Abstract
Objective:
To pool data from completed amyotrophic lateral sclerosis (ALS) clinical trials and create an open-access resource that enables greater understanding of the phenotype and biology of ALS.
Methods:
Clinical trials data were pooled from 16 completed phase II/III ALS clinical trials and one observational study. Over 8 million de-identified longitudinally collected data points from over 8,600 individuals with ALS were standardized across trials and merged to create the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database. This database includes demographics, family histories, and longitudinal clinical and laboratory data. Mixed effects models were used to describe the rate of disease progression measured by the Revised ALS Functional Rating Scale (ALSFRS-R) and vital capacity (VC). Cox regression models were used to describe survival data. Implementing Bonferroni correction, the critical p value for 15 different tests was p = 0.003.
Results:
The ALSFRS-R rate of decline was 1.02 (±2.3) points per month and the VC rate of decline was 2.24% of predicted (±6.9) per month. Higher levels of uric acid at trial entry were predictive of a slower drop in ALSFRS-R (p = 0.01) and VC (p < 0.0001), and longer survival (p = 0.02). Higher levels of creatinine at baseline were predictive of a slower drop in ALSFRS-R (p = 0.01) and VC (p < 0.0001), and longer survival (p = 0.01). Finally, higher body mass index (BMI) at baseline was associated with longer survival (p < 0.0001).
Conclusion:
The PRO-ACT database is the largest publicly available repository of merged ALS clinical trials data. We report that baseline levels of creatinine and uric acid, as well as baseline BMI, are strong predictors of disease progression and survival.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder that affects motor neurons in the brain and spinal cord. People with ALS have progressive voluntary muscle weakness involving the arms, legs, speech, swallowing, and breathing.1
Because ALS is a rare disease with an annual incidence of 2/100,000,2 clinical trials have typically been relatively small, with the largest studies including fewer than 1,000 participants.3 Therefore, aggregation of studies is needed to allow enough statistical power to answer important questions about ALS natural history and clinical symptoms in order to overcome some of the barriers associated with drug development for orphan diseases.
The Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database was designed to provide just such an opportunity. This database represents the largest aggregation of ALS clinical trial data available. Sixteen phase II and III ALS trials and one large observational study, conducted over the past 2 decades, are currently included, and this number is expected to increase. The database has been made publicly available with the goal of facilitating research that might leverage its remarkable statistical power for meaningful disease insights. We describe the methods for creating the PRO-ACT database, provide a detailed description of its contents, summarize the baseline (trial entry) characteristics of the population and longitudinal clinical outcomes, and identify novel predictors of disease progression in this large cohort of people with ALS.
METHODS
Database design.
Trials selection.
All phase II and III ALS clinical trials completed between 1990 and 2010 with at least 80 subjects were eligible for inclusion in the PRO-ACT database (figure 1). Key decision makers for each trial were contacted and asked to donate de-identified trial datasets. Standard protocol approvals, registrations, and patient consents were obtained by the participating medical centers. De-identified data from these trials were donated to the PRO-ACT database for research purposes only and under the explicit conditions that Prize4Life would maintain the anonymity of subjects. In the rare cases where donated data were not already anonymized, donated data were further anonymized following the Health Insurance Portability and Accountability Act de-identification conventions for personal health information: any potential patient initials or dates of birth were removed, new randomized subject numbers were created, and wherever possible, trial-specific information was removed in the merging of datasets, including trial center identity and location, trial dates, or other identifying information.
Figure 1. Flow of data processing for inclusion in the PRO-ACT database.
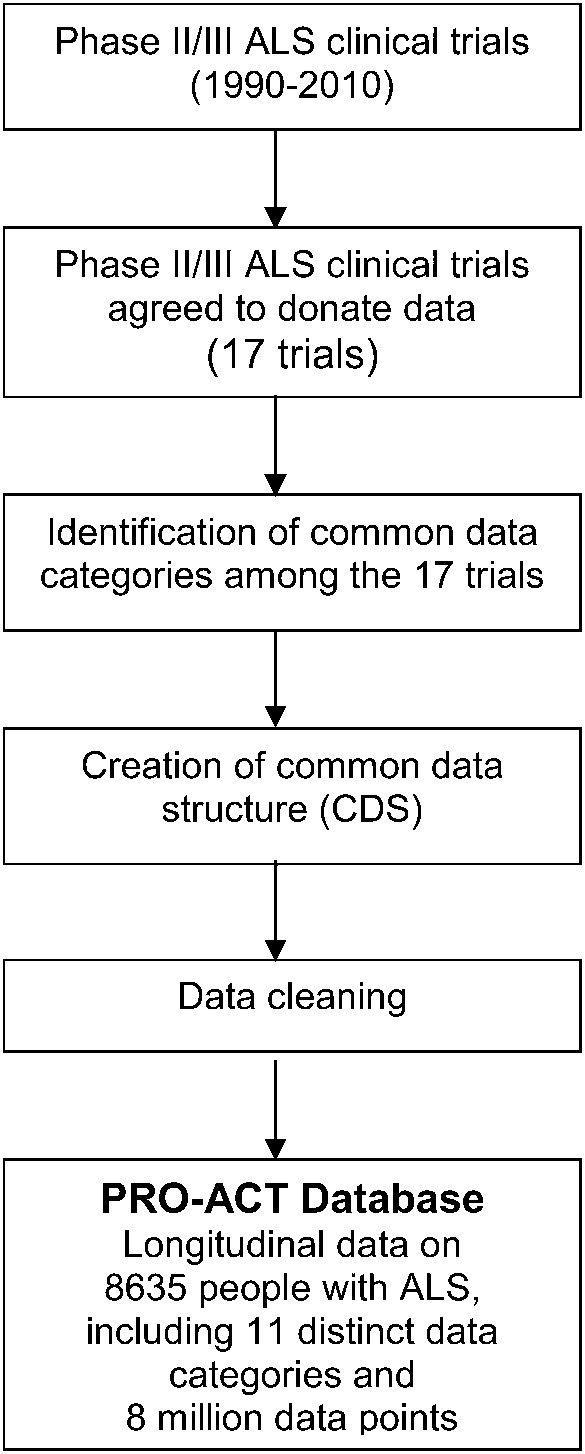
ALS = amyotrophic lateral sclerosis; PRO-ACT = Pooled Resource Open-Access ALS Clinical Trials.
Data categories and common data structure.
Datasets were transmitted electronically and adhered to appropriate data transfer agreements. An initial data review and inventory identified each trial's data categories, data format, and data subset completeness.
The National Institute of Neurological Disorders and Stroke common data elements were used as a starting template to create a comprehensive common data structure (CDS). Data types that were present in at least 4 different studies were included within the CDS of PRO-ACT. Data were then mapped to this final CDS to enable merging of the 16 distinct clinical trials and 1 observational study dataset into a single common data framework.
Data cleaning.
Commonsense data cleaning was performed on ALS Functional Rating Scale (ALSFRS) and laboratory data. Misspelled or synonymous names and units of measurement were merged, and units and text values were standardized. Unreadable or nonsense values (beyond the limits of physiologic possibility) were removed. Supplemental data on the Neurology® Web site at Neurology.org includes detailed description of the cleaning steps and the removed laboratory records.
Data analysis.
Means and percentages were calculated to describe baseline (defined as the time of trial entry) characteristics of the PRO-ACT population including age, sex, race, disease duration, site of onset, vital capacity (VC), ALSFRS, and riluzole use. Mixed effects models were used to describe the rate of disease progression measured by the revised ALSFRS (ALSFRS-R) and VC. Maximum likelihood estimations were used in the mixed effects models to handle missing data on repeated measures.4
Kaplan-Meier and Cox regression models were used to describe the survival data. Both disease duration and diagnosis timelines are available in PRO-ACT, but for the current analysis survival was calculated from the time of trial entry because symptom onset might be vague or inaccurate and because survival estimates from symptom onset might be misleading since they ignore deaths before enrollment. Multivariate analyses, controlling for known baseline predictors of disease progression including disease duration, age, sex, and baseline ALSFRS-R and VC, were conducted to identify predictors of ALSFRS-R, VC, and survival. A total of 15 baseline serum blood tests were preselected for the prediction analyses based on disease mechanisms of interest, including oxidative stress (uric acid), energy metabolism (glucose, creatine kinase, creatinine, cholesterol, triglycerides), inflammation (leukocyte with differential), and respiratory dysfunction (bicarbonate). Implementing Bonferroni correction to control for type I error, the critical p value for 15 different tests would be 0.05/15 (p = 0.003).
RESULTS
Database structure.
Twenty-seven clinical trials satisfied the inclusion criteria described above. Of those, a total of 16 privately and publicly funded phase II and III ALS clinical trials and 1 observational study were donated, most including data from both placebo and treatment arms (table 1). The PRO-ACT dataset currently includes longitudinal data from 8,635 people with ALS who enrolled in 17 different clinical studies with an average duration of 12 months.
Table 1.
Clinical studies included in Pooled Resource Open-Access ALS Clinical Trials
Within these trials, we identified 40 major data categories encompassing 760 different data fields (table e-1). A total of 11 data categories, including treatment assignment, shared by the widest number of different trials were selected for initial cleaning, mapping, merging, and inclusion within the PRO-ACT database (table 2). During data cleaning, 1,031 individual laboratory data records were removed (<0.1%). Baseline (trial entry) characteristics of subjects included in the PRO-ACT database are summarized in table 3. The complete dataset is publicly available online at www.ALSDatabase.org.
Table 2.
Data categories included in Pooled Resource Open-Access ALS Clinical Trials database
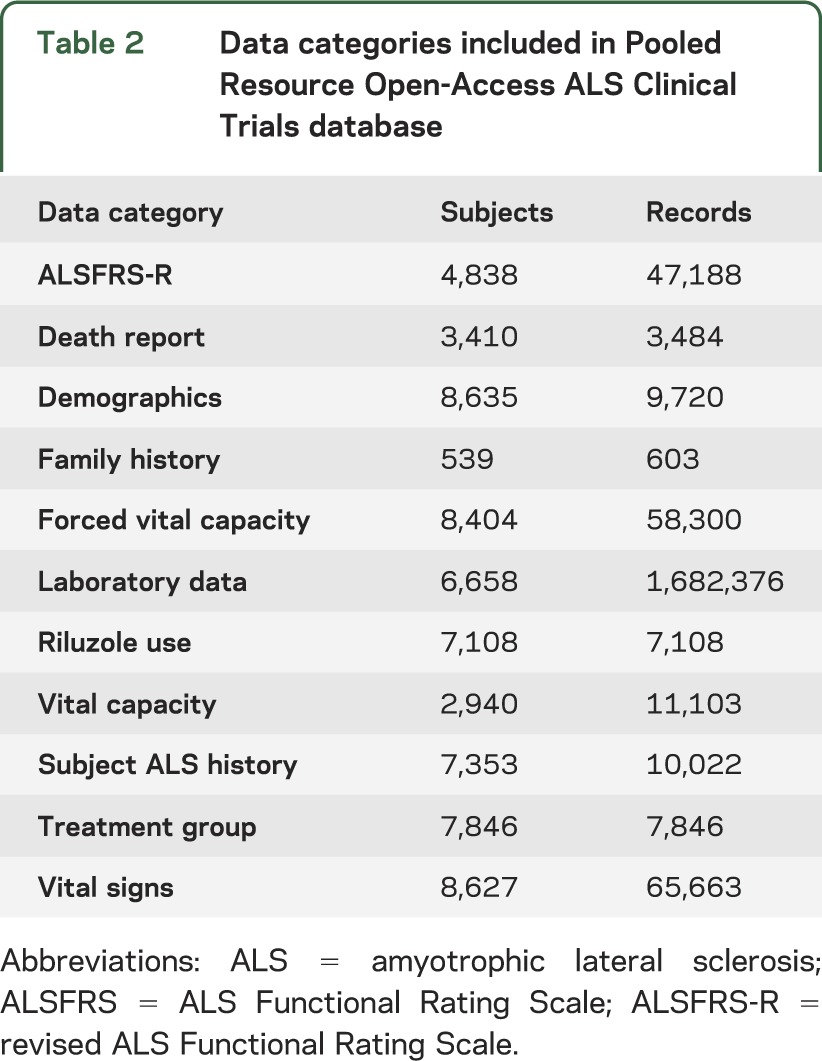
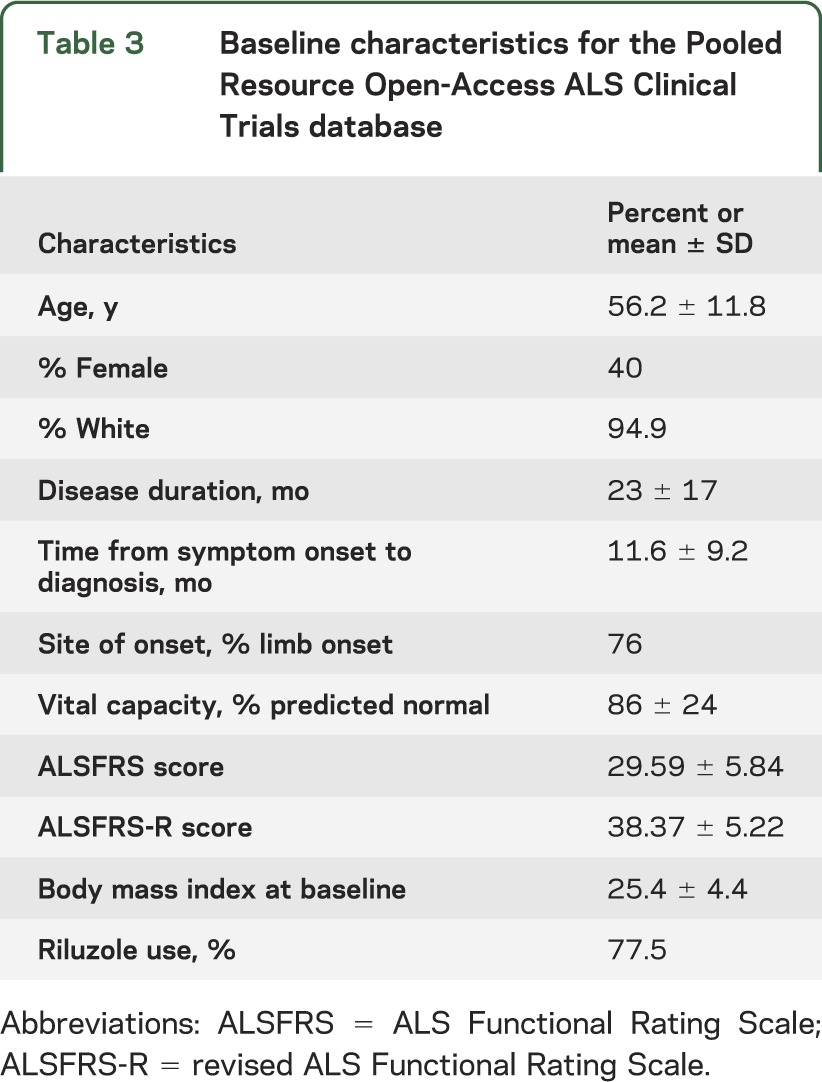
Table 3.
Baseline characteristics for the Pooled Resource Open-Access ALS Clinical Trials database
Predictors of disease progression.
Across all the studies in the database, the mean age at disease onset was 56 years, 40% of participants were female, 76% had limb onset, and riluzole was used by 78% of participants (table 3). The rate of functional decline measured by ALSFRS-R was 1.02 (±2.3) points per month and the rate of respiratory function decline measured by VC was 2.24% of predicted (±6.9) per month. Laboratory values analyzed did not show any discernible trend over time (data not shown).
Baseline levels of creatinine and uric acid at clinical trial onset were identified as independent predictors of rate of ALSFRS-R and VC decline, and of tracheostomy-free survival, after controlling for disease duration, age, sex, and baseline ALSFRS-R and VC in multivariate analysis. Using Bonferroni conservative p value cutoff of p = 0.003, only higher levels of uric acid and creatinine at trial entry continued to be significant predictors of slower drop of VC.
Higher levels of uric acid at the time of trial entry were predictive of a slower drop in ALSFRS-R (0.001 points per each mg/dL increase in uric acid, p = 0.01) and VC (0.006 points per each mg/dL increase in uric acid, p < 0.0001) and of longer survival (0.1% improved survival per each mg/dL increase in uric acid, hazard ratio [HR] 0.999, p = 0.02). Similarly, higher levels of creatinine at trial entry were predictive of a slower drop in ALSFRS-R (0.36 points per each mg/dL increase in creatinine, p = 0.01) and VC (2.1 points per each mg/dL increase in creatinine, p < 0.0001) and longer survival (27% improved survival per each mg/dL increase in creatinine, HR 0.73, p = 0.01).
Higher bicarbonate (p = 0.0047), lower lymphocyte count (p = 0.04), and lower triglyceride (p = 0.035) levels at baseline were associated with faster respiratory decline measured by VC, after adjusting for age, sex, time from symptom onset to diagnosis, and baseline ALSFRS-R and VC. None of these predictors satisfies the Bonferroni-corrected p value of 0.003. Baseline values of chloride, glucose, creatine kinase, total cholesterol, total leukocyte counts, totals or percentages of neutrophils, lymphocytes, or monocytes were not significant predictors of disease progression measured by ALSFRS-R.
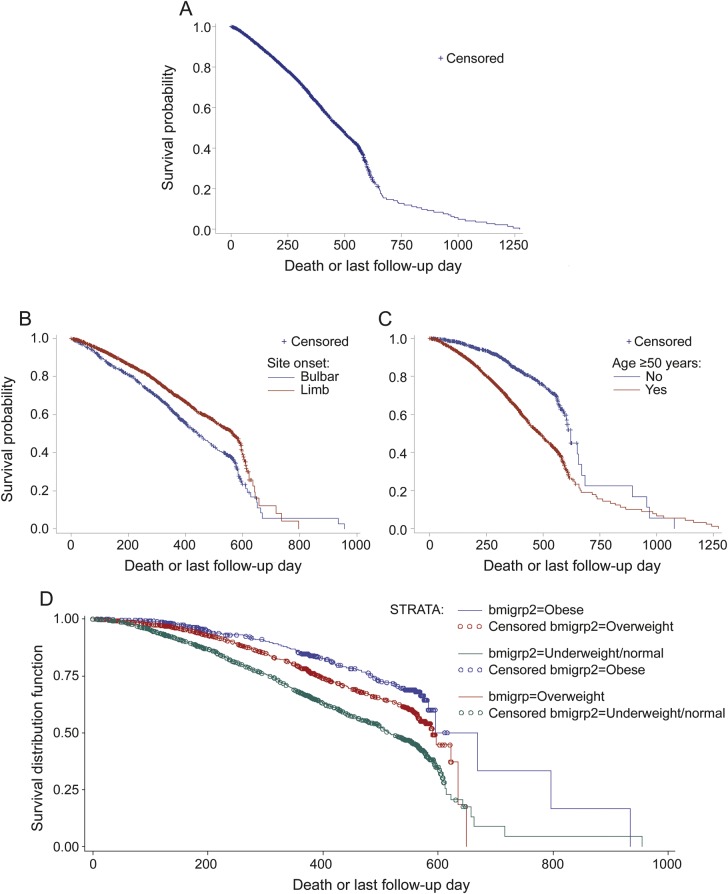
The median survival time in the PRO-ACT database was 479 days (interquartile range 279–622) from trial entry (figure 2A), with bulbar onset (HR 1.24, p = 0.04), older age at symptom onset (HR 2.25, p < 0.0001), and shorter time from symptom onset to diagnosis (HR 0.78, p < 0.0001) serving as independent predictors of shorter survival (figure 2, B and C).
Figure 2. Kaplan-Meier curves, product-limit survival estimates.
(A) Kaplan-Meier curve for all amyotrophic lateral sclerosis (ALS) trials. The median survival time in the Pooled Resource Open-Access ALS Clinical Trials database is 479 days from trial entry. (B) Kaplan-Meier curves for site of onset. People with limb-onset ALS (red) had better survival (p < 0.0001) compared to people with bulbar-onset ALS (blue). This survival advantage for people with limb-onset ALS remained significant (p = 0.04) even after controlling for age, sex, and time from symptom onset to diagnosis. (C) Kaplan-Meier curves for age at onset. People with younger age (<50 years) at ALS onset (blue) show longer survival (p < 0.0001) compared to people with older age (≥50 years) at ALS onset (red). This survival advantage for younger age remained significant (p < 0.0001) even after controlling for site of onset, sex, and time from symptom onset to diagnosis. (D) Kaplan-Meier curves for body mass index (BMI). People who were obese (hazard ratio [HR] 0.46, p < 0.0001) or overweight (HR 0.65, p < 0.0001) at trial entry had less risk of dying compared to people who were underweight or had normal BMI.
Higher body mass index (BMI) at trial entry was associated with longer survival (p < 0.0001) (figure 2D). People who were obese (BMI ≥ 30) showed a 54% lower risk of dying compared to those with normal or low BMI (BMI < 25) (p < 0.0001, HR 0.46, 95% confidence interval [CI] 0.38–0.56). Similarly, people who were overweight (BMI between 25 and 30) showed a 35% lower risk of dying compared to those with normal or low BMI (BMI < 25) (p < 0.0001, HR 0.65, 95% CI 0.58–0.73).
DISCUSSION
The current drug development paradigm is expensive and complicated. Pharmaceutical companies and academic institutions have valuable clinical research data that can be pooled and merged so as to increase their utility. In fact, large pharmaceutical companies have already started creating consortia for sharing clinical trials data, such as Yale Open Data Access and Project DataSphere.5 Similar efforts were instrumental in developing and characterizing a novel clinical outcomes measure for multiple sclerosis6 and raising critical questions about a commonly used, imaging-based surrogate marker of disease progression.7 Data sharing is especially valuable in rare diseases with complex gene–environment interactions and variable disease progression rates such as ALS.
The PRO-ACT database includes 16 ALS clinical trials, 1 large observation study, and over 8 million de-identified longitudinally collected data points from over 8,600 people with ALS. The baseline characteristics and rates of decline of ALSFRS-R and VC for the PRO-ACT population align well with the data from the largest clinical reports.8 This database is a valuable ALS research tool for any investigator interested in ALS clinical research.9 The PRO-ACT database holds the potential to provide insight into ALS phenotypes, underlying biology, novel biomarkers, and predictors of disease progression.10 Furthermore, the PRO-ACT database could serve as a source of well-matched historical controls for ALS trials and offer ways to improve future ALS clinical trial design through trial simulations and the identification of novel stratifiers/subpopulations, leading to more informed treatment assignment and more nuanced inclusion/exclusion criteria. The recent development of outcome prediction algorithms through the ALS Prediction Prize4Life crowdsourcing challenge is just one example of how the large sample size of this database can be leveraged to create important new clinical research tools.
The PRO-ACT database allows us to examine new and emerging predictive features that can help shed light on disease mechanisms and patient subgroups, and point to potential biomarkers of ALS progression. Our analyses of baseline laboratory values suggest that higher levels of creatinine and uric acid at the time of trial entry are predictors of slower decline in disease progression and longer survival time. These results provide evidence of the prognostic value of uric acid and creatinine, and verify previously published reports.11,12 Oxidative stress is one of the proposed mechanisms of ALS13,14 and uric acid is a potent antioxidant that could play a role in reducing oxidative stress.11 Levels of uric acid and creatinine have been reported to be generally lower in people with ALS compared to healthy controls, and previously published small studies have reported correlations between higher uric acid levels and slower disease progression and longer survival.11,12,15 It is particularly interesting that higher uric acid levels have also been proposed as a neuroprotective treatment for Parkinson disease16–18 and Huntington disease.19 A safety and tolerability trial of inosine, which increases endogenous uric acid levels, in Parkinson disease (Safety of Urate Elevation in Parkinson's Disease [SURE-PD]) was recently completed (clinicaltrials.gov; NCT00833690). Given the emerging data about the protective benefits of higher uric acid in Parkinson disease and the data confirming the correlation between higher uric acid and slower disease progression in our analysis, the benefit of increasing endogenous levels of uric acid using inosine in patients with ALS should be evaluated.
Our analyses using the PRO-ACT database also provide further evidence to support the prognostic value of BMI in ALS. Biochemical pathways associated with hypermetabolism have been associated with ALS20 and our data suggest that a higher BMI at baseline is an independent predictor of longer survival in ALS, though it does not appear to directly predict the rate of decline of ALSFRS-R or VC. This result confirms and extends previously published studies on the association between BMI and survival in people with ALS.21 A phase II clinical trial aimed at exploring the safety and benefit of a high fat/high calorie diet in ALS was recently completed (clinicaltrials.gov; NCT00983983) and our BMI finding supports further research into the link among nutrition, weight, and ALS progression.
Large clinical trial databases can provide important insights into the natural history of diseases and offer great power to detect small but meaningful signals. One of the strengths of a large database is to identify important disease pathways even when the effect sizes are small, especially in a highly heterogeneous disease like ALS. This concept applies to all large clinical studies and the interpretation of results should always take the effect size into consideration.
The PRO-ACT database also has limitations. Numerous studies have highlighted key differences between the population of patients with ALS participating in clinical trials and clinic-based populations.22,23 Compared to the general ALS patient population, the clinical trials population tends to be younger, with more limb onset and better prognosis. This selection bias is true for all clinical trials and is usually attributed to the inclusion and exclusion criteria of the trials and to any additional factors that can influence the enrollment decision made by the patients and investigators.
Compared to the ALS Patient Care Database, which included 1,857 clinic-based patients with ALS enrolled at 83 clinics,24 PRO-ACT subjects (8,635) were approximately 2 years younger (PRO-ACT 56.2 years; ALS Patient Care Database 58.6 years) and had shorter time from symptom onset to diagnosis (PRO-ACT 11.6 months; ALS Patient Care Database 14 months). The percentage of female subjects was similar (PRO-ACT 40%; ALS Patient Care Database 41%). The ALS Patient Care Database represents a clinic-based population, which could also be different from the general ALS population. Investigators planning on using the PRO-ACT database should be aware of the differences between the clinical trials ALS population and general clinic populations.
Whereas the PRO-ACT database includes a rich variety of data, there are many important missing data types. For example, cognitive status, genetic data, and the use of ambulatory assistive devices are not included in the PRO-ACT database.
The PRO-ACT database is the largest publicly available repository of longitudinal ALS clinical trials data. Preliminary analysis confirms the utility of a large clinical trial data collection for both hypothesis generation and confirmation. Our analyses of the data support the correlation of higher baseline BMI, creatinine, and uric acid with better prognosis. The database is publicly available and will continue to support analyses that can move the field of ALS research forward in critical and unpredictable directions.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Prize4Life for initiating and leading this effort to collect, merge, and widely share ALS clinical trials datasets; the NCRI for bringing PRO-ACT to life; and the thousands of people with ALS who participated in the ALS clinical trials that make up the PRO-ACT database.
GLOSSARY
- ALS
amyotrophic lateral sclerosis
- ALSFRS
ALS Functional Rating Scale
- ALSFRS-R
revised ALS Functional Rating Scale
- BMI
body mass index
- CDS
common data structure
- CI
confidence interval
- HR
hazard ratio
- PRO-ACT
Pooled Resource Open-Access ALS Clinical Trials
- VC
vital capacity
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Nazem Atassi: study concept and design, critical revision of the manuscript for important intellectual content. James Berry: study concept and design, critical revision of the manuscript for important intellectual content. Amy Shui: data analysis. Neta Zach: study concept and design, acquisition and standardization of data, data analysis, critical revision of the manuscript for important intellectual content. Alexander Sherman: acquisition and standardization of data. Ervin Sinani: acquisition and standardization of data. Jason Walker: acquisition and standardization of data. Igor Katsovskiy: acquisition and standardization of data. David Schoenfeld: data analysis and critical revision of the manuscript for important intellectual content. Merit Cudkowicz: critical revision of the manuscript for important intellectual content. Melanie Leitner: acquisition and standardization of data, study concept and design, data analysis, revision of the manuscript for important intellectual content.
STUDY FUNDING
The authors thank the ALS Therapy Alliance (ATA) for funding this project, the companies that donated clinical trials data, and the Northeast ALS (NEALS) consortium for providing a number of de-identified clinical trials datasets.
DISCLOSURE
N. Atassi received fellowship grants from the American Academy of Neurology (AAN), Muscular Dystrophy Association (MDA), and the Anne Young Fellowship, has research grants from the Harvard Neuro-Discovery Center and ALS Therapy Alliance (ATA), and provided consulting for Biogen IDEC. J. Berry received fellowship grants from the American Academy of Neurology (AAN), Muscular Dystrophy Association (MDA), has research grants from ALS Therapy Alliance (ATA), and provided consulting for Biogen IDEC. A. Shui reports no disclosures relevant to the manuscript. N. Zach is an employee of the 501(c)3 nonprofit Prize4Life. A. Sherman, E. Sinani, J. Walker, I. Katsovskiy, and D. Schoenfeld report no disclosures relevant to the manuscript. M. Cudkowicz served on the data and safety monitoring board for Synapse and Trophos and was a consultant for TEVA, Millennium, GlaxoSmithKline, Biogen Idec, and Cytokinetics, Inc. M. Leitner is an employee of the 501(c)3 nonprofit Prize4Life. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rowland L, Shneider N. Amyotrophic lateral sclerosis. N Engl J Med 2001;344:1688–1700. [DOI] [PubMed] [Google Scholar]
- 2.McGuire V, Longstreth W, Jr, Koepsell T, van Belle G. Incidence of amyotrophic lateral sclerosis in three counties in western Washington state. Neurology 1996;47:571–573. [DOI] [PubMed] [Google Scholar]
- 3.Cudkowicz M, Katz J, Moore D, et al. Toward more efficient clinical trials for amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2010;11:259–265. [DOI] [PubMed] [Google Scholar]
- 4.Molenberghs G, Kenward MG. The Direct Likelihood Method: Missing Data in Clinical Studies. Hoboken, NJ: John Wiley & Sons; 2007:75–92. [Google Scholar]
- 5.Bhattacharjee Y. Pharma firms push for sharing of cancer trial data. Science 2012;338:29. [DOI] [PubMed] [Google Scholar]
- 6.Fischer JS, Rudick RA, Cutter GR, Reingold SC; Force NMSCOAT. The Multiple Sclerosis Functional Composite measure (MSFC): an integrated approach to MS clinical outcome assessment. Mult Scler 1999;5:244–250. [DOI] [PubMed] [Google Scholar]
- 7.Daumer M, Neuhaus A, Morrissey S, Hintzen R, Ebers GC. MRI as an outcome in multiple sclerosis clinical trials. Neurology 2009;72:705–711. [DOI] [PubMed] [Google Scholar]
- 8.Gordon PH, Salachas F, Lacomblez L, et al. Predicting survival of patients with amyotrophic lateral sclerosis at presentation: a 15-year experience. Neurodegenerative Dis 2013;12:81–90. [DOI] [PubMed] [Google Scholar]
- 9.Sherman AV, Gubitz AK, Al-Chalabi A, et al. Infrastructure resources for clinical research in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:53–61. [DOI] [PubMed] [Google Scholar]
- 10.Gomeni R, Fava M. Amyotrophic lateral sclerosis disease progression model. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:119–129. [DOI] [PubMed] [Google Scholar]
- 11.Keizman D, Ish-Shalom M, Berliner S, et al. Low uric acid levels in serum of patients with ALS: further evidence for oxidative stress? J Neurol Sci 2009;285:95–99. [DOI] [PubMed] [Google Scholar]
- 12.Paganoni S, Zhang M, Quiroz Zárate A, et al. Uric acid levels predict survival in men with amyotrophic lateral sclerosis. J Neurol 2012;259:1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogdanov M, Brown RH, Matson W, et al. Increased oxidative damage to DNA in ALS patients. Free Radic Biol Med 2000;29:652–658. [DOI] [PubMed] [Google Scholar]
- 14.Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med 2010;48:629–641. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda K, Hirayama T, Takazawa T, Kawabe K, Iwasaki Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern Med 2012;51:1501–1508. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Wu G, Schwarzschild M. Urate in Parkinson's disease: more than a biomarker? Curr Neurol Neurosci Rep 2012;12:367–375. [DOI] [PubMed] [Google Scholar]
- 17.Ascherio A, LeWitt PA, Xu K, et al. URate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol 2009;66:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol 2008;65:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auinger P, Kieburtz K, McDermott MP. The relationship between uric acid levels and Huntington's disease progression. Mov Disord 2010;25:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawton K, Cudkowicz M, Brown M, et al. Biochemical alterations associated with ALS. Amyotroph Lateral Scler 2012;13:110–118. [DOI] [PubMed] [Google Scholar]
- 21.Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills A-M. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve 2011;44:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beghi E, Millul A, Logroscino G, Vitelli E, Micheli A, Group FTS. Outcome measures and prognostic indicators in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2008;9:163–167. [DOI] [PubMed] [Google Scholar]
- 23.Chiò A, Canosa A, Gallo S, et al. ALS clinical trials: do enrolled patients accurately represent the ALS population? Neurology 2011;77:1432–1437. [DOI] [PubMed] [Google Scholar]
- 24.Miller RG, Anderson FA, Bradley WG, et al. The ALS patient care database: goals, design, and early results. Neurology 2000;54:53. [DOI] [PubMed] [Google Scholar]
- 25.Cudkowicz ME, Shefner JM, Simpson E, et al. Arimoclomol at dosages up to 300 mg/day is well tolerated and safe in amyotrophic lateral sclerosis. Muscle Nerve 2008;38:837–844. [DOI] [PubMed] [Google Scholar]
- 26.Shefner JM, Cudkowicz ME, Schoenfeld D, et al. A clinical trial of creatine in ALS. Neurology 2004;63:1656–1661. [DOI] [PubMed] [Google Scholar]
- 27.Cudkowicz ME, Shefner JM, Schoenfeld DA, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol 2006;60:22–31. [DOI] [PubMed] [Google Scholar]
- 28.Miller R, Moore DH, Young L, Group WS. Placebo-controlled trial of gabapentin in patients with amyotrophic lateral sclerosis. Neurology 1996;47:1383–1388. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal SP, Zinman L, Simpson E, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.BDNF Study Group (Phase III). A controlled trial of recombinant methionyl human BDNF in ALS. Neurology 1999;52:1427–1433. [DOI] [PubMed] [Google Scholar]
- 31.ALS CNTF Treatment Study (ACTS) Group. A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. Neurology 1996;46:1244–1249. [DOI] [PubMed] [Google Scholar]
- 32.Bensimon G, Lacomblez L, Meininger V; The ALSRSG. A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med 1994;330:585–591. [DOI] [PubMed] [Google Scholar]
- 33.Bensimon G, Lacomblez L, Delumeau JC, Bejuit R, Truffinet P, Meininger V. A study of riluzole in the treatment of advanced stage or elderly patients with amyotrophic lateral sclerosis. J Neurol 2002;249:609–615. [DOI] [PubMed] [Google Scholar]
- 34.Miller R, Bradley W, Cudkowicz M, et al. Phase II/III randomized trial of TCH346 in patients with ALS. Neurology 2007;69:776–784. [DOI] [PubMed] [Google Scholar]
- 35.Pascuzzi RM, Shefner J, Chappell AS, et al. A phase II trial of talampanel in subjects with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2010;11:266–271. [DOI] [PubMed] [Google Scholar]
- 36.Cudkowicz ME, Shefner JM, Schoenfeld DA, et al. A randomized, placebo-controlled trial of topiramate in amyotrophic lateral sclerosis. Neurology 2003;61:456–464. [DOI] [PubMed] [Google Scholar]
- 37.Paillisse C, Lacomblez L, Dib M, Bensimon G, Garcia‐Acosta S, Meininger V. Prognostic factors for survival in amyotrophic lateral sclerosis patients treated with riluzole. Amyotroph Lateral Scler 2005;6:37–44. [DOI] [PubMed] [Google Scholar]
- 38.Desnuelle C, Dib M, Garrel C, Favier A. A double-blind, placebo-controlled randomized clinical trial of alpha-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis: ALS Riluzole-Tocopherol Study Group. Amyotroph Lateral Scler Other Mot Neuron Disord 2001;2:9–18. [DOI] [PubMed] [Google Scholar]
- 39.Meininger V, Bensimon G, Bradley WG, et al. Efficacy and safety of xaliproden in amyotrophic lateral sclerosis: results of two phase III trials. Amyotroph Lateral Scler Other Mot Neuron Disord 2004;5:107–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.