The incidence of cognitive impairment in colorectal cancer patients prior to chemotherapy is >3 times that of aged-matched healthy controls (45% vs 15%, P<0.001). Cognitive impairment was not associated with fatigue, anxiety/depression, or cognitive symptoms. Despite comprehensive correlative markers, the aetiology of cognitive impairment in cancer patients before adjuvant treatment remains unknown.
Keywords: cognitive function, fatigue, colorectal cancer, quality of life, survivorship
Abstract
Background
Cognitive impairment and fatigue have been associated with cancer and its treatment. We present baseline data from a large longitudinal study that evaluates cognitive function, fatigue, and potential underlying mechanisms following diagnosis of colorectal cancer (CRC).
Patients and methods
We evaluated CRC patients with stage I–III disease before or after surgery, participants with limited metastatic disease and healthy controls (HC). Neuropsychological evaluation included clinical and computerised tests. Participants completed questionnaires for fatigue and quality of life (QOL)-(FACT-F), anxiety/depression, and cognitive symptoms (FACT-Cog). Ten cytokines, clotting factors, sex hormones, carcinoembryonic antigen (CEA), and apolipoprotein E genotype were evaluated. Primary end points were cognitive function on clinical tests evaluated by a Global Deficit score (GDS) and fatigue. Associations between test results, demographic, and disease related factors were explored.
Results
We assessed 291 participants with early-stage disease [median age 59 (23–75) years, 63% men], 72 with metastatic disease, and 72 HC. Using GDS, 45% (126/281) of participants with early-stage CRC had cognitive impairment versus 15% (11/72) of HC (odds ratio 4.51, 95% confidence interval 2.28–8.93; P < 0.001), with complex processing speed, attention/working memory, and verbal learning efficiency being most affected. Women with early-stage CRC had greater cognitive impairment than men [55/105 (52%) versus 71/176 (40%), P < 0.050]. Cognitive symptoms were self-reported by 21% (59/286) of early-stage patients versus 17% (12/72) of HC; fatigue by 52% (149/287) of early-stage patients and 26% (19/72) of HC (P < 0.0001). Women reported more fatigue than men (P = 0.003). Fatigue, QOL, anxiety/depression, and cognitive symptoms were associated with each other (r = 0.43–0.71), but not with neuropsychological performance. Most cytokines were elevated in cancer patients. Cognitive function was not associated with cytokines, sex hormones, clotting factors, CEA, or apolipoprotein E genotype.
Conclusions
The incidence of cognitive impairment was three to five times higher in CRC patients than HC, with women having higher impairment rates than men. The cognitive impairment profile suggests dysfunction primarily in fronto-subcortical brain systems.
Trial registration
introduction
There is consistent evidence that some women with breast cancer suffer cognitive decline at diagnosis and/or after chemotherapy, and that effects may be sustained [1–4]. The aetiology of cognitive impairment is unknown, but possible mechanisms include release of cytokines, hormonal changes, blood clotting in small cerebral vessels, and neurotoxic effects from chemotherapy [5].
Fatigue is also associated with cancer and its treatment, with most studies being in women with breast cancer [6]. Mechanisms leading to cancer-related fatigue are largely unknown, but there is evidence to implicate inflammatory cytokines [7].
There have been no large prospective studies of cognitive function and fatigue in people with colorectal cancer (CRC), which affects both men and women; such changes may be subtle, but have profound effects on physical and social function and quality of life (QOL). The goal of the present article was to characterise the incidence and severity of these symptoms soon after diagnosis of CRC, compared with healthy controls (HC), and to evaluate putative mechanisms.
methods
patients
Here we present baseline findings of a prospective, longitudinal study of cognitive function and fatigue in patients with localised CRC (group 1). We also recruited patients with limited metastatic or locally recurrent CRC (group 2) before chemotherapy. Comparisons were made with HC (Figure 1 ).
Figure 1.
Consort diagram.
Participants were aged 18–75 years, with good performance status (Eastern Co-operative Oncology Group levels 0–1). Subjects were excluded if they had prior malignancy, co-morbidities that might impact cognitive performance, history of major psychiatric disorders or alcohol abuse, abnormal haematological, renal or liver function, or poor English fluency. Group 2 patients could have received adjuvant chemotherapy and/or radiotherapy ≥12 months previously; they were excluded if they had central nervous system metastases.
Participants with CRC were recruited from 2003 to 2010 from eight hospitals in Toronto, Canada and six hospitals in Sydney, Australia. HC were recruited from hospital visitors and the community in Sydney. Each institution's Research Ethics Board approved the study, and all participants gave written informed consent.
assessments
Baseline assessments of participants in group 1 occurred after surgery and before any adjuvant chemotherapy; except patients with rectal cancer planned for neoadjuvant chemoradiation, who were assessed before receiving any treatment.
Cognitive function was assessed with the following measures: (i) battery of clinical neuropsychological tests, (ii) computer-based Cambridge Neuropsychological Test Automated Battery (CANTAB) [8] and (iii) modified Six Elements Test (SET) [9] (supplementary Table S1, available at Annals of Oncology online). Tests were selected for their psychometric properties, and where possible on availability of demographically corrected normative data and alternate forms for repeated assessments. Testing was conducted by a trained research assistant and took ∼90 min.
Participants' perceptions of their cognitive function were evaluated with the Functional Assessment of Cancer Therapy–Cognitive (FACT-Cog) version 2 [10]. A summary score was calculated. The FACT-G questionnaire and FACT-fatigue (F) subscale were used to evaluate QOL and fatigue [11, 12]. The 12-item General Health Questionnaire (GHQ) assessed anxiety and depression [13].
The following blood tests were obtained for subjects with localised CRC and HC: haemoglobin; creatinine; liver function tests; carcinoembryonic antigen; sex hormones [oestradiol, follicle-stimulating hormone (FSH), luteinising hormone (LH)] in women; testosterone, FSH, LH in men); cytokines [interleukin (IL)-1β, IL-6, IL-2, IL-4, IL-8, IL-10, IL-12, tumour-necrosis factor-α, interferon-γ, and granulocyte-macrophage colony-stimulating factor]; homocysteine and other markers of blood clotting [thrombin–anti-thrombin (TAT), prothrombin fragment-1 and -2, and D dimers]. Genotyping for apolipoprotein-є4 (apo-E4) was carried out. Cytokines were analysed by Luminex technology using a human multiplex kit (Affymetrix, Inc., Santa Clara, CA) and mean immunofluorescence levels are reported. Cytokines, apolipoprotein genotyping, and blood clotting tests, with the exception of D dimers, were analysed in a central laboratory.
statistical analysis
Demographically adjusted T-scores for the clinical neuropsychological tests, corrected for age, education and gender, were generated using data from HC [14, 15]. T-scores were used to examine and compare the prevalence of neuropsychological performance between groups. Principal components analysis (Varimax with Kaiser normalisation) was applied to organise tests into specific cognitive domains as follows: complex processing speed, verbal learning and memory, visual learning and memory, and attention and working memory (supplementary Table S1, available at Annals of Oncology online). To characterise the performance, T-scores were converted to a deficit score ranging from 0 (no impairment) to 5 (severe impairment) and averaged to derive a Global Deficit score (GDS) to reflect overall neuropsychological performance [16].
For the CANTAB analysis, z-scores were derived from normative data [8], except for Verbal Recognition Memory, which was derived from our HC due to lack of normative data. Principal components analysis of CANTAB results generated six components. z-scores were converted to deficit scores and averaged, similar to our approach with the clinical neuropsychological tests.
Global cognitive impairment on clinical tests and CANTAB was defined as GDS of >0.5 [16]. Impairment on individual cognitive tests was defined as a T-score <40 or z-score ≤−1 and overall, by impairment in ≥2 domains. Following recommendations of the International Cognition and Cancer Task Force (ICCTF), we also determined the incidence of cognitive impairment defined as 2 standard deviation (SD) below the HC on at least one cognitive test (or one component for CANTAB), or >1.5 SD below on two or more tests [17]. Equations of Ingraham and Aiken [18] were used to determine whether the frequency of observed cognitive impairment exceeded expectation based on use of multiple measures.
A score <1.5 SD below the HC mean on the FACT-Cog was classified as perceived cognitive impairment. Fatigue of at least moderate severity was defined as a standardised FACT-F subscale score of <68/100, which is 1SD below the mean for the general United States population [19]. Anxiety or depressive symptoms were defined by a score <50/100 on the GHQ [13].
The primary end points, determined a priori, were cognitive function assessed by the GDS derived from the clinical neuropsychological tests, and fatigue assessed by the FACT-F. Secondary end points included: cognitive function assessed by clinical raw scores, T-scores, CANTAB, and SET; QOL; anxiety, depression, and patients' perception of cognitive function. Potential causative factors were studied in an exploratory manner.
Missing data were handled according to published guidelines for each questionnaire. On the clinical neuropsychological tests if one (of 10) result was unavailable the group mean raw score was imputed to determine the deficit score. If more than one result was missing the GDS could not be calculated. On CANTAB if <50% of tests were missing an impairment rating was calculated by imputing the mean of their other results within the same domain.
Tests between groups were carried out using Kruskal–Wallis test for continuous variables, Cochran–Armitage test for trend for ordinal variables, and exact χ2 tests for categorical variables. Spearman rank sum correlation coefficients were used to determine associations. The GDS, raw scores and T/z-scores were used to evaluate associations of neuropsychological performance with patient-reported outcomes. Nonparametric tests were used for comparisons with cytokines due to non-normal distribution. All P values are two-sided, and were not adjusted for multiple testing. Analyses were carried out in SAS v9.0 (Cary, NC).
sample size
Based on an earlier breast cancer study the planned sample size of group 1 was 240 with 50% to receive chemotherapy, providing 80% power (α = 0.05) to detect or rule out a difference in expected rates of cognitive impairment of 20% and 4%, and >90% power to detect a 10% difference on the fatigue subscale, at 12 months between groups receiving chemotherapy or not in the longitudinal study, allowing for attrition [20]. Recruitment of 75 patients with limited metastatic disease was planned for an exploratory analysis, based on feasibility.
After study initiation, we noted a higher rate of baseline cognitive impairment in CRC patients than predicted by population-based normative data. We therefore modified the study to include a concurrent group of 72 HC and increased the sample of CRC patients in group 1 to 290 based on expected baseline rates of cognitive impairment of 30% in CRC patients versus 10% in HC.
results
A total of 291 patients with early-stage CRC, 72 with metastatic CRC, and 72 HC completed baseline assessments. Supplementary Table S2, available at Annals of Oncology online, details characteristics of the participants.
cognitive function
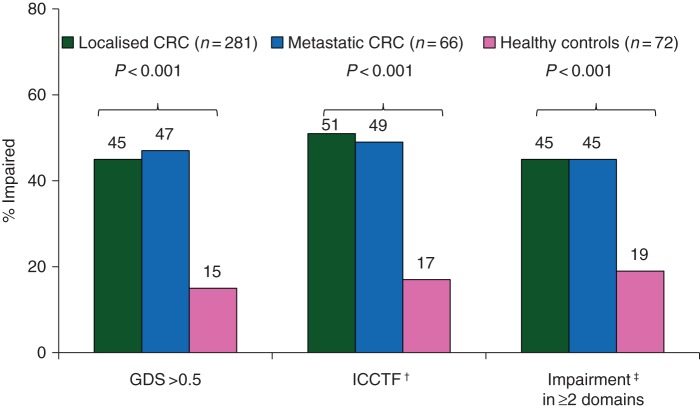
Mean neuropsychological test scores and proportions of subjects with cognitive impairment based on varying criteria are outlined in Tables 1 and 2, supplementary Table S3, available at Annals of Oncology online, and Figure 2. Using the GDS criteria (the primary end point), 15% (11/72) of controls had cognitive impairment on clinical neuropsychological tests compared with 45% (126/281) of group 1 [odds ratio (OR) 4.51, 95% confidence interval (CI) 2.28–8.93; P < 0.001] and 47% (31/66) of metastatic patients (OR 4.91, 95% CI 2.20–10.97; P < 0.001 for comparison with HC). Using ICCTF criteria, rates of cognitive impairment were 17% in controls compared with 51% in group 1 (P < 0.001), and 49% in group 2.
Table 1.
Neurocognitive impairment rates according to various criteria by study group
| Neurocognitive domain/test | Group 1 Localised CRC |
Group 2 Metastatic CRC |
Healthy controls | OR (95% CI) (Group 1 versus HC) |
P-value |
|---|---|---|---|---|---|
| Clinical tests | N = 281 | N = 66 | N = 72 | ||
| Overall neurocognitive impairment | |||||
| Global Deficit score >0.5a | 126 (45%)b | 31 (47%) | 11 (15%) | 4.51 (2.28–8.93) | <0.001 |
| ICCTF definitionc | 143 (51%) | 32 (49%) | 12 (17%) | 5.18 (2.67–10.05) | <0.001 |
| Deficit in ≥2 domainsd | 126 (45%) | 31 (47%) | 11 (15%) | 3.37 (1.80–6.32) | <0.001 |
| Cognitive domains—Deficit score >0.5 | |||||
| Attention and working memory | 82 (30%) | 14 (21%) | 12 (17%) | 2.06 (1.05–4.03) | 0.035 |
| Processing speed | 142 (51%) | 34 (51%) | 11 (15%) | 5.67 (2.86–11.22) | <0.001 |
| Verbal learning and memory | 95 (34%) | 19 (29%) | 7 (10%) | 5.67 (2.86–11.22) | <0.001 |
| Visual learning and memory | 48 (17%) | 13 (20%) | 10 (14%) | 1.28 (0.61–2.67) | 0.515 |
| Individual testse | |||||
| Digit Span | 40 (14%) | 10 (15%) | 10 (14%) | 1.03 (0.49–2.17) | 0.94 |
| Spatial Span | 81 (29%) | 19 (29%) | 11 (15%) | 2.25 (1.12–4.49) | 0.022 |
| Letter–number sequencing | 81 (29%) | 17 (26%) | 13 (18%) | 1.84 (0.96–3.53) | 0.068 |
| Digit symbol | 129 (46%) | 31 (47%) | 11 (15%) | 4.71 (2.38–9.32) | <0.001 |
| TMTA | 143 (51%) | 28 (42%) | 14 (19%) | 4.29 (2.29–8.05) | <0.001 |
| TMTB | 110 (39%) | 28 (42%) | 11 (15%) | 3.57 (1.80–7.08) | <0.001 |
| HVLT Total recall | 111 (40%) | 24 (36%) | 13 (18%) | 2.96 (1.55–5.66) | 0.001 |
| HVLT delayed recall | 93 (33%) | 18 (27%) | 14 (19%) | 2.05 (1.09–3.87) | 0.027 |
| HVLT retention percentf | 81 (29%) | 11 (17%) | 11 (15%) | ||
| BVMT total recall | 60 (21%) | 15 (23%) | 13 (18%) | 1.23 (0.63–2.40) | 0.54 |
| BVMT delayed recall | 63 (22%) | 17 (26%) | 13 (18%) | 1.31 (0.68–2.55) | 0.42 |
| BVMT retention percentf | 27 (10%) | 4 (6%) | 4 (6%) | ||
| Number of Impaired Clinical Tests | n = 281 | n = 66 | n = 72 | <0.001 | |
| 0 | 42 (15%) | 12 (18%) | 26 (36%) | ||
| 1 | 39 (14%) | 10 (15%) | 18 (25%) | ||
| 2 | 33 (12%) | 9 (14%) | 10 (14%) | ||
| 3 | 42 (15%) | 7 (11%) | 3 (4%) | ||
| 4 | 46 (16%) | 9 (14%) | 8 (11%) | ||
| ≥5 | 79 (28%) | 19 (29%) | 7 (10%) | ||
| CANTAB | N = 265 | N = 66 | N = 71 | ||
| Overall neurocognitive impairment | |||||
| Global Deficit score >0.5g | 79 (30%) | 20 (31%) | 9 (13%) | 2.96 (1.40–6.24) | 0.004 |
| ICCTF definitionc | 102 (38%) | 22 (33%) | 12 (17%) | 3.08 (1.58–6.00) | <0.001 |
| Cognitive domain deficit score | |||||
| Attention and Complex reaction time | 37 (14%) | 7 (11%) | 3 (4%) | 3.68 (1.10–12.30) | 0.023 |
| Attention and Simple reaction time | 58 (22%) | 16 (24%) | 10 (14%) | 1.71 (0.82–3.54) | 0.18 |
| Discriminability—memory | 70 (26%) | 12 (18%) | 16 (23%) | 1.23 (0.66–2.29) | 0.54 |
| Verbal learning and memoryg | 62 (23%) | 13 (20%) | 7 (10%) | 2.79 (1.22–6.41) | 0.013 |
| Spatial working memory | 60 (23%) | 13 (20%) | 2 (3%) | 10.10 (2.40–42.41) | <0.001 |
| Discriminability learning | 35 (13%) | 10 (15%) | 3 (4%) | 3.45 (1.03–11.56) | 0.035 |
| Individual testse | |||||
| Motor Screening Mean latency | 35 (13%) | 5 (8%) | 4 (6%) | 2.55 (0.87–7.43) | 0.095 |
| RVP Total False alarms | 47 (18%) | 11 (17%) | 9 (13%) | 1.49 (0.69–3.20) | 0.37 |
| RTI Five Choice Reaction Time | 65 (25%) | 22 (33%) | 5 (7%) | 4.29 (1.66–11.11) | <0.001 |
| RTI Five Choice Movement Time | 93 (35%) | 22 (33%) | 26 (37%) | 0.94 (0.54–1.61) | 0.89 |
| RVP Total hits | 84 (32%) | 24 (36%) | 16 (23%) | 1.60 (0.86–2.95) | 0.15 |
| RVP Total misses | 83 (31%) | 24 (36%) | 16 (23%) | 1.57 (0.85–2.90) | 0.19 |
| RVP mean latency | 57 (22%) | 20 (30%) | 8 (11%) | 2.16 (0.98–4.76) | 0.062 |
| VRM Delayed recognition False positives | 70 (26%) | 12 (18%) | 16 (23%) | 1.23 (0.66–2.29) | 0.54 |
| VRM Delayed recognition correct targets | 51 (19%) | 12 (18%) | 11 (15%) | 1.30 (0.64–2.65) | 0.61 |
| VRM Immediate recognition correct targets | 33 (12%) | 6 (9%) | 4 (6%) | 2.38 (0.82–6.96) | 0.13 |
| VRM Immediate free recall—total correct | 103 (39%) | 21 (32%) | 12 (17%) | 3.13 (1.60–6.10) | <0.001 |
| VRM Immediate recognition total false positives | 35 (13%) | 10 (15%) | 3 (4%) | 3.45 (1.03–11.56) | 0.035 |
| SWM total errors | 75 (28%) | 19 (29%) | 6 (8%) | 4.28 (1.78–10.29) | <0.001 |
| Spatial Working Memory Strategy score | 60 (23%) | 16 (24%) | 5 (7%) | 3.86 (1.49–10.03) | 0.002 |
| Number of Impaired CANTAB Tests | <0.001d | ||||
| 0 | 40 (15%) | 12 (18%) | 19 (27%) | ||
| 1 | 35 (13%) | 9 (14%) | 16 (23%) | ||
| 2 | 39 (15%) | 11 (17%) | 13 (18%) | ||
| 3 | 31 (12%) | 5 (8%) | 8 (11%) | ||
| 4 | 38 (14%) | 5 (8%) | 6 (8%) | ||
| 5 | 34 (13%) | 5 (8%) | 6 (8%) | ||
| ≥6 | 48 (18%) | 19 (29%) | 3 (4%) | ||
a413/419 had complete data on all 10 clinical tests; 6 [5 localised colorectal cancer (CRC) and 1 metastatic CRC] had missing/invalid data on letter–number sequencing or TMTB, and the group mean raw score was used instead.
bComparison of impairment in the post-surgical versus pre-surgical patients was 47% versus 36% (Pearson χ2 = 1.24, P = 0.266).
cImpairment defined as 2 SD below the mean of healthy controls (HC) on one test or 1.5 SD below the mean of HC on two tests.
dImpairment defined as T < 40 for individual tests, and within a domain as impairment on ≥50% of tests.
eImpairment defined as T-score <40 or z-score ≤−1.0 (or ≤1 SD below the HC on verbal learning and memory tests).
fNot included in computation of domain T-score.
gThe healthy control group was used to derive the z-score for the Verbal Recognition Memory (VRM) tests as no normative data were available. CANTAB GDS was recalculated omitting these tests with similar results (data not shown).
ICCTF, International Cognition and Cancer Task Force; WAIS III, Wechsler Adult Intelligence Scale, Third edition; WMS III, Wechsler Memory Scale, Third edition; Letter–Number Sequencing, WAIS-III Letter–Number Sequencing; Digit Span, WMS-III Digit Span; Spatial Span, WMS-III Spatial Span; HVLT, Hopkin's Verbal Learning Test-Revised; BVMT, Brief Visuospatial Memory Test-Revised; Digit Symbol, WAIS-III Digit Symbol; TMTA, Trail Making Test Part A; TMTB, Trail Making Test Part B; RVP, Rapid Visual Information Processing; RTI, Reaction Time Five Choice; SWM, Spatial Working Memory.
Table 2.
Mean (standard deviation) of global deficit score and T-scores/z-scores (standard deviation) by study group
| Neurocognitive domain | Group 1 | Group 2 | Healthy controls (HC) |
P-value Group 1 versus HC |
|---|---|---|---|---|
| Localised CRC | Metastatic CRC | |||
| Clinical Tests | N = 281 | N = 66 | N = 72 | |
| Global Deficit score | 0.61 (0.59) | 0.57 (0.55) | 0.23 (0.39) | <0.001 |
| Attention and working memory | 47.7 (8.8) | 48.4 (7.7) | 50.1 (8.2) | 0.10 |
| Digit Span | 52.0 (10.5) | 51.4 (10.0) | 51.4 (10.2) | 0.837 |
| Spatial Span | 46.1 (11.8) | 47.4 (11.5) | 49.7 (10.0) | 0.059 |
| Letter–number sequencing | 45.0 (10.5) | 46.5 (10.3) | 49.3 (10.4) | 0.008 |
| Processing speed | 41.5 (8.4) | 41.9 (10.2) | 49.8 (8.4) | <0.001 |
| Digit symbol | 41.3 (11.4) | 41.1 (12.7) | 49.7 (9.9) | <0.001 |
| Trail A | 39.9 (9.2) | 40.8 (10.3) | 49.8 (10.0) | <0.001 |
| Trail B | 43.3 (10.4) | 43.8 (12.3) | 49.8 (10.1) | <0.001 |
| Verbal learning and memory | 43.6 (10.2) | 44.2 (9.7) | 49.8 (8.9) | <0.001 |
| HVLT Total recall | 42.6 (11.1) | 42.8 (10.3) | 49.8 (10.0) | <0.001 |
| HVLT delayed recall | 44.6 (10.7) | 45.7 (10.3) | 49.8 (10.0) | 0.001 |
| HVLT Retention Percenta | 49.6 (14.1) | 51.1 (13.3) | 49.9 (10.0) | 0.701 |
| Visual learning and memory | 49.0 (11.7) | 47.5 (8.9) | 49.8 (9.4) | 0.445 |
| BVMT total recall | 49.1 (12.1) | 48.0 (9.1) | 49.7 (10.0) | 0.674 |
| BVMT delayed recall | 48.9 (12.5) | 46.9 (9.9) | 49.8 (10.0) | 0.319 |
| BVMT Retention Percenta | 58.5 (11.6) | 58.0 (10.5) | 59.3 (10.2) | 0.769 |
| CANTAB | N = 265 | N = 66 | N = 71 | |
| Global Deficit score | 0.41 (0.51) | 0.42 (0.54) | 0.19 (0.32) | <0.001 |
| Attention and Complex reaction time | −0.36 (0.97) | −0.37 (1.54) | −0.11 (0.51) | 0.16 |
| Motor Screening | −0.11 (0.76) | 0.07 (0.63) | 0.10 (0.55) | 0.058 |
| RTI Reaction Time | −0.29 (1.13) | −0.39 (1.19) | 0.19 (0.84) | 0.001 |
| RTI Movement Time | −0.55 (1.38) | −0.38 (1.23) | −0.72 (1.17) | 0.24 |
| RVP Total False Alarms | −0.47 (2.82) | −0.79 (4.69) | −0.03 (0.82) | 0.53 |
| Attention and simple reaction time | −0.28 (1.01) | −0.36 (0.97) | 0.00 (0.93) | 0.032 |
| RVP total hits | −0.37 (1.14) | −0.49 (1.01) | −0.14 (1.04) | 0.20 |
| RVP total misses | −0.37 (1.14) | −0.49 (1.01) | −0.14 (1.04) | 0.20 |
| RVP mean latency | −0.07 (1.15) | −0.14 (1.26) | 0.28 (0.97) | 0.068 |
| Discriminability - Memory | ||||
| VRM Delayed recognition: false positives | −0.14 (1.25) | −0.04 (1.30) | 0 (1) | 0.37 |
| Verbal learning and memoryb | −0.20 (0.86) | −0.15 (0.86) | 0 (0.80) | 0.11 |
| VRM Delayed Recognition—correct targets | −0.06 (0.94) | −0.06 (0.93) | 0 (1) | 0.82 |
| VRM Immediate Recognition—correct targets | −0.15 (1.06) | −0.10 (1.31) | 0 (1) | 0.35 |
| VRM Immediate Free recall—total correct | −0.38 (1.24) | −0.33 (1.11) | 0 (1) | 0.024 |
| SWM | −0.24 (0.88) | −0.23 (0.85) | 0.26 (0.87) | <0.001 |
| SWM total errors | −0.32 (0.94) | −0.28 (0.89) | 0.15 (0.79) | 0.001 |
| SWM Strategy | −0.15 (0.95) | −0.21 (0.96) | 0.37 (1.07) | <0.001 |
| Discriminability learning | ||||
| VRM Immediate Recognition - total false positives | −0.43 (1.78) | −0.32 (1.25) | 0 (1) | 0.086 |
| Six Element Test (SET) Score (SD) | 3.3 (1.0) | 3.1 (1.1) | 3.3 (1.0) | 0.87 |
aNot included in computation of domain T-score.
bVerbal recognition memory (VRM)—as no normative data were available for determining a z-score, the healthy control group was used. CANTAB GDS was recalculated omitting this test with similar results (data not shown).
HC, Healthy Controls. ICCTF, International Cognition and Cancer Task Force; WAIS III, Wechsler Adult Intelligence Scale, Third edition; WMS III, Wechsler Memory Scale, Third edition; Letter–Number Sequencing, WAIS-III Letter–Number Sequencing; Digit Span, WMS-III Digit Span; Spatial Span, WMS-III Spatial Span; HVLT, Hopkin's Verbal Learning Test-Revised; BVMT, Brief Visuospatial Memory Test-Revised; Digit Symbol, WAIS-III Digit Symbol; TMTA, Trail Making Test Part A; TMTB, Trail Making Test Part B; RVP, Rapid Visual Information Processing; RTI, Reaction Time Five Choice; SWM, Spatial Working Memory.
Figure 2.
Overall cognitive impairment rates by study group using various impairment criteria. †Defined as 2 standard deviation (SD) below the mean of healthy controls on one test or 1.5 SD below the mean of healthy controls on two tests. ‡Defined as T < 40 for individual tests and impairment on ≥50% of tests within a domain. CRC, colorectal cancer.
Overall 45% of group 1 patients had impairment in at least two cognitive domains, compared with 15% of HC. The proportion with deficits in individual cognitive domains was: verbal learning and memory 34% versus 10%; visual learning and memory 17% versus 14%; processing speed 51% versus 15%, and attention and working memory 30% versus 17%. Effect sizes were greatest for measures of verbal learning and memory (Hopkins Verbal Learning Test—total and delayed scores), and processing speed (Digit Symbol and Trail Making Test Part A and B). There was no significant difference in cognitive impairment between groups 1 and 2, or between those in group 1 by stage of disease, time from surgery, evaluation of patients pre- versus post-surgery, or by country of residence.
Cancer patients had more cognitive impairment than HC when evaluated by CANTAB (GDS 0.41 versus 0.19, P < 0.001), with 30% with localised CRC having a GDS >0.5 compared with 13% of HC (P < 0.004), and 38% impaired using ICCTF criteria compared with 17% of HC (P < 0.001). There was a weak association between performance as indicated by the GDS for the clinical neuropsychological tests and CANTAB (r = 0.26 group 1, r = 0.37 HC). There was no significant difference between the groups on the SET.
After adjusting for group, more women had cognitive impairment on the clinical GDS than men (44% versus 37%, P < 0.045), with more impairment in women with localised CRC than men (52% versus 40%, P = 0.050). There was no significant difference in impairment rates by gender for CANTAB. Increasing age in CRC patients was associated with greater impairment (P < 0.001) on clinical tests and CANTAB, even after adjusting for changes in age expected in a normal population.
patient-reported outcomes
Patient-reported outcomes are summarised in supplementary Table S4, available at Annals of Oncology online. Perceived cognitive impairment rates were 21% in group 1, 18.5% in group 2, and 17% in HC (P = 0.51). There was no significant difference in FACT-Cog scores between men and women in group 1, but subjects ≥60 years old reported more cognitive deficits than younger subjects (P = 0.009). Only a weak association was observed between cognitive symptoms and neuropsychological performance by GDS on clinical tests (r = 0.19) or CANTAB (r = −0.18) for group 1 or HC.
Fatigue was reported by 52% of group 1 patients with no difference between those evaluated pre- or post-surgery. The incidence of fatigue in HC was 26% (P < 0.001 for comparison with group 1) and 68% of patients with metastatic disease were fatigued. Women reported significantly more fatigue than men (P = 0.005) after adjusting for group.
Cancer patients, particularly those with metastatic disease, had poorer QOL and more symptoms of anxiety and depression than HC. No significant difference was observed in QOL by gender or age in group 1.
Moderate association was found between self-reported cognitive function and each of fatigue (r = 0.43), QOL (r = 0.49), and anxiety and depression (r = −0.44) in group 1, but these symptoms were not associated with neuropsychological performance (supplementary Table S5, available at Annals of Oncology online).
blood results
Haemoglobin levels in group 1 were significantly lower than in HC (median 131 versus 142, P < 0.001) and were weakly associated with fatigue (r = 0.21). Prothrombin fragments and D dimers were significantly higher in group 1 than in HC (P < 0.0001) (supplementary Table S6, available at Annals of Oncology online). Higher oestradiol levels were weakly related to fatigue (r = −0.26) in women in group 1 but not in HC. There was no association between oestradiol levels and GDS.
Median levels of most cytokines were significantly higher in cancer patients than HC (supplementary Table S6 and Figure S1, available at Annals of Oncology online) and were related to stage of disease. They were also higher in pre-surgical patients than those post surgery. No cytokines were associated significantly with neuropsychological performance by GDS. There was a weak association of each of the cytokines with the Trail Making Test Parts A and B (r = −0.21 to 0.34) in group 1, but not with fatigue or anxiety/depression.
apolipoprotein E4
In group 1, 61/260 (24%) patients with genotyping had apolipoprotein E4 alleles, (homozygotes E4/4 n = 3), with 17/72 (24%) in HC (E4/4 n = 3). There was no significant difference in the proportion with cognitive impairment on GDS or ICCTF criteria, or on individual clinical tests, based on absence or presence of E4 alleles.
discussion
This large study evaluated cognitive function and fatigue at or soon after diagnosis of localised CRC, when compared with HC, and a sub-study of patients with metastatic/recurrent disease. We found that 45% of patients with localised CRC had impairment on clinical neuropsychological tests and 30% on CANTAB, compared with 15% and 13% of HC using GDS criteria. There was 51% and 17% impairment using ICCTF criteria on clinical tests in localised CRC patients and HC, respectively. The incidence varies depending on criteria used to define impairment, but regardless, is at least three times higher that of HC.
We found no significant difference in rates of cognitive impairment by disease stage, or between patients pre- and post-surgery. It appears that cancer per se rather than disease stage contributes to cognitive impairment, and that causes other than surgery and anaesthesia, fatigue, or anxiety and depression are responsible for the high rates of cognitive impairment in CRC patients at baseline.
The main cognitive domains affected were complex information processing speed, auditory working memory, and verbal learning efficiency (but not memory or retention of information acquired). This overall profile of cognitive impairment suggests dysfunction primarily in fronto-subcortical brain systems (as is the case in neuromedical conditions such as multiple sclerosis and HIV) as opposed to involving more cortical brain systems (such as Alzheimer's disease).
Two other studies evaluated cognitive function before adjuvant chemotherapy in CRC patients, but both are single arm studies. The first, evaluated 81 patients reporting impairment in 37% on ICCTF criteria, with Trail Making Test Part A and B particularly impaired [21]. The second [22] included 57 patients, and reported no incidence of cognitive impairment based on the Mini Mental State Examination, but this is a dementia screening test and is not sensitive for the subtle impairment seen in cancer patients. Interestingly, their patients and ours had similar mean times on the Trail Making Test Parts A and B; significantly higher than our HC, suggesting impairment was present. Our rates of cognitive impairment are higher than studies of women with breast cancer, where up to 35% of women had cognitive impairment before chemotherapy [4, 23].
A difficulty in testing patients with cancer is finding a battery that is brief, evaluates multiple domains, and is sensitive to subtle cognitive impairment. One aim in using both clinical neuropsychological and CANTAB batteries was to determine whether the computer-based CANTAB might achieve this, and whether its results were well correlated with standard clinical tests. The association of the GDS between the two batteries was weak to moderate, suggesting these two methods are not interchangeable.
We found more cognitive impairment on clinical neuropsychological tests and CANTAB with older age. This is consistent with findings in breast cancer suggesting older patients and those with lower cognitive reserve are at higher risk of impairment [24].
Our study found a weak association between objective and subjective cognitive impairment. In contrast to objective measures of cognitive impairment, rates of self-reported cognitive symptoms in CRC patients were not significantly different from HC. Our analysis was limited to comparing summary scores for perceived and objective cognitive performance, and we plan further analysis of associations between individual cognitive domains and FACT-Cog subscales. Other small studies found no association of anxiety, depression, fatigue, or QOL with neuropsychological performance [20]. In our large study, none of these variables was associated with objective cognitive impairment on clinical tests using the GDS.
Mechanistic aspects of this study were exploratory. Using the clinical GDS, we found no evidence to suggest that the candidate mechanisms tested were causing cognitive impairment. Levels of each cytokine were significantly higher in cancer patients than HC; greatest in those with more advanced stage, and those evaluated pre-surgery. There was no association between global cognitive impairment and higher cytokine levels, but there was a weak association between cytokine levels and impairment on the Trail Making Test Parts A and B, measuring processing speed for complex information and cognitive flexibility. These results contradict smaller breast cancer studies reporting an association between cognitive impairment and both apolipoproteinE4 allele and elevated cytokines [25, 26]. It is unclear if these findings are tumour specific, if the mechanism for baseline impairment is different from post-chemotherapy impairment, or their findings were due to chance with small sample sizes.
Strengths of the study include: large sample size; CRC population; evaluation of gender differences; inclusion of a HC group; multiple approaches to cognitive assessment, and extensive correlative biomarkers. A limitation of our study was having more women than men in our HC group, but it was well matched with CRC patients on age and education. We found greater cognitive impairment in women than men with CRC. There was no difference in cognitive impairment by gender in the HC, and rates are consistent with the general population. All HC were recruited from Australia, but there were no differences in the primary end points for group 1 between the two countries. A major challenge in cognitive studies is analysing multiple test results. Multiple significance testing was minimised by using summary statistics for primary end points, but we also analysed raw and domain scores. We chose not to adjust P values and to regard all secondary end points as exploratory. We acknowledge that some differences may be due to chance alone and advise P values >0.01 should be interpreted with caution.
In conclusion, our results show that almost half of CRC patients have cognitive impairment and a similar proportion report fatigue at or soon after diagnosis, and these rates are significantly higher than in controls. Women had greater rates of cognitive impairment than men. A weak association was found between objective cognitive function and cognitive symptoms. Self-reported cognitive impairment was associated with fatigue, poorer QOL, and anxiety and depression, but there was no association between objective neuropsychological performance and: fatigue, anxiety and depression, or QOL. The causes of baseline cognitive impairment in cancer patients remain unknown.
funding
This work was supported by: National Cancer Institute of Canada (grant number #15261, 2004), American Society of Clinical Oncology Young Investigator Award to JV (2004), National Health Medical Research Council, Australia (grant number 457386, 2007) and the Cancer Institute New South Wales (grant number 05/CRF/1-06, 2006; grant number 09/RIG1-13, 2010) to JV. Study sponsors had no role in the conduct or reporting of the study, and researchers had full independence from the funders.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
Collaborators, study co-ordinators and participants from the following hospitals:
Toronto: Princess Margaret, Toronto General, Toronto Western, Mount Sinai, Sunnybrook, Credit Valley, Humber River, St Michael's, Toronto East General
Sydney: Concord Repatriation & General, Royal Prince Alfred, Bankstown-Lidcombe, Royal North Shore, Prince of Wales, Nepean.
David Laurence for his work as data manager for the study. We express our gratitude to Anya Umlauf and Robert Heaton, UCSD, for their assistance with the generation of normative adjustments for the clinical neuropsychological tests.
references
- 1.Schagen SB, Muller MJ, Boogerd W, et al. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98:1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- 2.Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 3.Hermelink K, Untch M, Lux MP, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109:1905–1913. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- 4.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 7.Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun. 2007;21:863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins TW, James M, Owen AM, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychol Soc. 1998;4:474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- 9.Wilson BA, Alderman N, Burgess PW, et al. Behavioural Assessment of the Dysexecutive Syndrome. Edmunds, England: Thames Valley Test Company; 1996. [Google Scholar]
- 10.Wagner L, Sweet J, Butt Z, et al. Measuring patient self-reported cognitive function: development of the Functional Assessment of Cancer Therapy—Cognitive Function instrument. J Support Oncol. 2009;7:W32–W39. [Google Scholar]
- 11.Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 12.Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34:13–19. [PubMed] [Google Scholar]
- 13.Goldberg DP, Williams P. Windsor: NFER-Nelson Publishing Company Ltd. 1991. A User's Guide to the General Health Questionnaire. [Google Scholar]
- 14.Norman MA, Moore DJ, Taylor M, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011;33:793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heaton R, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults Professional Manual. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 16.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 17.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 18.Ingraham L, Aiken CB. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology. 1996;10:120–124. [Google Scholar]
- 19.Cella D, Lai JS, Chang CH, et al. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 20.Tchen N, Juffs HG, Downie FP, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol. 2003;21:4175–4183. doi: 10.1200/JCO.2003.01.119. [DOI] [PubMed] [Google Scholar]
- 21.Cruzado JA, Lopez-Santiago S, Martinez-Marin V, et al. Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients. Support Care Cancer. 2014;22:1815–1823. doi: 10.1007/s00520-014-2147-x. [DOI] [PubMed] [Google Scholar]
- 22.Andreis F, Ferri M, Mazzocchi M, et al. Lack of a chemobrain effect for adjuvant FOLFOX chemotherapy in colon cancer patients. A pilot study. Support Care Cancer. 2013;21:583–590. doi: 10.1007/s00520-012-1560-2. [DOI] [PubMed] [Google Scholar]
- 23.Wefel JS, Lenzi R, Theriault RL, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 24.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 26.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30(Suppl):S99–108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.