1.
Treatment algorithm for locally relapsed or metastatic DTC
Treatment of lung micrometastases with RAI therapy is highly effective and potentially curative, sessions should be repeated every 6–12 months as long as there is uptake of iodine, and complete remission is achieved or disease progression is documented, with reduction in iodine concentration or on reaching high cumulative doses of RAI [10]. RAI therapy is also effective for macrometastases in the lung and other sites, but complete responses are unusual and prognosis remains poor. Treatment cycles should be repeated while clinical benefit is observed (reduction in tumor burden, decrease in thyroglobulin levels, and symptomatic improvement) and until metastatic disease loses the iodine uptake capability or on reaching high cumulative doses. Doses of RAI therapy for metastatic disease are empiric, from 100 to 200 mCi.
Up to two-thirds of patients with distant metastases or unresectable locoregional disease will become RAI-refractory. The main criteria to define RAI-refractoriness include: RAI non-avid tumor lesions, loss of uptake of previous RAI-avid lesions, evidence of tumor progression after 12–16 months of RAI therapy, and FDG-PET scan uptake. Until recently, no effective therapy was available in RAI-refractory setting, and classical chemotherapy combinations did not show significant activity in many clinical trials. The better knowledge of molecular biology of thyroid cancer has allowed to the development of new targeted agents directed to the angiogenesis process and the main pathways involved in thyroid cancer pathogenesis [11]. Two randomized, placebo-controlled phase III studies have demonstrated the efficacy of multikinase inhibitors, sorafenib [12] and lenvatinib [13], in RAI-refractory DTC patients. Sorafenib and lenvatinib demonstrated a statistically significant improvement in progression-free survival, with significant increase in response rates in all subgroups of patients with RAI-refractory DTC. Sorafenib has been the first effective drug in this setting approved by regulatory authorities worldwide (category 1), and lenvatinib is expected to be approved in the near future. Many other targeted therapies are in clinical development in RAI-refractory DTC, including multikinase inhibitors with a different spectrum of kinase activity to avoid resistance to previous tyrosine kinase inhibitors and inhibitors of specific alterations of DTC patients, such as BRAF or MEK inhibitors for BRAF mutant population.
Recommendations: Treatment of lung micrometastases with RAI therapy is highly effective and potentially curative, and sessions should be repeated every 6–12 months as long as uptake of iodine is present, complete remission is achieved or disease progression is documented, with reduction in iodine concentration or on reaching high cumulative doses of RAI. Sorafenib has been the first effective drug in this setting approved by regulatory authorities worldwide in RAI-refractory DTC patients.
Medullary thyroid carcinoma (MTC)
MTC is a neuroendocrine-derived malignancy that accounts for 2–8 % of primary thyroid tumors depending on the series. It is estimated that around 50 patients are diagnosed with advanced MTC patients every year in Spain [11]. With regard to the activity of systemic chemotherapy in patients with metastatic MTC, only 29 % of patients remained free of progression at 5 months after initiation of treatment with a median overall survival of about 2 years [14].
Recently, it has been revealed the importance of the tyrosine kinase receptor RET (Rearranged During Transfection) presented in the pathogenesis of MTC. RET oncogene mutations occur in approximately 30–50 % of sporadic MTC and in virtually all hereditary MTC. In addition to these mutations, MTCs are associated with other conditions of the tumor microenvironment, such as high vascularization, as well as high levels of vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and the platelet-derived growth factor (PDGF). This fact shows that these tumors show a high capillary microdensity allowing neoplastic cells to enter the blood vessels and spread to distant metastases [15].
While hereditary MTCs are traditionally diagnosed by bilateral and multicentric tumors, sporadic MTCs usually appear as a single nodule or as a unilateral palpable cervical lymph node. In addition, a small group of patients may present with systemic manifestations due to the secretion of various peptides and vasoactive substances that might produce similar symptoms to those of a carcinoid syndrome with diarrhea, palpitations, facial flushing, etc. Peptides that these tumors can produce and release into the bloodstream comprise calcitonin, chromogranin A, neurotensin, bombesin, somatostatin, vasoactive intestinal peptide, carcinoembryonic antigen, calcitonin gene-related peptide, and adrenocorticotropic hormone [16]. About 50 % of patients with MTC have tumors that extend beyond the thyroid, mostly across the regional nodal level, and 13 % have metastases at diagnosis. About 90 % of patients with metastatic disease will die due to disease progression.
Most new treatments against advanced MTC target the cell membrane tyrosine kinase receptors. These receptors are responsible for controlling the tumoral cell vital functions such as cell differentiation, cell survival, proliferation, and motility. The main tyrosine kinase receptor involved in MTC is the one derived from RET gene. Several multitargeted kinase inhibitors are able to inhibit not only RET but also other receptors involved in tumor proliferation and angiogenesis [11]. Some of these multitargeted agents have shown activity in the MTC field [17, 18].
The paradigm for treating patients with advanced MTC has changed in recent years. Thus, recently vandetanib has been commercialized for the treatment of aggressive and symptomatic patients with MTC in patients with locally advanced or metastatic unresectable disease based on data from the ZETA study [17]. Vandetanib treatment was able to significantly increase progression-free survival compared to the placebo in the first-line systemic treatment of patients with locally advanced or metastatic MTC (30.5 months vs. 19.3 months, HR, 0.46, 95 % CI, 0.31–0.69, P < 0.001).
The phase III clinical trial called EXAM (Efficacy of XL184 in Advanced Medullary Thyroid Cancer) has shown that cabozantinib increases progression-free survival compared with placebo in patients with progressing advanced or metastatic MTC (11.2 months vs. 4 months, HR 0.28, 95 % CI 0.19–0.40, P < 0.001) [18]. The increase in PFS was observed consistently in all subgroups of patients analyzed including age, treatment with a prior tyrosine kinase inhibitor, presence or absence of RET mutation and in sporadic and hereditary tumors.
Direct comparison of the pivotal clinical trials of cabozantinib and vandetanib is not methodologically correct, being impossible to extrapolate definitive conclusions about which drug is more active and better tolerated. The profile of patients treated in these studies is not superimposable.
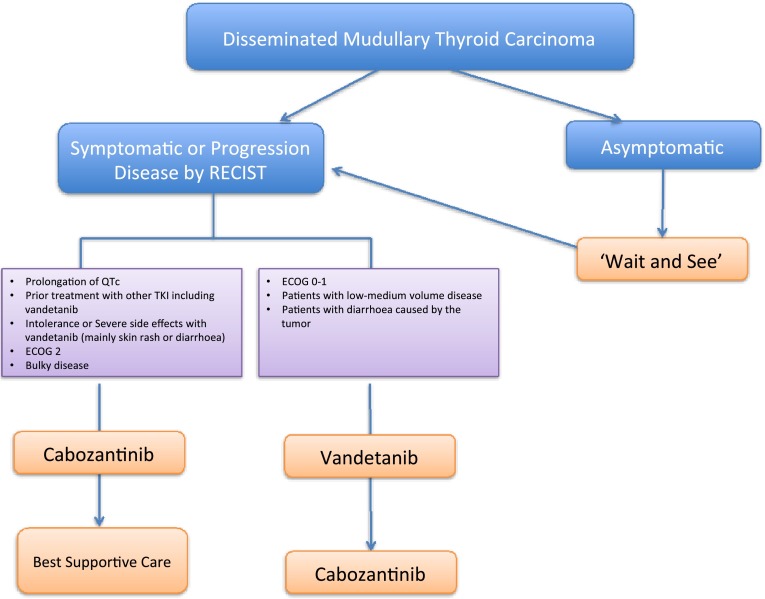
Based on the different safety profile and baseline characteristics of the patients recruited in both EXAM and ZETA trial and based on the clinical experience, the SEOM proposes the following algorithm of treatment for non-resectable and advanced MTC patients. (Figs. 1, 2) Both, vandetanib and cabozantinib exert a IB level of evidence for the indication and there is a need to find molecular biomarkers that might help us to select the best options for each patients and also the best sequencing treatment strategy for them.
Fig. 2.
Algorithm of treatment for the management of advanced MTC
Multidisciplinary approach, experience in dealing with advanced MTC and skills in the management of multitargeted agents emerge as crucial factors for the correct management of advanced MTC patients in the daily clinical practice.
Recommendations: Vandetanib and cabozantinib exert a IB level of evidence for the indication of locally advanced or metastatic unresectable MTC treatment.
Undifferentiated/Anaplastic thyroid carcinoma (ATC)
The undifferentiated/anaplastic thyroid carcinoma represents 1–2 % of malignant epithelial thyroid tumors. So far, it is one of the most lethal malignancies in adults, with a mortality approaching 100 % and a median survival of 3–5 months after diagnosis [19].
The ATC has a different genetic profile to differentiated thyroid tumors. Some of these mutations, such as RAS and BRAF, are common in differentiated and undifferentiated thyroid tumors, suggesting that might occur in early stages of carcinogenesis. About 90 % of the mutations of BRAF are detected in the V600E variant. Other mutations such as PI3K and PTEN can also be observed, although to a lesser extent. Other genes such as TP53 and β-catenin (CTNNB1) often have somatic mutations in the ATC.
Histological and immunohistochemical study of samples during the diagnosis is required to exclude other forms of differentiated thyroid cancer and potentially curable, as well as other tumor types that may result in a differential diagnosis.
All patients with ATC are designated as stage IV [20], being able to distinguish between stage IVA (intrathyroidal tumors), IVB, (extrathyroidal extension) and IVC (existence of distant metastases).
We always have to keep in mind that patients with ATC may develop a quick symptomatic deterioration of their general conditions and performance status. For that reason, best supportive care may be the only therapeutic option in most patients. There is no randomized clinical trial on treatment of ATC alone because of their low prevalence. As a consequence, the low grade of evidence is in general the rule. Multiple descriptive studies with small sample size have had conflicting results. They were performed mostly in differentiated thyroid carcinoma but including some cases of ATC.
Recommendation: Histological and immunohistochemical study of samples is required to exclude other forms of differentiated thyroid cancer and potentially curable in the ATC diagnosis. Preoperative radiological studies have to be performed in ATC including ultrasound, CT scan or MRI (for neck and chest), and PET/CT scan.
Treatment of resectable localized or locally advanced ATC (IVA and resectable IVB)
In patients with resectable disease at diagnosis, surgical resection (total thyroidectomy together with central and lateral cervical lymphadenectomy) should be performed followed by adjuvant treatment with radiotherapy or concurrent chemoradiotherapy. In the case of incidental ATC diagnosis, is not proven that performing total thyroidectomy after partial thyroidectomy improves the prognosis of the disease.
Adjuvant radiation therapy after surgical resection of ATC has shown significant increase in survival [21]. Multiple studies suggest a possible benefit of combined chemoradiotherapy after additional surgery, although any randomized controlled study has succeeded in demonstrating significant benefit compared to radiotherapy alone, so there is no established standard treatment. Schemes commonly used in combination chemotherapy in the adjuvant setting include taxanes, platinum-based regimens, and anthracyclines.
Therefore, adjuvant treatment should be used in all patients with adequate performance status after complete surgical resection (excluding incidental ATC). In those patients with incomplete gross resection (R2), subsequent salvage surgery can be considered.
Recommendation: Lobectomy or near-total thyroidectomy with lateral and central node dissection should be performed in ATC. Definitive radiation therapy with or without concomitant chemotherapy should be offered to ATC patients with adequate performance status.
Treatment of locally advanced ATC (unresectable IVB) and advanced ATC (IVC)
Patients with unresectable ATC diagnosis without distant metastases may benefit from neoadjuvant or adjuvant radiotherapy or chemoradiotherapy. If complete or partial response is observed it might consider a subsequent surgery, provided a complete gross resection (R0/R1) possible [22].
The priority in ATC patients with distant metastases should be proper control of symptoms, as no randomized study to demonstrate benefit in survival or quality of life. Cytotoxic agents that have shown increased activity in ATC are the taxanes with the highest response rate (50 %) [23] and anthracyclines.
Several agents have been shown to have potential benefit in preclinical studies, but many of them have failed to demonstrate efficacy in clinical trials. Nevertheless, there are recent positive results suggesting an improved outcome of these patients in the near future. Imatinib has shown variable activity in different cell lines of ATC. In a phase II clinical trial with 11 patients, disease control was observed in 6 of 8 patients evaluated, with a survival rate of 46 % and a progression-free survival of 27 % at 6 months [24]. The combretastatin A4 has achieved satisfactory results in phase II trial in combination with carboplatin and paclitaxel, showing 35 % reduction in mortality risk and one-year progression-free survival in 26 % of the patients [25]. In a phase II clinical trial, Sorafenib has shown 10 % partial response rate and clinical benefit in 7 of 10 patients, with a progression-free survival of 1.9 months and a median survival of 3.9 months [26].
Recommendation: Neoadjuvant radiotherapy with or without chemotherapy should be offered in unresectable IVB patients. If response is observed, salvage surgery has to be performed. Systemic chemotherapy based on taxanes and/or anthracyclines should be offered in patients with good performance status. New clinical trials may offer an opportunity to improve the outcome in advanced ATC patients. Other options are systemic chemotherapy, chemoradiotherapy, radiotherapy alone or best supportive care.
Conflict of interest
All the authors declare that they do not have conflict of interest.
References
- 1.American Cancer Society . Cancer facts and figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. eds. SEER cancer statistics review, 1975–2009 (vintage 2009 populations). Bethesda, MD: National Cancer Institute 2012.
- 3.Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol. 2010;6:1771–1779. doi: 10.2217/fon.10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagataki S, Nystrom E. Epidemiology and primary prevention of thyroid cancer. Thyroid. 2002;12:889–896. doi: 10.1089/105072502761016511. [DOI] [PubMed] [Google Scholar]
- 5.Eustatia-Rutten CF, Corssmit EP, Biermasz NR, Pereira AM, Romijn JA, Smit JW. Survival and death causes in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2006;91:313–319. doi: 10.1210/jc.2005-1322. [DOI] [PubMed] [Google Scholar]
- 6.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W, European Thyroid Cancer Taskforce European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 7.Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006;91:926–932. doi: 10.1210/jc.2005-1651. [DOI] [PubMed] [Google Scholar]
- 8.Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–1673. doi: 10.1056/NEJMoa1108586. [DOI] [PubMed] [Google Scholar]
- 9.Biondi B, Filetti S, Schlumberger M. Thyroid-hormone therapy and thyroid cancer: a reassessment. Nat Clin Pract Endocrinol Metab. 2005;1:32–40. doi: 10.1038/ncpendmet0020. [DOI] [PubMed] [Google Scholar]
- 10.Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD, et al. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med. 1996;37:598–605. [PubMed] [Google Scholar]
- 11.Grande E, Diez JJ, Zafon C, Capdevila J. Thyroid cancer: molecular aspects and new therapeutic strategies. J Thyroid Res. 2012;2012:847108. doi: 10.1155/2012/847108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014. [DOI] [PMC free article] [PubMed]
- 13.Schlumberger M, Tahara M, Wirth L, Robinson B, Brose M, Elisei R, et al. A phase 3, multicenter, double-blind, placebo-controlled trial of lenvatinib (E7080) in patients with 131I-refractory differentiated thyroid cancer (SELECT) Proc Am Soc Clin Oncol. 2014;32:LBA6008. [Google Scholar]
- 14.Schlumberger M, Bastholt L, Dralle H, Jarzab B, Pacini F, Smit JWA. 2012 European Thyroid Association Guidelines for Metastatic Medullary Thyroid Cancer. Eur Thyroid J. 2012;1:5–14. doi: 10.1159/000336977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sipos JA, Shah MH. Thyroid cancer: emerging role for targeted therapies. Ther Adv Med Oncol. 2010;2(1):3–16. doi: 10.1177/1758834009352667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoff AO, Hoff PM. Medullary thyroid carcinoma. Hematol Oncol Clin North Am. 2007;21(3):475–488. doi: 10.1016/j.hoc.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neff RL, Farrar WB, Kloos RT, Burman KD. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am. 2008;37:525. doi: 10.1016/j.ecl.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 20.AJCC Cancer Staging Manual, 7th ed. Springer, New York, 2010.
- 21.Chen J, Tward JD, Shrieve DC, Hitchcock YJ. Surgery and radiotherapy improves survival in patients with anaplastic thyroid carcinoma: analysis of the surveillance, epidemiology, and end results 1983–2002. Am J Clin Oncol. 2008;31:460. doi: 10.1097/COC.0b013e31816a61f3. [DOI] [PubMed] [Google Scholar]
- 22.Pierie JP, Muzikansky A, Gaz RD, Faquin WC, Ott MJ. The effect of surgery and radiotherapy on outcome of anaplastic thyroid carcinoma. Ann Surg Oncol 2002;156:57–64. [DOI] [PubMed]
- 23.Kawada K, Kitagawa K, Kamei S, Inada M, Mitsuma A, Sawaki M, et al. The feasibility study of docetaxel in patients with anaplastic thyroid cancer. Jpn J Clin Oncol. 2010;40:596–599. doi: 10.1093/jjco/hyq025. [DOI] [PubMed] [Google Scholar]
- 24.Ha HT, Lee JS, Urba S, Koenig RJ, Sisson J, Giordano T, et al. A phase II study of imatinib in patients with advanced anaplastic thyroid cancer. Thyroid. 2010;20:975–980. doi: 10.1089/thy.2010.0057. [DOI] [PubMed] [Google Scholar]
- 25.Sosa JA, Elisei R, Jarzab B, Balkissoon J, Lu SP, Bal C, et al. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid. 2014;24:232–240. doi: 10.1089/thy.2013.0078. [DOI] [PubMed] [Google Scholar]
- 26.Savvides P, Nagaiah G, Lavertu P, Fu P, Wright JJ, Chapman R, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013;23:600–604. doi: 10.1089/thy.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]