Abstract
Objectives:
The purpose of this study is to evaluate the relative risk of abnormal dopamine transporter (DAT) imaging for subjects with and without hyposmia and the feasibility of acquiring a large, community-based, 2-tiered biomarker assessment strategy to detect prodromal Parkinson disease (PD).
Methods:
In this observational study, individuals without a diagnosis of PD, recruited through 16 movement disorder clinics, underwent tier 1 assessments (olfactory testing, questionnaires). Tier 2 assessments (neurologic examination, DAT imaging, and other biomarker assessments) were completed by 303 subjects. The main outcome of the study is to compare age-expected [123I]β-CIT striatal binding ratio in hyposmic and normosmic subjects.
Results:
Tier 1 assessments were mailed to 9,398 eligible subjects and returned by 4,999; 669 were hyposmic. Three hundred three subjects (203 hyposmic, 100 normosmic) completed baseline evaluations. DAT deficit was present in 11% of hyposmic subjects compared with 1% of normosmic subjects. Multiple logistic regression demonstrates hyposmia (odds ratio [OR] 12.4; 95% confidence interval [CI] 1.6, 96.1), male sex (OR 5.5; 95% CI 1.7, 17.2), and constipation (OR 4.3; 95% CI 1.6, 11.6) as factors predictive of DAT deficit. Combining multiple factors (hyposmia, male sex, and constipation) increased the percentage of subjects with a DAT deficit to >40%.
Conclusion:
Subjects with DAT deficit who do not meet criteria for a diagnosis of PD can be identified by olfactory testing. Sequential biomarker assessment may identify those at risk of PD. Selecting hyposmic individuals enriches the population for DAT deficit, and combining hyposmia with other potential risk factors (male sex, constipation) increases the percentage of subjects with a DAT deficit compatible with prodromal PD.
The prodromal stage of Parkinson disease (PD) occurs several years in advance of typical motor symptoms and involves nigral dopamine degeneration and likely brainstem and olfactory bulb pathology. Detecting loss of dopaminergic integrity during the prodromal period offers the possibility to test potential disease-modifying therapies in the earliest stages of disease.
Studies have highlighted the pathologic, clinical, and imaging manifestations of prodromal PD. Braak has proposed that prodromal neuropathologic changes in PD may begin in extranigral structures, including the olfactory bulb or brainstem nuclei.1–4 Early nonmotor symptoms may precede motor symptoms and allow identification of individuals with early neurodegeneration.5–7 Imaging studies have demonstrated a 40% to 60% loss of dopaminergic neuronal integrity at PD motor symptom threshold.8,9 Studies in unaffected subjects with PD mutations, hyposmia, and a first-degree relative with PD or REM behavior disorder (RBD) demonstrate abnormal dopaminergic imaging in advance of motor symptoms.10–16 Ponsen et al.17 explored sequential biomarker assessment of olfaction and dopamine transporter (DAT) imaging in PD relatives, identifying a small at-risk cohort. In the Parkinson Associated Risk Syndrome (PARS) Study, this approach was expanded to allow larger-scale testing using an at-home test of olfaction to enrich the population for DAT imaging.
Our previous report18 demonstrates that hyposmic subjects were significantly more likely to endorse nonmotor features (anxiety, depression, constipation, and RBD symptoms). This report describes the risk of DAT deficit for subjects with and without hyposmia and the feasibility of utilizing a large, community-based, sequential biomarker assessment strategy to detect prodromal PD.
METHODS
PARS is an ongoing large-scale observational study evaluating a 2-tiered strategy to identify individuals at risk of PD. The study is coordinated at the Institute for Neurodegenerative Disorders (New Haven, CT).
Standard protocol approvals, registrations, and patient consents.
This study received approval by Western Institutional Review Board, the Human Research Protection Office at the US Army Medical Research Material and Command, and the local institutional review boards at participating centers (see list of coinvestigators on the Neurology® Web site at Neurology.org). The Clinicaltrials.gov registry number is NCT00387075.
Study population.
Individuals were recruited by 16 movement disorder clinics through methods previously described.18 Specifically, patients with PD from participating movement disorder clinics were asked to recruit first-degree relatives who completed a demographic form and brief questionnaire to determine eligibility. The remainder of the recruitment included mailings locally to clinics and posting on the PARS Web site or other media. Eligibility criteria included no diagnosis of PD or other neurodegenerative disorder, age older than 50 years (or within 10 years of onset of an affected PD relative), and no known reason for abnormal olfaction (e.g., nasal trauma, sinus infection, sinus surgery). Subjects were informed that they would not receive olfactory or imaging data in this study.
Tier 1 assessments: Olfactory screening and self-report questionnaires.
Eligible subjects were mailed an informed consent form, a self-administered assessment of nonmotor and subtle motor symptoms, and a 40-item University of Pennsylvania Smell Identification Test (UPSIT).18–20 UPSIT raw scores were calculated based on the number of correct identifications. Cohort-specific norms were developed based on the first 2,200 subjects enrolled. Subjects scoring ≤15th percentile based on age and sex were identified as hyposmic and those in the >15th percentile were considered normosmic. A bowel movement frequency questionnaire adapted from the Honolulu Asian Aging Study was used to assess constipation.21 Using this questionnaire, constipation was defined as less than one bowel movement per day. The PD Symptom Rating Scale (SRS)20 was used to assess subtle motor symptoms (e.g., changes in handwriting, gait, ability to get out of a chair). Endorsing 2 or more items on the PD symptom self-report questionnaire was considered positive. RBD was assessed by the RBD questionnaire developed by Comella,22 and scoring was described previously.18 The RBD questionnaire was completed by a bed partner, and subjects experiencing any symptom more than once per month were considered to have RBD.
Tier 2 assessments: Clinical and imaging evaluations.
All hyposmic subjects were invited to participate in the clinical imaging cohort. Normosmic subjects were matched with consenting hyposmic subjects by age (within 5 years) and sex to create a 2:1 ratio of hyposmic/normosmic subjects. All subjects in the clinical imaging cohort provided informed consent and underwent baseline clinical evaluations at the movement disorder clinics. Diagnostic questionnaires were completed by investigators unaware of olfactory status and DAT imaging data. Prodromal PD was defined operationally as the presence of one motor feature (bradykinesia, rigidity, or resting tremor) and one PD nonmotor symptom or the presence of 2 PD nonmotor symptoms (depression, anxiety, cognitive dysfunction, olfactory deficit, excessive sweating, orthostasis, constipation, urinary frequency, or incontinence). Site investigators assigned a best current diagnosis for each subject as no neurologic disease, PD, or parkinsonian syndrome (progressive supranuclear palsy, multiple system atrophy, striatonigral degeneration, corticobasal degeneration, and diffuse Lewy body disease). The parkinsonian syndromes are expected to have DAT loss and may be clinically indistinguishable from PD at an early stage.
DAT imaging using [123I]β-CIT SPECT was performed at a single imaging center (Institute for Neurodegenerative Disorders) using previously described methods.23 At the imaging visit, subjects also underwent a battery of clinical tests including a videotaped Unified Parkinson’s Disease Rating Scale (UPDRS) examination, cognitive and autonomic assessments for heart rate variability, and collection of blood and CSF for future research analyses.
Imaging outcome measure.
Analysis of the SPECT images included evaluation of the striatal binding ratio using a standardized region-of-interest analysis method previously described.24 DAT striatal binding declines with age25 and therefore the imaging outcome was adjusted by determining the percent of age-expected [123I]β-CIT binding in the lowest putamen compared with a previously acquired database of 99 healthy subjects.25,26 Baseline scans were categorized as DAT deficit (≤65% age-expected lowest putamen [123I]β-CIT binding), indeterminate (65%–80% age-expected lowest putamen [123I]β-CIT binding), or no DAT deficit (>80% age-expected lowest putamen [123I]β-CIT binding).25,26 Nuclear medicine experts completed the image analysis masked to all clinical information.
Statistical analyses.
Descriptive statistics including means, SDs, medians, and percentages when appropriate were calculated for the UPSIT, demographic variables, risk factors, imaging, and questions on PD symptoms. Chi-square tests, Cochran-Armitage trend tests, Fisher exact test, and t tests were used to compare groups. Multiple logistic regression was used to evaluate factors in prespecified primary models to predict DAT deficit scan status, and adjusted odds ratios (ORs) were calculated by including additional prodromal features in the primary models.
All p values reported were based on 2-tailed statistical tests. All analyses were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
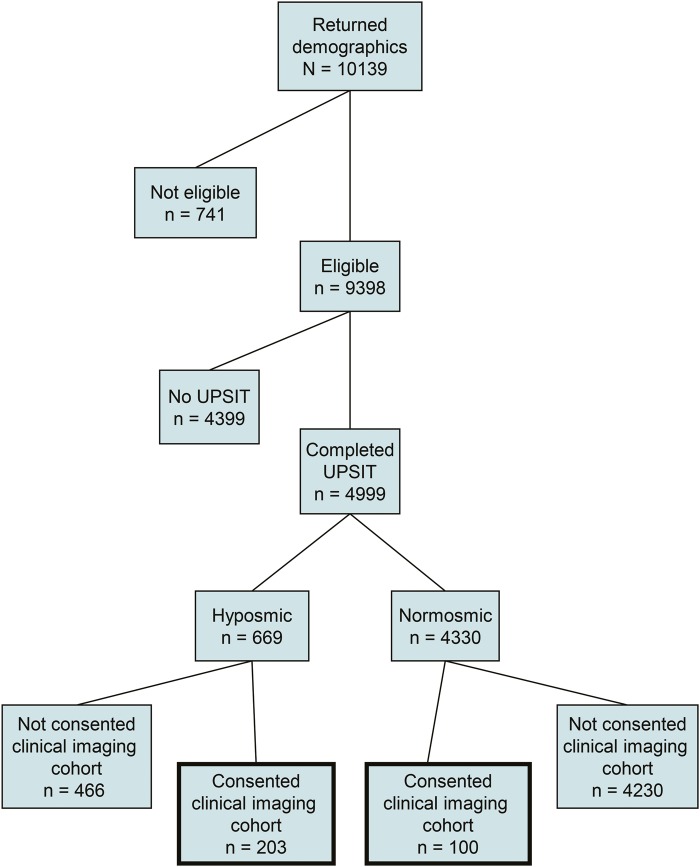
Demographic information was provided by 10,139 individuals. Subsequently, 741 were found to be ineligible because of a diagnosis of PD or other neurologic condition (333), age (319), previous sinus surgery or other sinus condition (72), or other medical concerns.17 Eligible individuals (n = 9,398) were mailed UPSITs and questionnaires, and 4,999 completed and returned these assessments (figure 1). Six hundred sixty-nine of these subjects were categorized as hyposmic, and 203 (30%) consented for participation in clinical and imaging evaluations. One hundred normosmic subjects were matched to the hyposmic subjects for age and sex and consented for tier 2 assessments.
Figure 1. PARS Study flowchart.
Flowchart illustrating the overall design of the PARS Study. PARS = Parkinson Associated Risk Syndrome; UPSIT = University of Pennsylvania Smell Identification Test.
Subject characteristics.
Participating hyposmic subjects (n = 203) were more likely than nonparticipating hyposmic subjects (n = 466) to report a decreased sense of smell (94/202, 47% vs 162/466, 35%) but did not differ by sex (106/203, 52% male vs 249/466, 53% male), age (64.7 ± 8.3 vs 64.4 ± 9.8), smoking status (90/198, 45% never smoked vs 208/459, 45% never smoked), or having one or more first-degree relatives with PD (95/203, 47% vs 196/466, 42%) Participating normosmic subjects (n = 100) did not differ from nonparticipating normosmic subjects (n = 4,230): self-identified decreased sense of smell (10/100, 10% vs 402/4,215, 10%), sex (44/100, 44% male vs 2,156/4,230, 51% male), age (62.9, 10.3 vs 63.9, 9.5), smoking status (52/100, 52% never smoked vs 1,927/4,151, 46% never smoked), and having one or more first-degree relatives with PD (51/100, 51% vs 1,886/4,230, 45%).
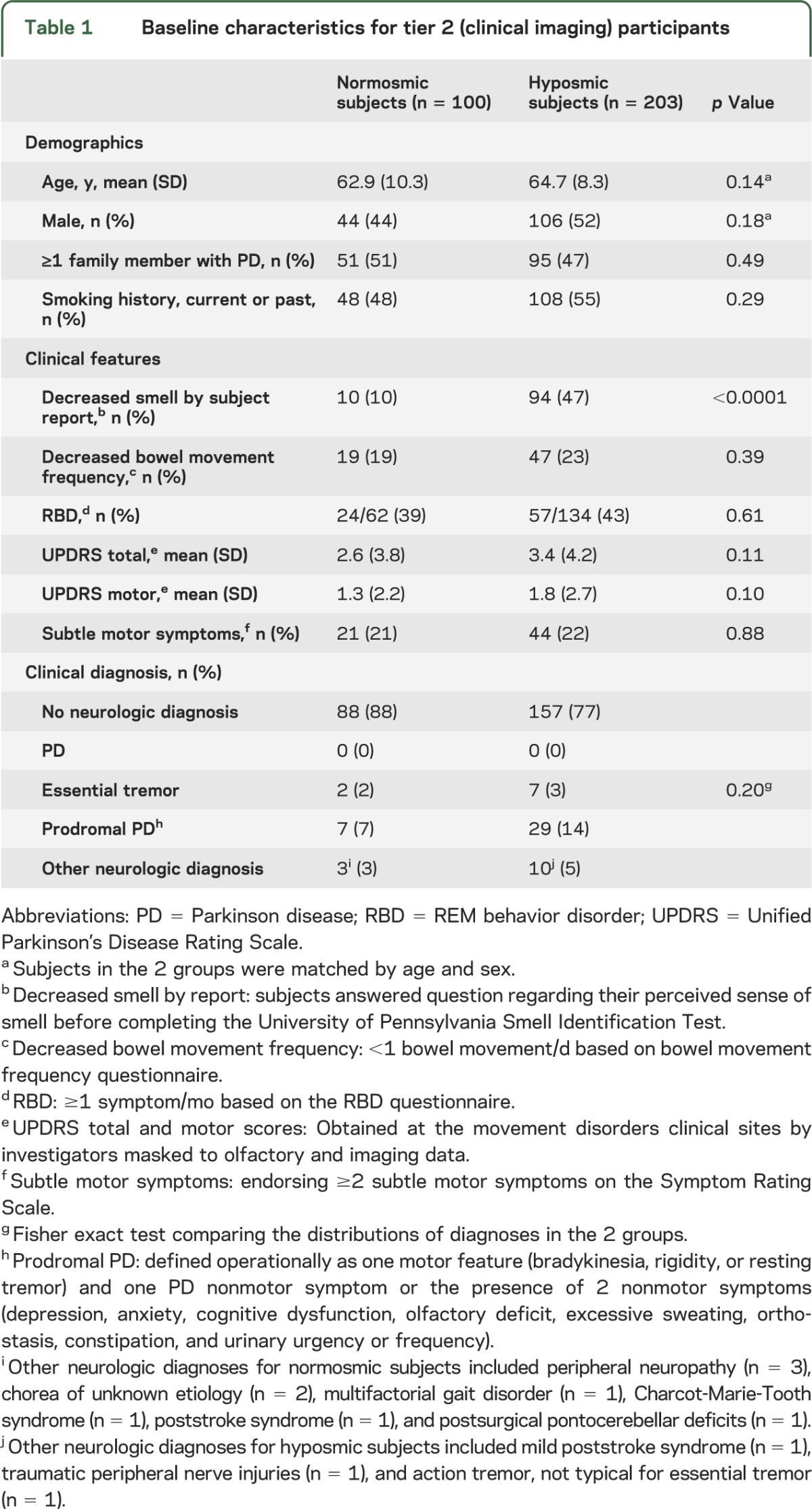
Participants in the tier 2 assessments (table 1) had no significant differences between normosmic and hyposmic subjects regarding age, sex, having a family member with PD, or smoking status. Hyposmic subjects endorsed a decrease in their sense of smell more frequently than normosmic subjects (94/203, 47% vs 10/100, 10%; p < 0.0001). On clinical evaluation, the mean scores for the UPDRS and its subscales were not significantly different between the 2 groups (mean UPDRS score for hyposmic subjects was 3.4 and normosmic subjects 2.6; p = 0.11). None of the participants (hyposmic or normosmic) were given a diagnosis of PD by the clinical investigators at baseline evaluation. No neurologic diagnosis was assigned in 77% of hyposmic subjects compared with 88% of normosmic subjects. A diagnosis of prodromal PD was assigned in a higher percentage of hyposmic subjects (14%) than normosmic subjects (7%).
Table 1.
Baseline characteristics for tier 2 (clinical imaging) participants
[123I]β-CIT SPECT imaging data.
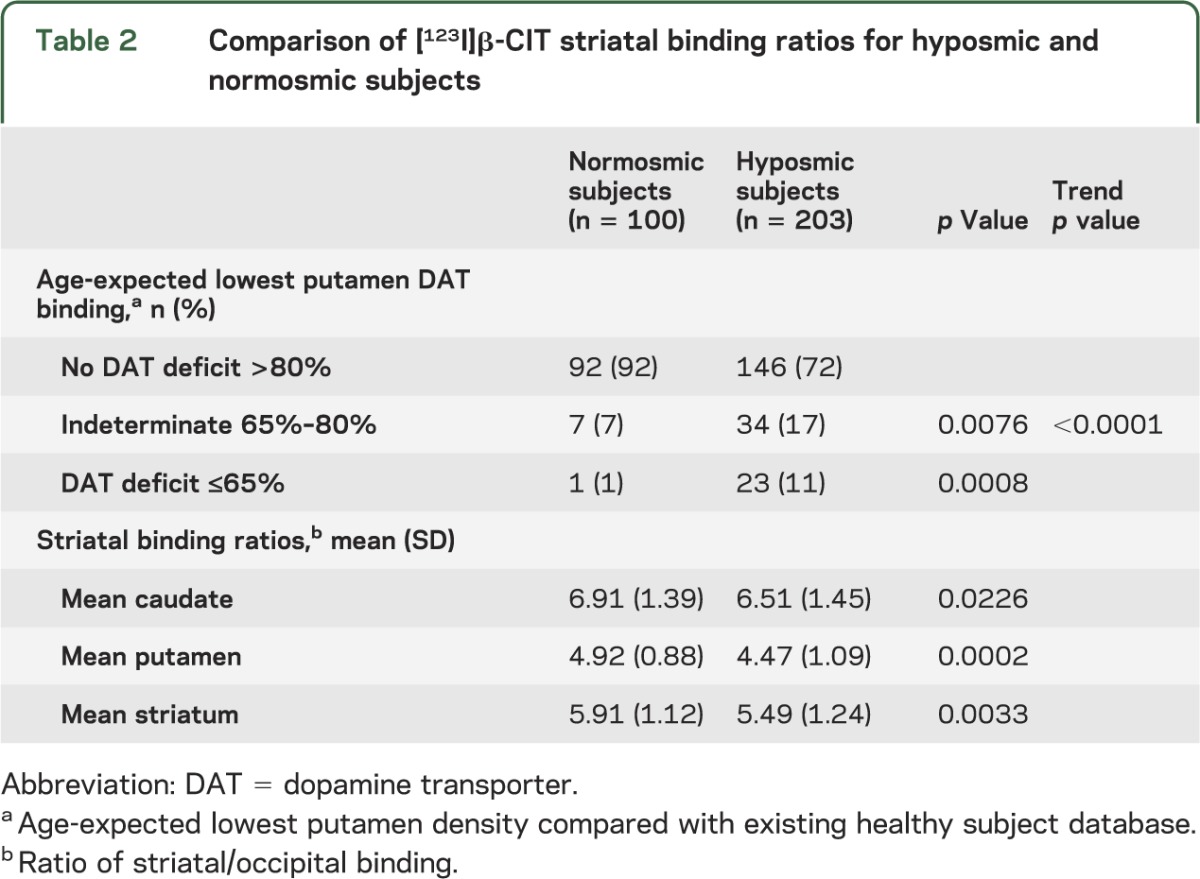
DAT deficit (lowest putamen DAT binding ≤65% of age-expected) was found in 11% (23/203) of hyposmic subjects compared with 1% (1/100) of normosmic subjects, and significantly more hyposmic subjects were classified in the indeterminate range (65%–80% of age-expected binding) compared with normosmic subjects (17% vs 7%, p value for trend <0.0001) (table 2). The mean [123I]β-CIT striatal binding ratio was significantly lower for hyposmic compared with normosmic subjects (p = 0.0033). Similar findings were demonstrated in the mean caudate and putamen.
Table 2.
Comparison of [123I]β-CIT striatal binding ratios for hyposmic and normosmic subjects
Factors predicting DAT deficit in hyposmic subjects.
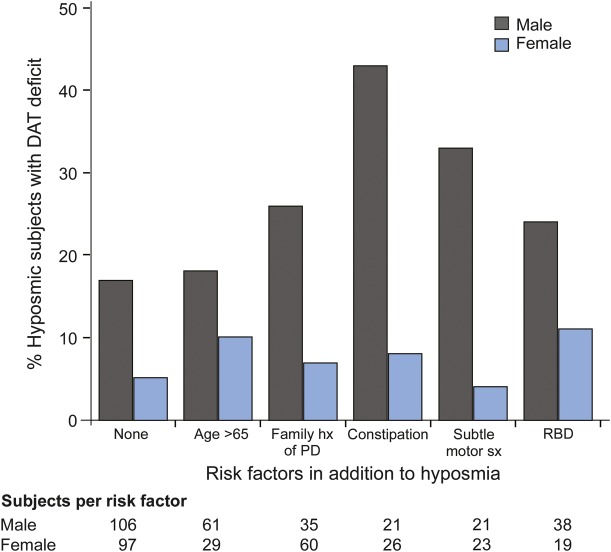
Proportions of hyposmic subjects with a DAT deficit based on a combination of prodromal factors are provided in figure 2. Among hyposmic males, those with constipation have the highest rate of DAT deficit (43%), followed by subtle motor symptoms (endorsing ≥2 items on the SRS) at 33%, family history of PD 25%, and RBD 24%. Hyposmic females with RBD had the highest proportion with DAT deficit (11%), followed by those older than 65 years (10%).
Figure 2. Factors influencing the risk of DAT deficit.
Factors influencing the risk of DAT deficit (≤65% age-expected DAT binding in lowest putamen); family hx of PD = one or more first-degree relatives with PD; constipation ≤1 bowel movement/d; subtle motor sx = endorsing ≥2 symptoms on the PD Symptom Rating Scale; RBD = endorsing any symptom more than once per month. DAT = dopamine transporter; PD = Parkinson disease; RBD = REM behavior disorder.
ORs (95% confidence intervals [CIs]) for factors influencing risk for a DAT deficit of either ≤65% or ≤80% age-expected lowest putamen binding are shown in table 3. Of the factors in the primary models, hyposmia had the highest odds of being associated with a DAT deficit. In a combined logistic regression analysis to predict DAT deficit (≤65% of age-expected lowest putamen DAT binding), hyposmia (OR 12.4; 95% CI 1.6, 96.1), male sex (OR 5.5; 95% CI 1.7, 17.2), and constipation (OR 4.3; 95% CI 1.6, 11.6) were significant predictors, but the CIs are wide because only one normosmic subject showed DAT deficit of ≤65%. Using the same regression model to predict DAT deficit ≤80% of age-expected lowest putamen DAT binding, hyposmia and male sex were the only significant predictors. Age, family history of PD, and subjective report of subtle motor symptoms (endorsing ≥2 items on the SRS) were not predictive of a DAT deficit.
Table 3.
ORs (95% CI) from multiple logistic regression models to predict DAT deficit at the level of ≤65% and ≤80% of age-expected binding
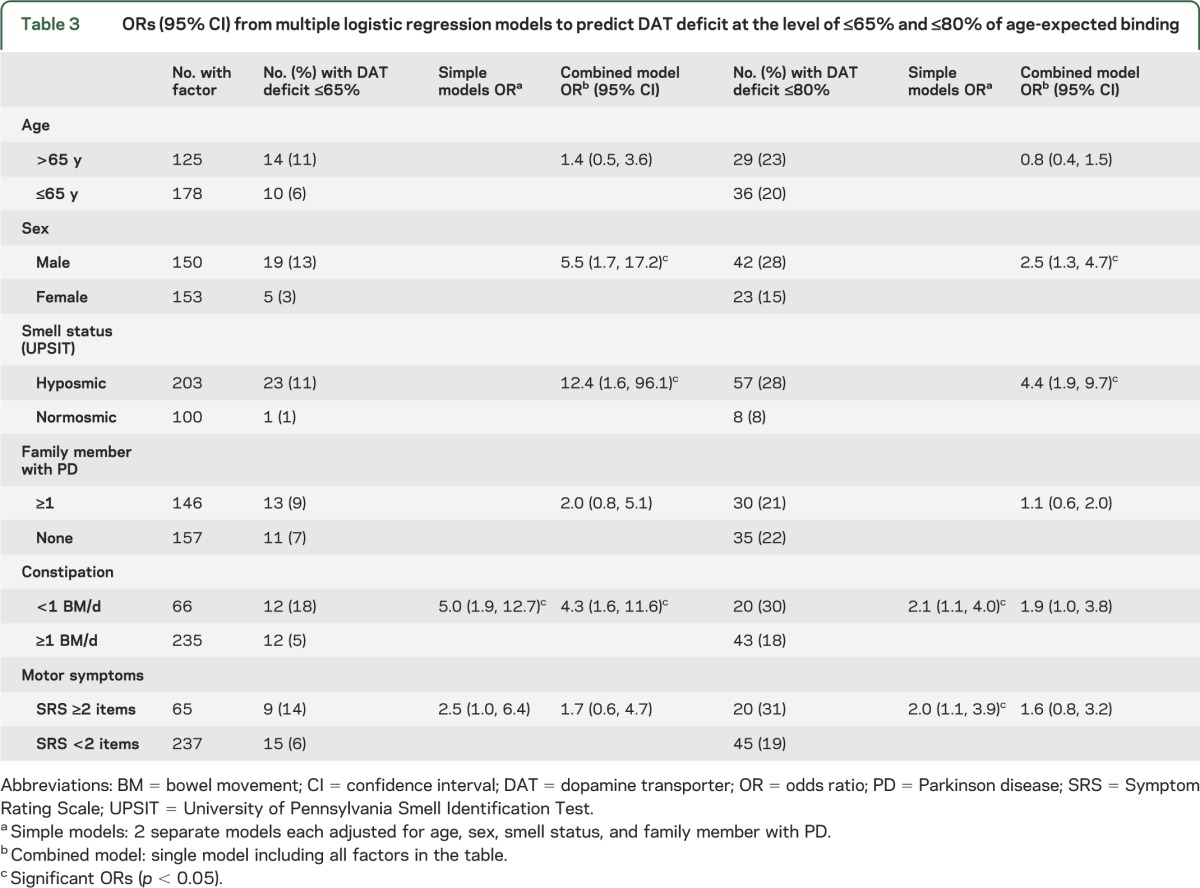
Bed partners completed the RBD questionnaire for only 196 of 301 subjects. In this subset, RBD was not a significant predictor of DAT deficit in either the simple or combined models, and the ORs and significance levels for the other variables were similar to those in table 3 (data not shown).
DISCUSSION
PARS Study baseline data demonstrate that large-scale, at-home olfactory testing can be utilized to identify a cohort of subjects with hyposmia, but not fulfilling clinical motor criteria for a diagnosis of PD, to enrich for detecting DAT deficit.
Among hyposmic subjects, 11% of the cohort demonstrated DAT deficit (≤65% of age-expected lowest putamen binding) compared with 1% among normosmic subjects, suggesting that hyposmia enriches for DAT deficit by about 10-fold. In prior large clinical trials of newly diagnosed PD, such as PRECEPT or ELLDOPA, the cutoff of ≤65% of age-expected [123I]β-CIT binding in the lowest putamen has reliably identified subjects with PD.27 Therefore, PARS subjects with ≤65% DAT binding in the lowest putamen for age are expected to be at high risk of developing manifest PD.28 The PARS cohort continues to be followed longitudinally to determine the timeline for development of possible PD motor signs and other nonmotor features.
A key goal of PARS is to examine the feasibility of identifying a large cohort of at-risk PD subjects that could ultimately be utilized to test therapies with potential to delay or prevent PD onset. This study utilized an inverse pyramid approach, contacting many potential subjects expecting that at subsequent study stages, only a percentage of subjects would continue participation. Using this strategy, PARS began with 10,139 enquiries to identify 24 subjects with DAT deficit (only 0.24% of the original cohort). Despite these small numbers, PARS data have successfully demonstrated the proof of concept that the study design and prescreening assessments are highly feasible, inexpensive, and scalable. Undiagnosed subjects were willing to provide consent and complete PARS tier 1 questionnaires and UPSITs. Hyposmia enriched the cohort for DAT deficit by about 10-fold. While only a modest percentage of hyposmic subjects manifested severe DAT deficit, the data suggest a method to increase the percentage of DAT deficit tier 2 subjects. For example, while 11% of subjects identified by hyposmia alone result in DAT deficit, the percentage of subjects with DAT deficit climbs to >40% when combining factors that increase risk, such as hyposmia, sex, and constipation (figure 2). While these data suggest that combining biomarkers in tier 1 increases the enrichment for DAT deficit, it does so at the potential cost of generalizability. In addition, the result of combining tier 1 biomarkers is that increased sample size may be required for subjects entering the PARS inverse pyramid at the study start to identify the same number of DAT deficit subjects as hyposmia alone. For example, the number of subjects with both hyposmia and constipation completing tier 2 screening (DAT imaging) is 47 compared with 203 for hyposmia alone, and accounts for only 50% of the DAT deficit subjects. Therefore, twice as many subjects would be required to complete tier 1 screening for the combined biomarker strategy to detect the same number with a DAT deficit as hyposmia alone. The PARS pyramid has been designed to scale up the numbers of contacts at the top of the pyramid to increase the DAT deficit sample in tier 2.
PARS data further demonstrate almost a 2.5-fold increase in hyposmic compared with normosmic subjects with lowest putamen DAT binding between 65% and 80% of age-expected binding. In prior clinical trials of newly diagnosed PD, subjects with these scans have been defined as indeterminate because it is unclear whether they will ultimately develop a diagnosis of PD.9,29,30 In PARS, we expect that some of these subjects will demonstrate progressive loss of DAT binding on follow-up imaging.28 Longitudinal assessment of the subjects with indeterminate scans will be particularly informative in refining the DAT imaging cutoff used to define risk of motor PD.
Several limitations of this study need to be acknowledged. Hyposmia was defined in PARS based on PARS data collected using the self-administered UPSIT at home, allowing the possibility that UPSITs may have not been completed independently. However, PARS age and sex normative data closely resemble data obtained as an operator administered test.31,32 In addition, we empirically chose the cutoff of ≤15% as severe hyposmia. This UPSIT cutoff effectively separated individuals with and without DAT deficit, but could be refined as additional longitudinal data become available. It should also be noted that the recruitment strategies used in this study led to approximately 50% of the PARS subjects having a first-degree relative with PD, a factor that has potential to influence the generalizability of our results.
PARS baseline data are consistent with similar studies examining biomarker strategies to define PD risk. The Honolulu Asian Aging Study demonstrates that subjects with constipation and/or hyposmia are more likely to develop PD and manifest incidental Lewy body pathology.21,33 In a recent study, 17 of 43 subjects diagnosed with RBD had abnormal DAT imaging and 6 of 17 developed PD within 2 to 3 years.5 While RBD was not found to predict for DAT deficit in PARS, this may be explained by the limited reliability of the RBD rating scale used. To our knowledge, this scale has not been validated with polysomnogram. The prevalence of RBD in PARS (39% for normosmic and 43% for hyposmic subjects) is higher than other population-based studies,34 suggesting that the scale used was sensitive, but lacked specificity. PARS baseline data from the large tier 1 cohort similarly shows that hyposmic subjects were significantly more likely than normosmic subjects to endorse clinical markers of PD risk including constipation, RBD symptoms, and subtle changes in motor function.18 PARS subjects with hyposmia and DAT deficit in the tier 2 cohort were even more likely than hyposmic subjects without DAT deficit to manifest constipation, RBD, early motor symptoms, and family history of PD consistent with premotor PD. While these exploratory findings need to be confirmed in a larger DAT deficit sample, they provide further evidence that DAT deficit subjects are likely to progress to manifest PD.
In the absence of clear diagnostic criteria for prodromal PD, we operationally defined prodromal PD as described in the methods for the purpose of this study. Given that we could not predict who would develop manifest PD, information regarding risk status was not provided to participants. Even in future studies, careful consideration would need to be given to providing this information given the lack of availability of disease-modifying therapies. We anticipated there would likely be several subjects rated as “prodromal PD,” based on our definition, who would never develop manifest PD. While PARS subjects with hyposmia and DAT deficit were more likely to endorse nonmotor and subtle motor signs on the SRS questionnaire, the SRS lacks specificity for a diagnosis of PD and the endorsed symptoms were not considered diagnostic of PD by the clinical investigators examining these subjects at baseline. The investigators did not assign a diagnosis of PD to any study subject at baseline. While motor signs reported in this study may represent early manifestations of PD not meeting diagnostic criteria, these signs were generally nonspecific and frequently reported in older subjects.35
PARS baseline data demonstrate that subjects with significant DAT loss, but not fulfilling clinical motor criteria for PD, can be identified based on an at-home test of olfaction. These subjects also manifest subtle motor and nonmotor symptoms consistent with, but not diagnostic of, early PD. Longitudinal evaluation of PARS subjects is crucial both to validate the PARS biomarker strategy and elucidate the progression of prodromal PD. PARS olfaction and imaging baseline data provide a critical proof of concept demonstrating that a sequential biomarker approach is a blueprint to assess PD before onset of manifest disease.
Supplementary Material
ACKNOWLEDGMENT
Danna Jennings, Andrew Siderowf, Matthew Stern, John Seibyl, Shirley Eberly, David Oakes, and Kenneth Marek had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis and the decision to submit the manuscript.
GLOSSARY
- CI
confidence interval
- DAT
dopamine transporter
- [123I]β-CIT
iodine-123 labeled 2β-carbomethoxy-3β-(4-iodophenyl)tropane
- OR
odds ratio
- PARS
Parkinson Associated Risk Syndrome
- PD
Parkinson disease
- RBD
REM behavior disorder
- SRS
Symptom Rating Scale
- UPDRS
Unified Parkinson’s Disease Rating Scale
- UPSIT
University of Pennsylvania Smell Identification Test
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Danna Jennings, as the primary author of the manuscript, contributed to the trial design, subject recruitment, and data collection and data analysis. Andrew Siderowf contributed to the development of the protocol, data collection and data analysis, and critical review of the manuscript. Matthew Stern contributed to the study design and data collection and data interpretation. John Seibyl was involved in the analysis and interpretation of the imaging data and review of the manuscript. Shirley Eberly contributed to data analysis and interpretation and critical revision of the manuscript. David Oakes contributed to the analysis and interpretation of data and critical review of the manuscript. Kenneth Marek was involved in the development of the protocol, data collection, data analysis, and review of and contribution to the manuscript.
STUDY FUNDING
Support for this study is provided by the Department of Defense award number W81XWH-06-067.
DISCLOSURE
D. Jennings is an employee of Molecular NeuroImaging, LLC. A. Siderowf is an employee of Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly and Co. M. Stern is a member of the scientific advisory board for Adamas and Civitas. He is a paid consultant to Adamas, Civitas, Ipsen, Merz, NuPathe, and Teva. He has received payment for participating in the educational videos “Parkinson's in the Crossfire” and “Parkinson's Update” funded by Teva and for a lecture series in Australia funded by Novartis. J. Seibyl is a paid consultant for GE Healthcare and has received payment for developing educational presentations for Bayer Healthcare. He holds an equity interest in Molecular NeuroImaging, LLC, and has current grants with the Department of Defense. S. Eberly and D. Oakes report no disclosures relevant to the manuscript. K. Marek has served as a consultant for Pfizer, GE Healthcare, Merck, Eli Lilly, Bristol-Myers Squibb, Piramal, Prothena, NeuroPhage, nLife, and Roche. He holds grants with the Department of Defense and The Michael J. Fox Foundation for Parkinson's Research. Dr. Marek has ownership interest in Molecular NeuroImaging, LLC. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Braak H, Del Tredici K, Rub U, de Vos R, Jansen Steur E, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 2.Halliday G, McCann H. The progression of pathology in Parkinson's disease. Ann NY Acad Sci 2010;1184:188–195. [DOI] [PubMed] [Google Scholar]
- 3.Beach T, Adler C, Lue L, et al. Correlation with nigrostriatal degeneration, cognitive impairment and motor function. Acta Neuropathol 2009;117:613–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beach T, Adler C, Sue L, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 2010;119:689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iranzo A, Valldeoriola F, Lomena F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol 2011;10:797–805. [DOI] [PubMed] [Google Scholar]
- 6.Przuntek H, Muller T, Riederer P. Diagnostic staging of Parkinson's disease: conceptual aspects. J Neural Transm 2004;111:201–216. [DOI] [PubMed] [Google Scholar]
- 7.Lang A, Obeso J. Challenges in Parkinson's disease: restoration of nigrostriatal system is not enough. Lancet Neurol 2004;3:309–316. [DOI] [PubMed] [Google Scholar]
- 8.Parkinson Study Group PRECEPT Investigators. Mixed linkage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology 2007;69:1480–1490. [DOI] [PubMed] [Google Scholar]
- 9.Parkinson Study Group. Levodopa and the progression of Parkinson's disease. N Engl J Med 2004;351:2498–2508. [DOI] [PubMed] [Google Scholar]
- 10.Khan N, Valente EM, Bentivoglio AR, et al. Clinical and subclinical dopaminergic dysfunction in PARK6-linked parkinsonism: an 18F-dopa PET study. Ann Neurol 2002;52:849–853. [DOI] [PubMed] [Google Scholar]
- 11.Adams J, van Netten H, Schulzer M, et al. PET in LRRK2 mutations: comparison to sporadic Parkinson's disease and evidence for presymptomatic compensation. Brain 2005;128:2777–2785. [DOI] [PubMed] [Google Scholar]
- 12.Stiansky-Kolster K, Doerr Y, Moller J, et al. Combination of “idiopathic” REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain 2005;128:126–137. [DOI] [PubMed] [Google Scholar]
- 13.Sossi V, de la Fuente-Fernandez R, Nandhagopal R, et al. Dopamine turnover increases in asymptomatic LRRK2 mutation carriers. Mov Disord 2010;25:2717–2723. [DOI] [PubMed] [Google Scholar]
- 14.Berendse H, Booij J, Francot G, et al. Subclinical dopaminergic dysfunction in asymptomatic Parkinson's disease patients' relatives with a decreased sense of smell. Ann Neurol 2001;50:34–41. [DOI] [PubMed] [Google Scholar]
- 15.Berg D, Godau J, Seppi K, et al. The PRIPS Study: screening battery for subjects at risk for Parkinson's disease. Eur J Neurol 2013;20:102–108. [DOI] [PubMed] [Google Scholar]
- 16.Sierra M, Sanchez-Juan P, Martinez-Rodriguez M, et al. Olfaction and imaging biomarkers in premotor LRRK2 G2019S-associated Parkinson disease. Neurology 2013;80:621–626. [DOI] [PubMed] [Google Scholar]
- 17.Ponsen M, Stofers D, Booij J, et al. Idiopathic hyposmia as a premotor sign of Parkinson's disease. Ann Neurol 2004;56:173–181. [DOI] [PubMed] [Google Scholar]
- 18.Siderowf A, Jennings D, Eberly S, et al. Association between impaired olfaction and other prodromal features of Parkinson's disease in the Parkinson AT-Risk Syndrome (PARS) Study. Mov Disord 2012;27:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984;32:489–502. [DOI] [PubMed] [Google Scholar]
- 20.Tanner C, Ellenberg J, Mayeux R, Ottman R, Langston J. A sensitive and specific screening method for Parkinson's disease. Neurology 1994;32:397–398. [Google Scholar]
- 21.Abbott R, Petrovitch H, White L, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 2001;57:456–462. [DOI] [PubMed] [Google Scholar]
- 22.Comella C, Nardine T, Deiederich N, Stebbins G. Sleep-related violence, injury, and REM sleep behavior disorder in Parkinson's disease. Neurology 1998;51:526–529. [DOI] [PubMed] [Google Scholar]
- 23.Seibyl J, Marek K, Quinlan D, et al. Decreased single-photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson's disease. Ann Neurol 1995;34:589–598. [DOI] [PubMed] [Google Scholar]
- 24.Seibyl J, Marek K, Sheff K, et al. Iodine-123-beta-CIT and iodine-123-FPCIT SPECT measurement of dopamine transporters in healthy subjects and Parkinson's patients. J Nucl Med 1998;39:1500–1508. [PubMed] [Google Scholar]
- 25.van Dyck C, Seibyl J, Malison R, et al. Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry 2002;10:36–43. [PubMed] [Google Scholar]
- 26.Jennings D, Seibyl J, Oakes D, Eberly S, Murphy J, Marek K. [123I] beta-CIT and single photon emission computed tomographic imaging vs clinical evaluation in parkinsonian syndrome. Arch Neurol 2004;61:1224–1229. [DOI] [PubMed] [Google Scholar]
- 27.Marek K, Seibyl J, Eberly S, et al. Longitudinal follow-up of SWEDD subjects in the PRECEPT Study. Neurology 2014;82:1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponsen M, Stoffers D, Wolters E, Booij J, Berendse H. Olfactory testing combined with dopamine transporter imaging as a method to detect prodromal Parkinson's disease. J Neurol Neurosurg Psychiatry 2010;81:396–399. [DOI] [PubMed] [Google Scholar]
- 29.Parkinson Study Group. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology 2007;69:1480–1490. [DOI] [PubMed] [Google Scholar]
- 30.Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA 2002;287:1653–1661. [DOI] [PubMed] [Google Scholar]
- 31.Doty RL, Perl DP, Steele JC, et al. Olfactory dysfunction in three neurodegenerative diseases. Geriatrics 1991;46(suppl 1):47–51. [PubMed] [Google Scholar]
- 32.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 1984;94:176–178. [DOI] [PubMed] [Google Scholar]
- 33.Petrovitch H, Abott R, Ross G, et al. Bowel movement frequency in late-life and substantia nigra neuron density at death. Mov Disord 2009;24:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boeve B, Molano J, Ferman T, et al. Validation of the Mayo Sleep Questionnaire to screen for REM behavior disorder in a community-based sample. J Clin Sleep Med 2013;9:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett D, Beckett L, Murray A, et al. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med 1996;334:71–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.