Abstract
Objective:
To compare rates of intracerebral hemorrhage (ICH) in HIV-infected and uninfected individuals in a large clinical care cohort and to assess risk factors associated with ICH.
Methods:
We identified incident ICH in HIV-infected and uninfected control cohorts from the Partners Health Care system using ICD-9-CM codes. We constructed Cox proportional hazards models to estimate adjusted hazard ratios for HIV infection and other predictors of ICH.
Results:
The incidence rate of ICH was 2.29 per 1,000 person-years in HIV-infected individuals compared with 1.23 per 1,000 person-years in uninfected individuals, with an unadjusted incidence rate ratio of 1.85 (95% confidence interval 1.37–2.47, p < 0.001). In a multivariable model, HIV infection was independently associated with a higher hazard of ICH, although its effect diminished with increasing age. Female sex was associated with a lower hazard of ICH in the uninfected cohort but not in the HIV cohort. CD4 count <200 × 106 cells/L and anticoagulant use were predictive of ICH.
Conclusions:
HIV infection conferred an increased adjusted hazard of ICH, which was more pronounced in young patients and in women.
Cardiovascular and cerebrovascular event rates are increased in HIV infection.1–7 Several studies pointing to an elevated risk of cerebrovascular disease in HIV-infected individuals have focused on ischemic stroke and excluded intracranial hemorrhage.5,8,9 Whereas ischemic stroke and cardiovascular disease often coexist and share a similar risk profile and underlying mechanism, the association between HIV and intracerebral hemorrhage (ICH), which has mechanisms distinct from ischemic stroke, is less clear. We examined the association of HIV with the risk of ICH by comparing the incidence of ICH in HIV-infected and uninfected individuals in a large US clinical care cohort. Our primary hypothesis was that the rate of ICH in HIV-infected individuals would be higher than in uninfected individuals. We further controlled for potential confounders and identified predictors of ICH in HIV-infected individuals.
METHODS
Study design and patient population.
We conducted an observational study of an HIV cohort derived from the Partners HealthCare System Research Patient Data Registry (RPDR), a clinical care database of all inpatient and outpatient encounters from Massachusetts General Hospital and Brigham and Women's Hospital. Other vascular outcomes have been studied in this cohort.3,5 HIV-infected individuals were identified from the RPDR using ICD-9-CM codes 042 or V08, previously validated in the cohort.5 The HIV cohort was matched by age, race, and sex in a 1:10 ratio to HIV-uninfected individuals, who constituted the control cohort. The control cohort was intentionally overpopulated to allow for the possibility of missing data and application of the exclusion criteria. Within the cohorts, individuals who were at least 18 years of age at the beginning of the observation period and who had at least 1 inpatient or 2 outpatient clinical encounters were eligible for the study. The observation period began on the latest of January 1, 1996, date of first ICD-9-CM code for HIV, or the first encounter for controls. Individuals were censored at the earliest of date of ICH, date of last encounter, or December 31, 2009.
Standard protocol approvals, registrations, and patient consents.
The Partners Human Research Committee and University of California, San Francisco Committee on Human Research approved the study.
Outcomes.
We identified the primary endpoint of first-ever ICH using ICD-9-CM code 431. This ICD-9-CM code has been previously demonstrated in a large administrative database to be 82% sensitive and 93% specific with positive predictive value of 80%.10 Individuals with concomitant ICD-9-CM codes for skull fracture or intracranial injury (800.xx–801.xx, 803.xx–804.xx, 851.xx–854.xx) from the same encounter were excluded. Patients were excluded from the study if an ICD-9-CM code for ICH occurred prior to the first ICD-9-CM code for HIV or before the start of the observation period.
Comorbid conditions that might impact the risk of ICH were also identified by ICD-9-CM codes documented at any time during the observation period, including hypertension (401.xx), diabetes (250.xx), chronic kidney disease (CKD) (585.1–6, 9; 403.xx, 404.xx), and endocarditis (421.xx, 424.9x). Ascertainment of hypertension and diabetes using ICD-9-CM codes has been previously validated in this cohort.11 Smoking status was ascertained by natural language processing, as described previously.12 Patients with no smoking-related documentation in the medical record were considered nonsmokers. We also obtained medication data on statins, antiplatelet agents, and anticoagulants. HIV parameters, including a history of any antiretroviral therapy (ART) use (ever vs never use by the end of the observation period), most recent CD4 count before the end of the observation period, CD4 count <200 × 106 cells/L, and detectable HIV viral load (HIV RNA ≥ 400 copies/mL) at any time during the observation period were determined.
Statistical analysis.
We calculated age- and sex-stratified incidence rates of ICH per 1,000 person-years (PY) of follow-up time in the HIV and control cohorts, using incidence rate ratios to compare rates between cohorts. We constructed a multivariable Cox proportional hazards regression analysis to model time to incident ICH, measured in years of age with the age at the start of the study being a “late entry” time, for the entire cohort of HIV-infected and uninfected individuals, adjusting for demographics and potential ICH-related factors. For the HIV cohort with available CD4 and viral load data (n = 2,278), we constructed a second multivariable Cox regression model of time to incident ICH, again measured in years of age. Given the smaller number of events (n = 26), we included only clinically relevant variables in a forward stepwise selection process. In each model, we tested the proportional hazards assumption by plotting scaled Schoenfeld residuals for each predictor against time, in addition to performing a test of nonzero slopes. We modeled variables with strong evidence for nonproportional effects on the hazard of ICH by allowing their effects to change with age. Because Cox regression does not explicitly estimate the effect of time (in our case age) on the risk of the outcome, we performed an auxiliary analysis fitting a parametric Weibull model to obtain a simple quantitative estimate of the effect of age among HIV-infected individuals. We used the STATA streg command and extracted the hazard ratio (HR) for age 60 vs age 40 to illustrate the estimated magnitude of the influence of age on hazard of ICH. For sensitivity analyses where we evaluated the effect of the addition of a variable to a model, a predetermined change-in-coefficient threshold of 10% was considered significant. All p values were 2-sided with <0.05 considered statistically significant for testing the primary hypothesis that HIV is associated with ICH after controlling for potential confounders. Statistical analyses were performed using Stata (StataCorp 2012; Stata Statistical Software: release 12; Stata Corporation, College Station, TX).
RESULTS
Demographic and clinical characteristics.
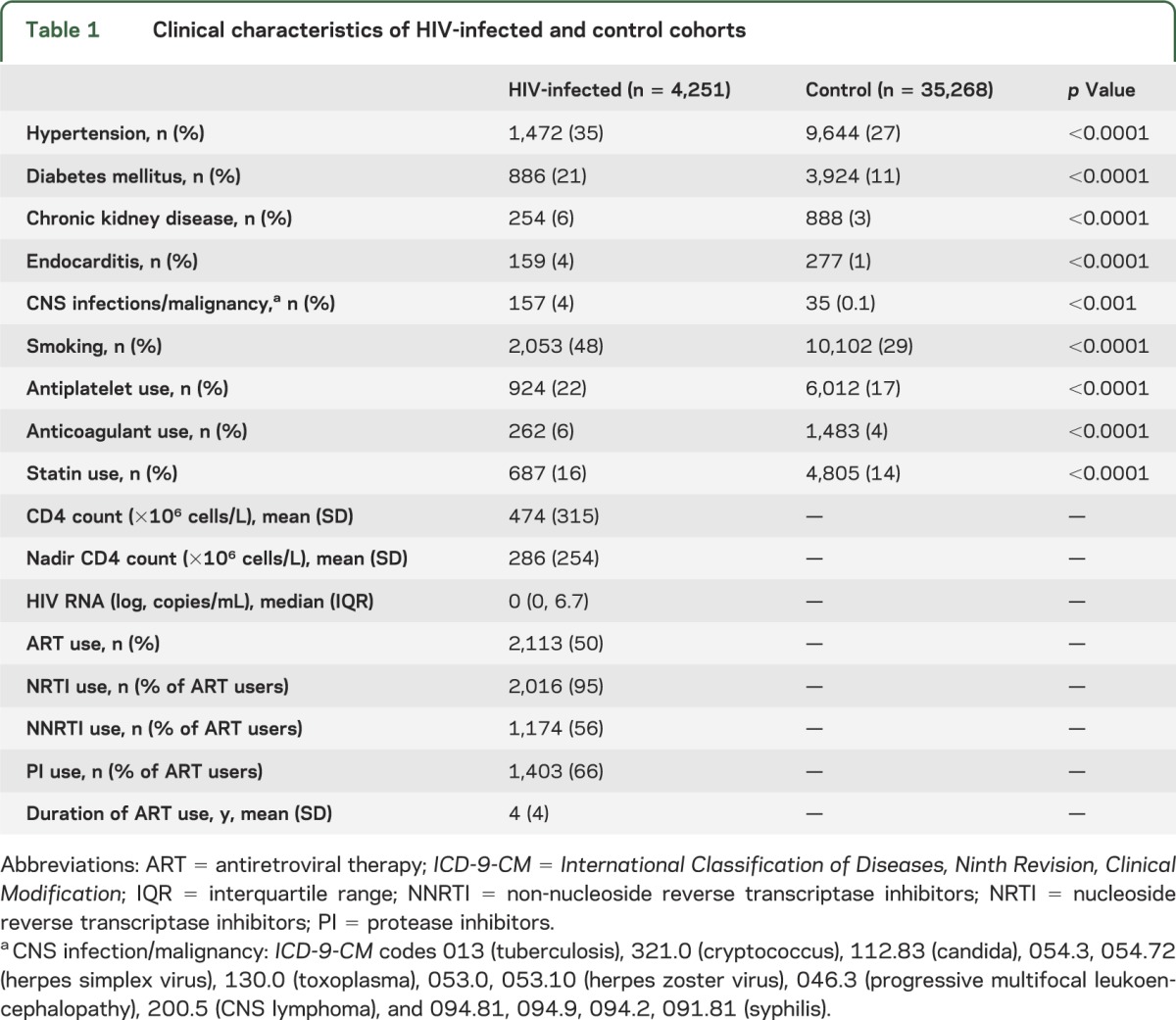
A total of 39,519 individuals were included in the study, of whom 4,251 were HIV-infected and 35,268 HIV-uninfected. These 2 cohorts contributed 237,760 PY of observation, with a median duration of follow up of 5.47 years (interquartile range 1.48, 10.25). The mean age was 41.6 years in the HIV cohort and 40.6 years in the control cohort. Women constituted 31% of the HIV cohort compared with 34% of controls. A total of 52% of the HIV and control cohorts were white. The prevalence of most comorbidities, including hypertension, diabetes mellitus, and smoking, was higher in the HIV cohort. Anticoagulation and statin use were also more common in the HIV cohort (table 1).
Table 1.
Clinical characteristics of HIV-infected and control cohorts
Rates of ICH.
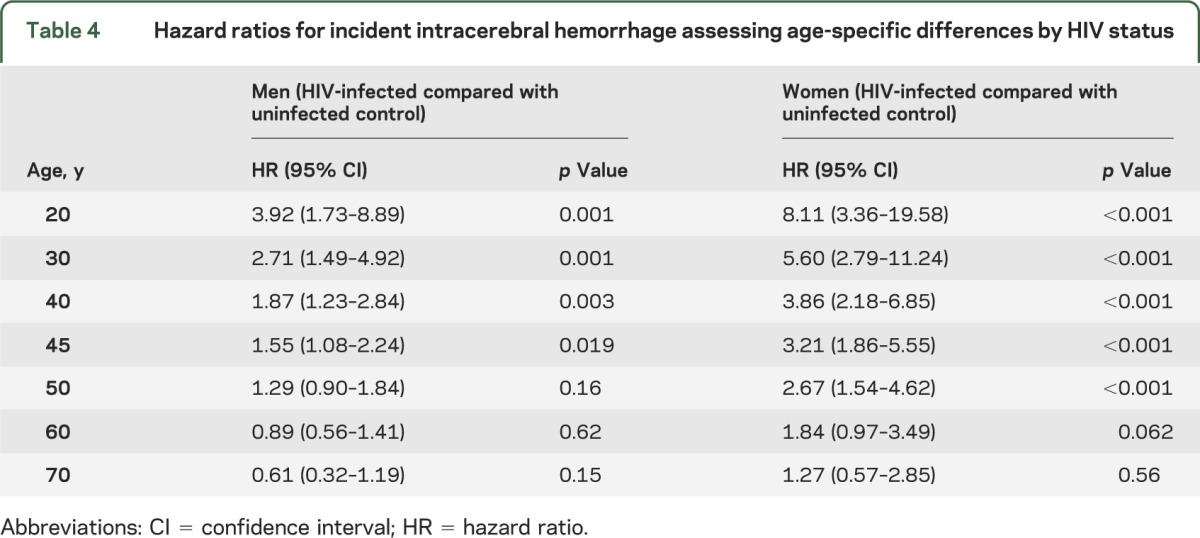
We identified 320 cases of ICH, 58 in the HIV and 262 in the control cohort. The incidence rate of ICH was 2.29 per 1,000 PY in HIV-infected compared with 1.23 per 1,000 PY in uninfected individuals, with an unadjusted incidence rate ratio (IRR) of 1.85 (table 2). After stratification by sex, a relative increase in ICH rates was also seen overall in HIV-infected men and women, although the IRRs comparing ICH rates in the HIV to control cohort were greater for women than for men overall and in most age groups (table 2). In a multivariable model, HIV was independently associated with ICH, with a 1.87 greater hazard of ICH at age 40 in men, after adjusting for age, race, hypertension, diabetes, CKD, endocarditis, CNS infections or malignancy, smoking, antiplatelet therapy, anticoagulation, and statin use (table 3). The effect of HIV on the hazard of ICH appeared to violate the proportional hazards assumption, decreasing by an estimated factor of 0.96 for every year that age increased. For example, at age 30, an HIV-infected man had 2.71 times the hazard of ICH compared with an uninfected man, whereas at age 50, the hazard of ICH for an HIV-infected man compared to an uninfected man was 1.29 and by age 60, the increased hazard was no longer present (table 4).
Table 2.
Incidence rates for intracerebral hemorrhage stratified by age group and sex
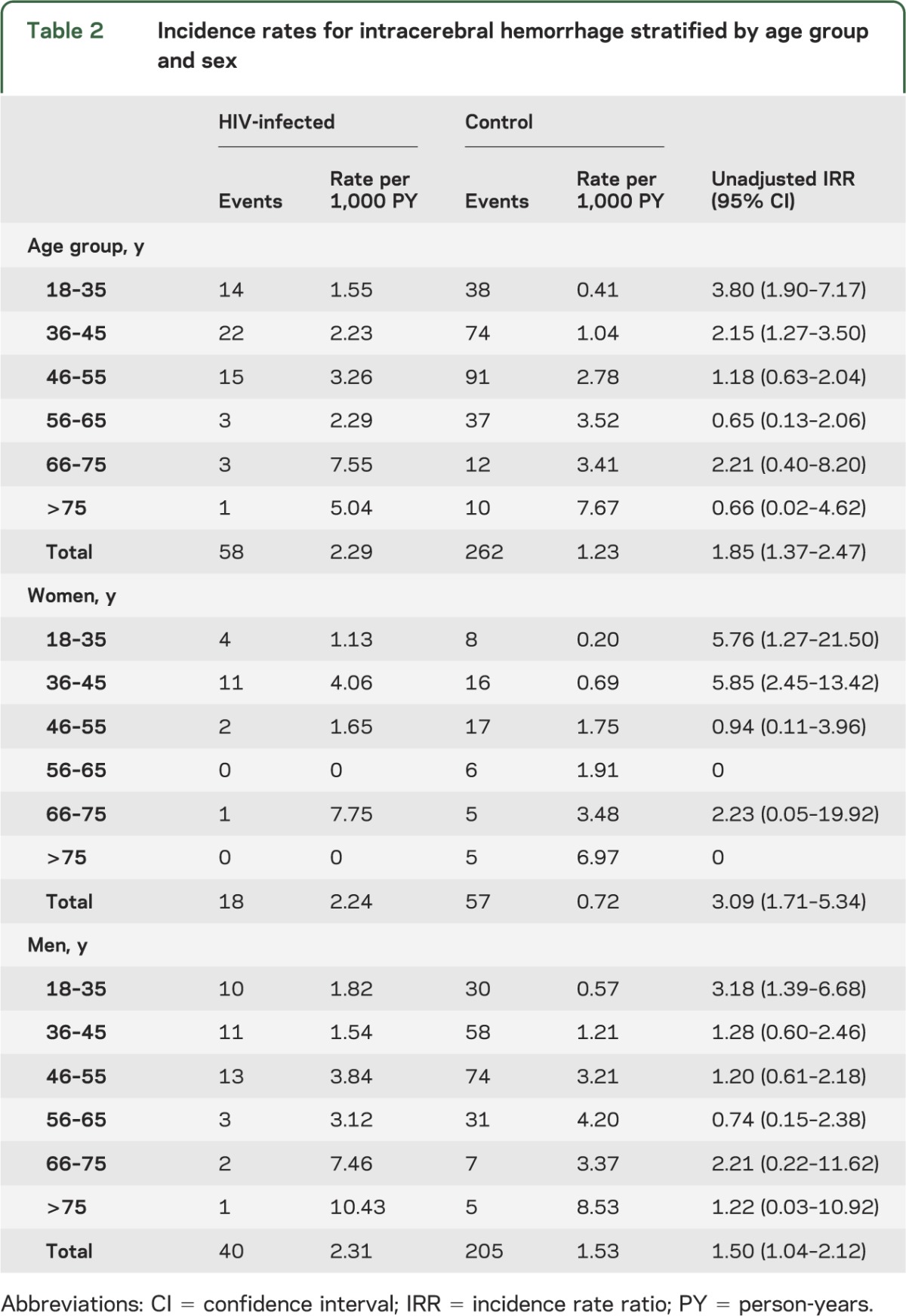
Table 3.
Hazard ratios for predictors of incident intracerebral hemorrhage among HIV-infected individuals and uninfected controls
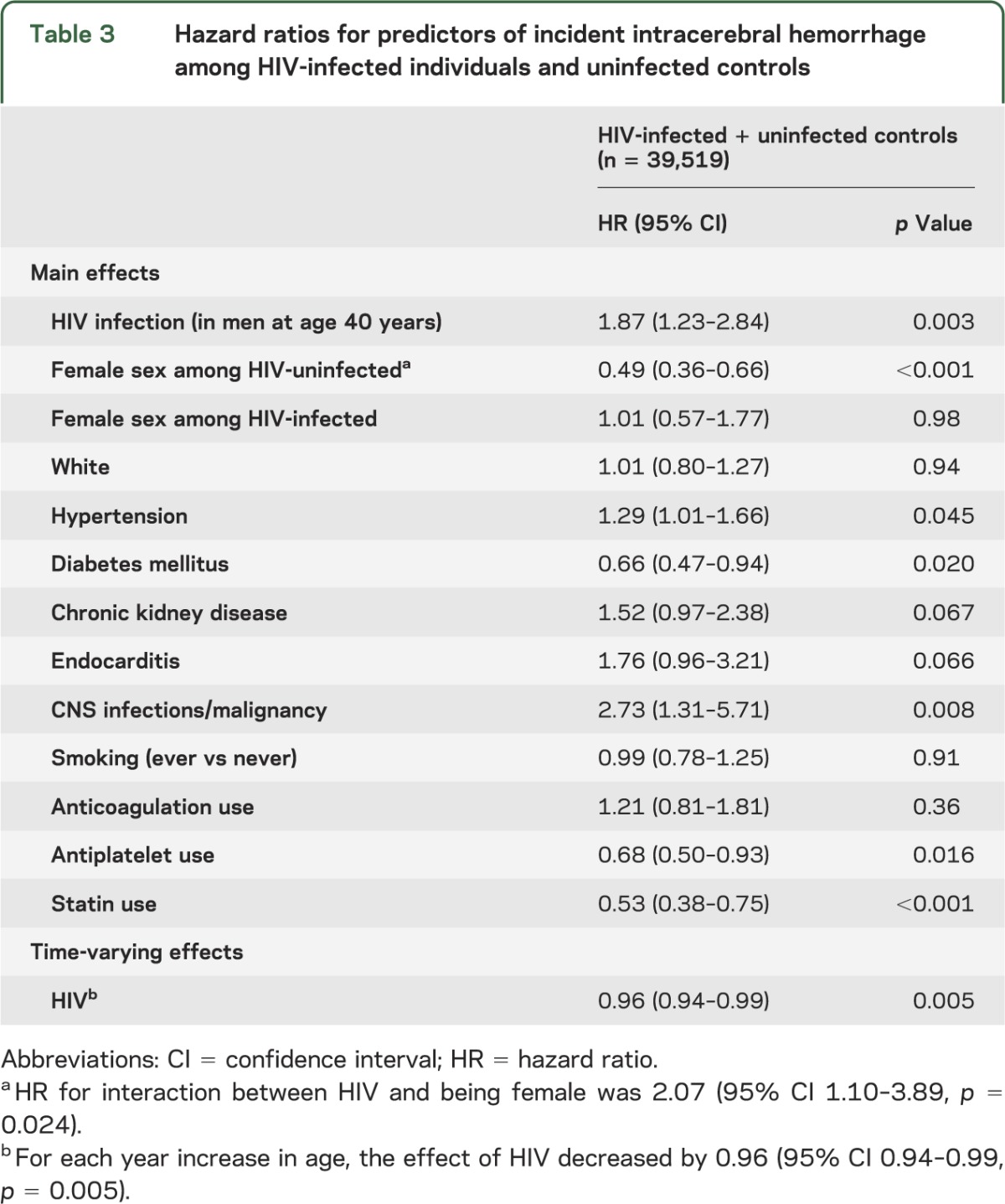
Table 4.
Hazard ratios for incident intracerebral hemorrhage assessing age-specific differences by HIV status
Predictors of ICH.
In addition to HIV status, hypertension predicted ICH (table 3). Diabetes mellitus was associated with a lower hazard of ICH, as were statin and antiplatelet use. We observed an interaction between female sex and HIV status on the hazard of ICH, with a protective effect of female sex on ICH in HIV-uninfected individuals, but an estimate of no protective effect among those with HIV (table 3). In a sensitivity analysis, we excluded individuals with a history of endocarditis or CNS infection or malignancy from the multivariate model. The effect of HIV on the hazard of ICH did not change significantly (HR 1.89, 95% CI 1.22–2.91, p = 0.004 for a man at age 40), nor did the HR for other covariates in the model.
Predictors of ICH among HIV-infected individuals.
In the HIV cohort, 2,278 HIV-infected individuals had available data on CD4 count and viral load. Using a forward stepwise selection process, we included sex, CD4 count <200 × 106 cells/L, anticoagulant use, ART use, and statin use in the multivariable model. CD4 count <200 × 106 cells/L at any point during the observation period was associated with a higher rate of ICH, as was anticoagulant use (table 5). We found strong evidence from fitting a parametric Weibull model to the HIV cohort, with the same covariates as those chosen by forward stepwise selection, that age influenced hazard of ICH in the HIV cohort (p = 0.038 vs constant hazard), as in the general population. For example, the ratio of hazards in HIV-infected individuals at age 60 compared with age 40 was 1.74 (95% CI 1.02–5.80).
Table 5.
Hazard ratios for predictors of incident intracerebral hemorrhage in the HIV cohort
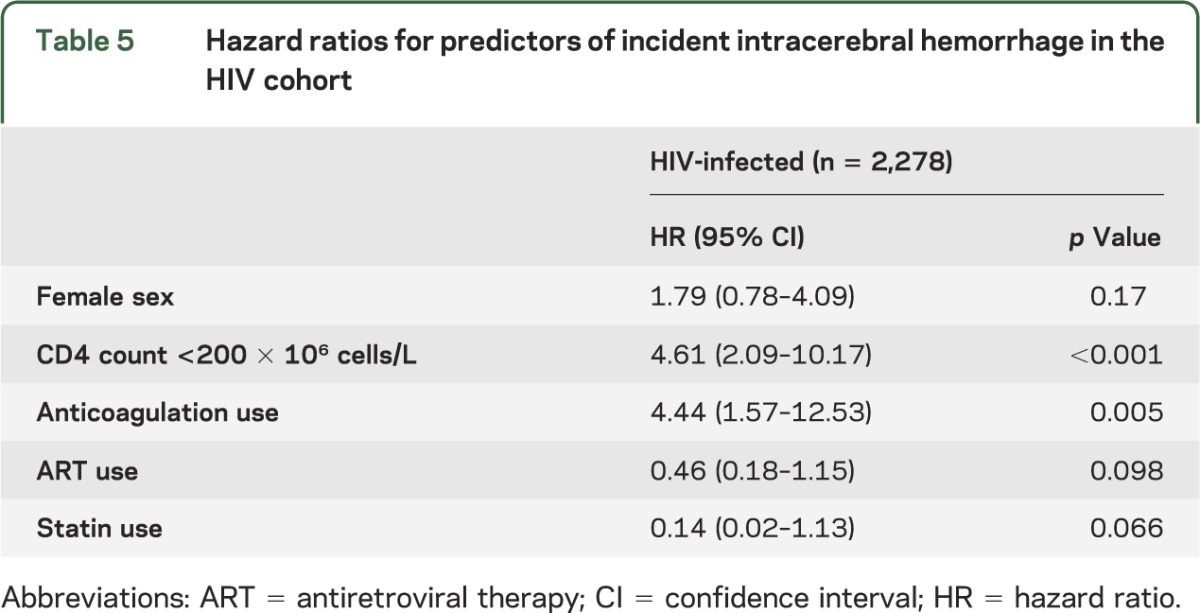
In a sensitivity analysis, we included having a detectable viral load in the HIV-only model. This had little effect on the hazard of ICH and did not alter the HR for any other predictors by more than 10%, other than CD4 count <200 × 106 cells/L, for which the HR changed from 4.61 (95% CI 2.09–10.17, p < 0.001) to 3.88 (95% CI 1.68–8.96, p = 0.002).
DISCUSSION
In a large US clinical care cohort, we found an increased rate of ICH among HIV-infected compared with uninfected individuals. The hazard of ICH associated with HIV was increased even after adjustment for recognized ICH risk factors, suggesting that a component of the risk may be mediated by factors unique to HIV. Indeed, among the HIV cohort, a CD4 count <200 × 106 cells/L was associated with a markedly higher risk of ICH, raising the possibility that more advanced HIV disease stage may increase ICH risk.
Several risk factors for ICH are more prevalent in HIV-infected individuals than in the general population, including hypertension,13–16 a strong independent predictor of ICH.17–21 Moreover, at younger ages, African Americans, who have been disproportionately affected by the HIV epidemic,22 have a greater risk of ICH compared with whites.17,20 The fact that ICH is associated with worse functional outcomes and higher case fatality rates than ischemic stroke23,24 could lead to considerable loss of quality-adjusted life-years, particularly for younger HIV-infected individuals. Few studies have specifically evaluated ICH risk in HIV-infected compared with uninfected individuals. Data from studies performed before combination ART are confounded by advanced disease and associated opportunistic infections that may increase ICH risk.25 Furthermore, ICH was less common than ischemic stroke in many of these studies,26,27 reflecting the typical breakdown of stroke by subtype and rendering it difficult to draw conclusions based on small numbers of events. Studies evaluating ICH from the current era of combination ART conflict. A Danish observational cohort of HIV-infected individuals compared with a population-based control group evaluated cerebral infarction separately from ICH.6 ICH incidence was increased in HIV-infected individuals with a much more pronounced effect among HIV-infected IV drug users, although information on other potential confounders including smoking and anticoagulant use was not included. Conversely, in the United States, data from the Nationwide Inpatient Sample indicate that the proportion of all ischemic strokes attributable to HIV-infected individuals rose in the United States from 1997 to 2006, whereas the proportion of ICH hospitalizations in HIV-infected individuals was stable to decreased.28 Our results support a study that observed a higher rate of a composite outcome of ICH and subarachnoid hemorrhage (SAH) in a Canadian HIV cohort compared with population-based controls.29 Our study advances the existing literature by focusing specifically on ICH as opposed to a composite outcome of ICH and SAH, an important distinction given differences in underlying pathophysiology and etiology, and by including HIV-related clinical factors.
Mounting evidence points to a link among inadequately controlled HIV, chronic inflammation, immune dysregulation, and accelerated atherosclerosis in HIV infection.30–37 Existing data conflict regarding the specific association between CD4 count and cerebrovascular and cardiovascular risk.5,6,33,38,39 Similar to our finding that a CD4 count <200 × 106 cells/L was associated with higher ICH risk, we previously found a higher CD4 count decreased the hazard of ischemic stroke, although this did not reach statistical significance.5 Furthermore, ART use, for which there is mixed evidence in relation to stroke risk,5,6,9,38 was associated with a lower, but not statistically significant, hazard of ICH (table 5). These findings suggest that more advanced or less well-controlled HIV infection may contribute to increased cerebrovascular risk. While inflammation may play a role in the development of ischemic stroke in HIV-infected individuals, an analogous association with ICH has not been explored. Inflammatory markers that are elevated in HIV and strongly predictive of mortality,40 however, have been shown to predict ICH in the general population.e1–e3 Lipohyalinosis, a destructive process affecting intracerebral small vessels marked by “a loss of normal arterial architecture, mural foam cells and, in acute cases, evidence of fibrinoid vessel wall necrosis,” may underlie both small vessel lacunar infarcts and a subset of primary ICH.e4–e6 Endothelial dysfunction in the setting of chronic inflammation and immune dysfunction is postulated to play a role in the pathogenesis of cerebral small vessel disease and lipohyalinosise7,e8 and may be a contributing factor in the association between HIV and increased ICH risk.
We found that HIV conferred a relatively greater risk of ICH in women compared with men. While absolute ICH rates were higher in men, the relative risk of ICH comparing HIV-infected to uninfected individuals was greater for women at any age. Examined another way, the protective effect of female sex on the hazard of ICH, which is in line with the known epidemiology of ICH in the general population up to 75 years of age,21,e9 was absent or less strong for HIV-infected women compared with uninfected women (multivariable model p value for interaction of HIV and sex, p = 0.024). Prior studies have demonstrated a greater risk of myocardial infarction and ischemic stroke in HIV-infected compared to uninfected women.2,3,5 Women have been shown to have lower rates of adherence and higher rates of viral rebound and treatment interruption, which could potentially contribute to the decreased protective effect from being a woman on ICH risk among HIV-infected individuals.e10–e15 Furthermore, sex-specific differences in the immune response to HIV have been elucidated, with higher levels of immune activation in HIV-infected women compared with men at a given level of viral suppressione16 and compared with uninfected women.e17 Additional investigation into differential effects of sex on ICH hazard among HIV-infected individuals is warranted.
As with myocardial infarction and ischemic stroke,1,3,5,38 the absolute rates of ICH among HIV-infected individuals increased with older age. In the overall cohort, however, we demonstrated that the effect of HIV status on the hazard of ICH decreased as age increased. A relatively greater effect of HIV in younger age groups has been demonstrated for other vascular outcomes, including myocardial infarction and ischemic stroke.1,5 The decrease in the effect of HIV on the hazard of ICH with older age may reflect a relatively greater contribution of traditional risk factors to ICH risk as individuals age, consonant with the rising incidence and prevalence of various comorbidities in aging HIV-infected individuals.e18,e19
As expected, hypertension was associated with a higher hazard of ICH in the overall model, and there was a trend towards an association with endocarditis and CKD. These comorbidities represent potentially modifiable risk factors that should be targeted in HIV-infected individuals, as in the general population. The decrease in the hazard of ICH associated with diabetes and statin use may reflect better engagement in the health care system of these individuals (and thus, for example, better control of associated comorbidities including hypertension). A similar explanation may underlie the protective effect of antiplatelet use.
In addition to unmeasured confounding that can occur in observational studies, limitations relate to the use of ICD-9-CM codes. Rates of ICH were higher in our cohort than previously published estimates, a finding that may reflect misclassification by ICD-9-CM codes or referral center bias. However, misclassification should be equally likely to occur in HIV-infected and uninfected cohorts (i.e., nondifferential) and thus would bias the result toward the null. Due to limitations associated with ICD-9-CM codes, we could not assess ICH size, location, or severity, or their relationship with hypertension stage or duration. Furthermore, we could not distinguish between ICH and hemorrhagic conversion of ischemic strokes. We lacked data on duration of HIV infection and illicit drug use; however, we included several clinically relevant covariates, including smoking status, which is unavailable for many observational studies. Our study is also potentially limited by defining various fixed predictors using information from the entire observation period. For example, an individual who does not incur ICH at a given time has the continued opportunity to be started on ART or develop hypertension, while those who incur ICH at that time have no such opportunity, leading to bias toward a protective effect. Finally, the Partners HIV cohort may not entirely reflect the greater population of HIV-infected individuals in the United States, or in resource-limited settings worldwide, although data on other vascular outcomes from this cohort have been consistently corroborated by those from other large HIV cohorts. Although the Partners HIV cohort has a greater percentage of whites than that reported for the United States,e20 nonwhite race/ethnicity and women are, overall, well-represented (48% and 31%, respectively).
In this large US clinical care cohort, HIV conferred an independent risk of ICH, adjusting for established ICH-related risk factors. This increased risk was relatively more pronounced for young patients and women. Further investigation into the mechanism for this relatively greater increase in risk of ICH among these traditionally low-risk demographic subsets is merited. Focusing preventive efforts on modifiable ICH risk factors among all HIV-infected individuals should remain a priority in the setting of an aging HIV-infected population.
Supplementary Material
GLOSSARY
- ART
antiretroviral therapy
- CKD
chronic kidney disease
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- ICH
intracerebral hemorrhage
- IRR
incidence rate ratio
- PY
person-years
- RPDR
Research Patient Data Registry
- SAH
subarachnoid hemorrhage
Footnotes
Editorial, page 1690
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
F.C. Chow participated in the study concept and design, performed the data analysis and interpretation, and drafted the manuscript. W. He performed the data analysis and provided critical revisions on the paper. P. Bacchetti performed the data analysis and interpretation and provided critical revisions on the paper. S. Regan performed the data analysis and interpretation and provided critical revisions on the paper. S.K. Feske participated in the study concept and design and provided critical revisions on the paper. J.B. Meigs participated in the study concept and design and provided critical revisions on the paper. S.K. Grinspoon participated in the study concept and design and provided critical revisions on the paper. V.A. Triant participated in the study concept and design, interpreted the data, supervised the study, and provided critical revisions on the paper.
STUDY FUNDING
Supported in part by NIH grants 5T32MH090847 (F.C.C.), K24 DK080140 (J.B.M.), P30 DK040561 (S.K.G.), and K01 AI073109 (V.A.T.).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 2003;33:506–512. [DOI] [PubMed] [Google Scholar]
- 2.Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS 2010;24:1228–1230. [DOI] [PubMed] [Google Scholar]
- 3.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis 2007;44:1625–1631. [DOI] [PubMed] [Google Scholar]
- 5.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012;60:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen LD, Engsig FN, Christensen H, et al. Risk of cerebrovascular events in persons with and without HIV. AIDS 2011;25:1637–1646. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr 2014;65:160–166. [DOI] [PubMed] [Google Scholar]
- 8.Evers S, Nabavi D, Rahmann A, Heese C, Reichelt D, Husstedt I-W. Ischaemic cerebrovascular events in HIV infection: a cohort study. Cerebrovasc Dis 2003;15:199–205. [DOI] [PubMed] [Google Scholar]
- 9.Corral I, Quereda C, Moreno A, et al. Cerebrovascular ischemic events in HIV-1-infected patients receiving highly active antiretroviral therapy: incidence and risk factors. Cerebrovasc Dis 2009;27:559–563. [DOI] [PubMed] [Google Scholar]
- 10.Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 11.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009;51:268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng QT, Goryachev S, Weiss S, Sordo M, Murphy SN, Lazarus R. Extracting principal diagnosis, co-morbidity and smoking status for asthma research: evaluation of a natural language processing system. BMC Med Inform Decis Mak 2006;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergersen BM, Sandvik L, Dunlop O, Birkeland K, Bruun JN. Prevalence of hypertension in HIV-positive patients on highly active retroviral therapy (HAART) compared with HAART-naïve and HIV-negative controls: results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infect Dis 2003;22:731–736. [DOI] [PubMed] [Google Scholar]
- 14.Seaberg EC, Muñoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 2005;19:953–960. [DOI] [PubMed] [Google Scholar]
- 15.Palacios R, Santos J, García A, et al. Impact of highly active antiretroviral therapy on blood pressure in HIV‐infected patients: a prospective study in a cohort of naive patients. HIV Med 2006;7:10–15. [DOI] [PubMed] [Google Scholar]
- 16.De Socio GV, Ricci E, Maggi P, et al. Prevalence, awareness, treatment, and control rate of hypertension in HIV-infected patients: the HIV-HY study. Am J Hypertens 2014;2:222–228. [DOI] [PubMed] [Google Scholar]
- 17.Sturgeon JD, Folsom AR, Longstreth WT, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 2007;38:2718–2725. [DOI] [PubMed] [Google Scholar]
- 18.Feldmann E, Broderick JP, Kernan WN, et al. Major risk factors for intracerebral hemorrhage in the young are modifiable. Stroke 2005;36:1881–1885. [DOI] [PubMed] [Google Scholar]
- 19.Woo D, Sauerbeck LR, Kissela BM, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke 2002;33:1190–1195. [DOI] [PubMed] [Google Scholar]
- 20.Howard G, Cushman M, Howard VJ, et al. Risk factors for intracerebral hemorrhage: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke 2013;44:1282–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ariesen MJ, Claus SP, Rinkel GJE, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke 2003;34:2060–2065. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Disparities in diagnoses of HIV infection between blacks/African Americans and other racial/ethnic populations: 37 states, 2005–2008. MMWR Morb Mortal Wkly Rep 2011;60:93–98. [PubMed] [Google Scholar]
- 23.Bhalla A, Wang Y, Rudd A, Wolfe CDA. Differences in outcome and predictors between ischemic and intracerebral hemorrhage: the South London stroke register. Stroke 2013;44:2174–2181. [DOI] [PubMed] [Google Scholar]
- 24.Dennis MS. Outcome after brain haemorrhage. Cerebrovasc Dis 2003;16(suppl 1):9–13. [DOI] [PubMed] [Google Scholar]
- 25.Cole JW, Pinto AN, Hebel JR, et al. Acquired immunodeficiency syndrome and the risk of stroke. Stroke 2004;35:51–56. [DOI] [PubMed] [Google Scholar]
- 26.Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology 2007;68:1257–1261. [DOI] [PubMed] [Google Scholar]
- 27.Mochan A, Modi M, Modi G. Stroke in black South African HIV-positive patients: a prospective analysis. Stroke 2003;34:10–15. [DOI] [PubMed] [Google Scholar]
- 28.Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology 2011;76:444–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durand M, Sheehy O, Baril J-G, LeLorier J, Tremblay CL. Risk of spontaneous intracranial hemorrhage in HIV-infected individuals: a population-based cohort study. J Stroke Cerebrovasc Dis 2013;22:e34–e41. [DOI] [PubMed] [Google Scholar]
- 30.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 2012;205:S375–S382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triant VA. HIV infection and coronary heart disease: an intersection of epidemics. J Infect Dis 2012;205:S355–S361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strategies for Management of Antiretroviral Therapy SMART Study Group, El-Sadr WM, Lundgren JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 33.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr 2010;55:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004;109:1603–1608. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS 2008;22:1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4 +T Cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis 2010;51:435–447. [DOI] [PubMed] [Google Scholar]
- 37.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012;308:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and clinical Features of cerebrovascular disease among HIV-infected adults in the Southeastern United States. AIDS Res Hum Retroviruses 2013;29:1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabin CA, Ryom L, De Wit S, et al. Associations between immune depression and cardiovascular events in HIV infection. AIDS 2013;27:2735–2748. [DOI] [PubMed] [Google Scholar]
- 40.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr 2010;55:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


