Abstract
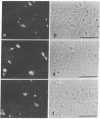
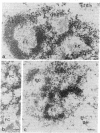
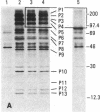
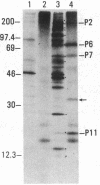
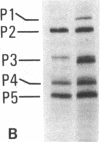
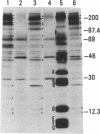
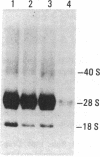
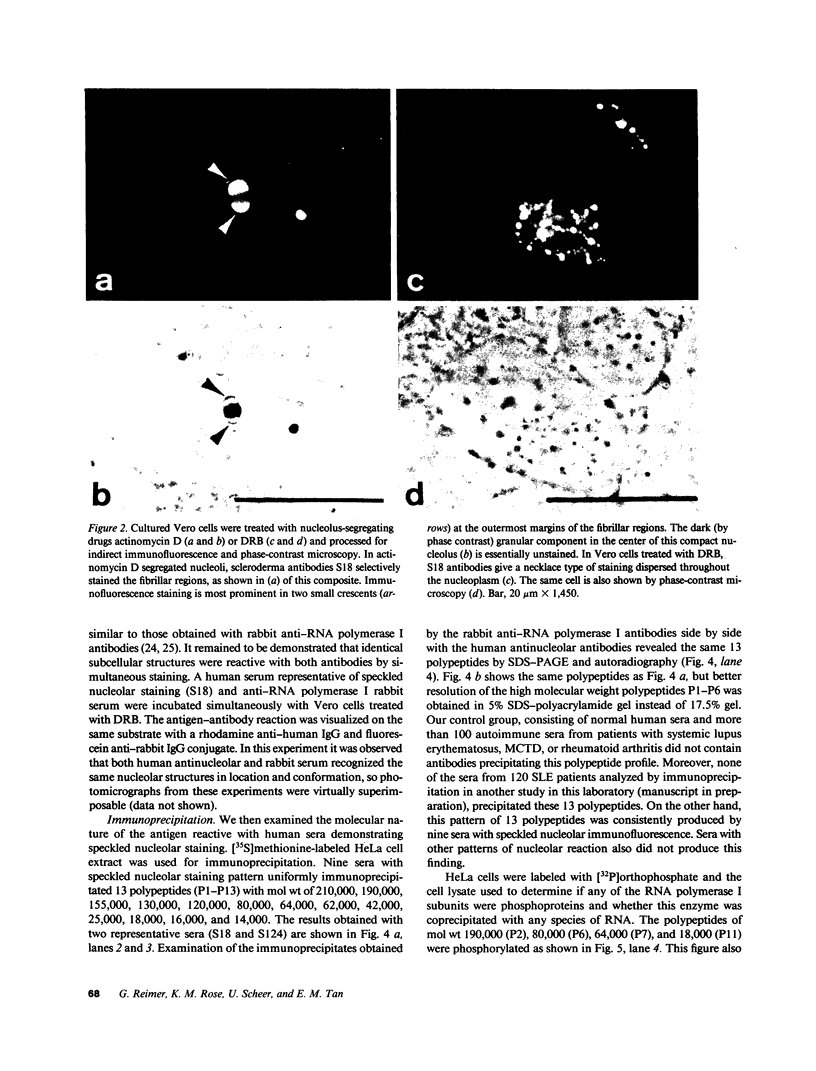
Autoantibodies to components of the nucleolus are a unique serological feature of patients with scleroderma. There are autoantibodies of several specificities; one type produces a speckled pattern of nucleolar staining in immunofluorescence. In actinomycin D and 5,6-dichloro-beta-D-ribofuranosylbenzimidazole-treated Vero cells, staining was restricted to the fibrillar and not the granular regions. By double immunofluorescence, specific rabbit anti-RNA polymerase I antibodies stained the same fibrillar structures in drug-segregated nucleoli as scleroderma sera. Scleroderma sera immunoprecipitated 13 polypeptides from [35S]methionine-labeled HeLa cell extract with molecular weights ranging from 210,000 to 14,000. Similar polypeptides were precipitated by rabbit anti-RNA polymerase I antibodies, and their common identities were confirmed in immunoabsorption experiments. Microinjection of purified IgG from a patient with speckled nucleolar staining effectively inhibited ribosomal RNA transcription. Autoantibodies to RNA polymerase I were restricted to certain patients with scleroderma and were not found in other autoimmune diseases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard W. Drug-induced changes in the interphase nucleus. Adv Cytopharmacol. 1971 May;1:49–67. [PubMed] [Google Scholar]
- Bernstein R. M., Steigerwald J. C., Tan E. M. Association of antinuclear and antinucleolar antibodies in progressive systemic sclerosis. Clin Exp Immunol. 1982 Apr;48(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- Cox J. V., Schenk E. A., Olmsted J. B. Human anticentromere antibodies: distribution, characterization of antigens, and effect on microtubule organization. Cell. 1983 Nov;35(1):331–339. doi: 10.1016/0092-8674(83)90236-2. [DOI] [PubMed] [Google Scholar]
- Douvas A. S., Achten M., Tan E. M. Identification of a nuclear protein (Scl-70) as a unique target of human antinuclear antibodies in scleroderma. J Biol Chem. 1979 Oct 25;254(20):10514–10522. [PubMed] [Google Scholar]
- Duceman B. W., Rose K. M., Jacob S. T. Activation of purified hepatoma RNA polymerase I by homologous protein kinase NII. J Biol Chem. 1981 Nov 10;256(21):10755–10758. [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan N., Cooke C., Rothfield N. The kinetochore is part of the metaphase chromosome scaffold. J Cell Biol. 1984 Jan;98(1):352–357. doi: 10.1083/jcb.98.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einck L., Bustin M. Functional histone antibody fragments traverse the nuclear envelope. J Cell Biol. 1984 Jan;98(1):205–213. doi: 10.1083/jcb.98.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoeur A. M., Chan E. K., Garrels J. I., Mathews M. B. Characterization and purification of lupus antigen La, and RNA-binding protein. Mol Cell Biol. 1985 Mar;5(3):586–590. doi: 10.1128/mcb.5.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick D. Nucleolar necklaces in chick embryo fibroblast cells. I. Formation of necklaces by dichlororibobenzimidazole and other adenosine analogues that decrease RNA synthesis and degrade preribosomes. J Cell Biol. 1975 May;65(2):398–417. doi: 10.1083/jcb.65.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick D. Nucleolar necklaces in chick embryo fibroblast cells. II. Microscope observations of the effect of adenosine analogues on nucleolar necklace formation. J Cell Biol. 1975 May;65(2):418–427. doi: 10.1083/jcb.65.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. Sequential association of nucleolar 7-2 RNA with two different autoantigens. J Biol Chem. 1983 Feb 10;258(3):1379–1382. [PubMed] [Google Scholar]
- Karawya E., Swack J., Albert W., Fedorko J., Minna J. D., Wilson S. H. Identification of a higher molecular weight DNA polymerase alpha catalytic polypeptide in monkey cells by monoclonal antibody. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7777–7781. doi: 10.1073/pnas.81.24.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs R. L., Reddy R., Cook R. G., Yeoman L. C., Tan E. M., Reichlin M., Busch H. Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine. J Biol Chem. 1985 Nov 15;260(26):14304–14310. [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature. 1983 Jul 14;304(5922):177–179. doi: 10.1038/304177a0. [DOI] [PubMed] [Google Scholar]
- Moroi Y., Peebles C., Fritzler M. J., Steigerwald J., Tan E. M. Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1627–1631. doi: 10.1073/pnas.77.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Spohn W. H., Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54(2):123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Pinnas J. L., Northway J. D., Tan E. M. Antinucleolar antibodies in human sera. J Immunol. 1973 Oct;111(4):996–1004. [PubMed] [Google Scholar]
- Pizer L. I., Deng J. S., Stenberg R. M., Tan E. M. Characterization of a phosphoprotein associated with the SS-B/La nuclear antigen in adenovirus-infected and uninfected KB cells. Mol Cell Biol. 1983 Jul;3(7):1235–1245. doi: 10.1128/mcb.3.7.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Bull Rheum Dis. 1981;31(1):1–6. [PubMed] [Google Scholar]
- Reddy R., Tan E. M., Henning D., Nohga K., Busch H. Detection of a nucleolar 7-2 ribonucleoprotein and a cytoplasmic 8-2 ribonucleoprotein with autoantibodies from patients with scleroderma. J Biol Chem. 1983 Feb 10;258(3):1383–1386. [PubMed] [Google Scholar]
- Reimer G., Rubin R. L., Kotzin B. L., Tan E. M. Anti-native DNA antibodies from autoimmune sera also bind to DNA in mitochondria. J Immunol. 1984 Nov;133(5):2532–2536. [PubMed] [Google Scholar]
- Ritchie R. F. Antinucleolar antibodies. Their frequency and diagnostic association. N Engl J Med. 1970 May 21;282(21):1174–1178. doi: 10.1056/NEJM197005212822104. [DOI] [PubMed] [Google Scholar]
- Rose K. M., Stetler D. A., Jacob S. T. Protein kinase activity of RNA polymerase I purified from a rat hepatoma: probable function of Mr 42,000 and 24,600 polypeptides. Proc Natl Acad Sci U S A. 1981 May;78(5):2833–2837. doi: 10.1073/pnas.78.5.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U., Hügle B., Hazan R., Rose K. M. Drug-induced dispersal of transcribed rRNA genes and transcriptional products: immunolocalization and silver staining of different nucleolar components in rat cells treated with 5,6-dichloro-beta-D-ribofuranosylbenzimidazole. J Cell Biol. 1984 Aug;99(2):672–679. doi: 10.1083/jcb.99.2.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U., Rose K. M. Localization of RNA polymerase I in interphase cells and mitotic chromosomes by light and electron microscopic immunocytochemistry. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1431–1435. doi: 10.1073/pnas.81.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shero J. H., Bordwell B., Rothfield N. F., Earnshaw W. C. High titers of autoantibodies to topoisomerase I (Scl-70) in sera from scleroderma patients. Science. 1986 Feb 14;231(4739):737–740. doi: 10.1126/science.3003910. [DOI] [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Stetler D. A., Jacob S. T. Phosphorylation of RNA polymerase I augments its interaction with autoantibodies of systemic lupus erythematosus patients. J Biol Chem. 1984 Nov 25;259(22):13629–13632. [PubMed] [Google Scholar]
- Stetler D. A., Rose K. M., Wenger M. E., Berlin C. M., Jacob S. T. Antibodies to distinct polypeptides of RNA polymerase I in sera from patients with rheumatic autoimmune disease. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7499–7503. doi: 10.1073/pnas.79.23.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Wandelt C., Grummt I. Formation of stable preinitiation complexes is a prerequisite for ribosomal DNA transcription in vitro. Nucleic Acids Res. 1983 Jun 11;11(11):3795–3809. doi: 10.1093/nar/11.11.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang V. W., Lerner M. R., Steitz J. A., Flint S. J. A small nuclear ribonucleoprotein is required for splicing of adenoviral early RNA sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1371–1375. doi: 10.1073/pnas.78.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]