Abstract
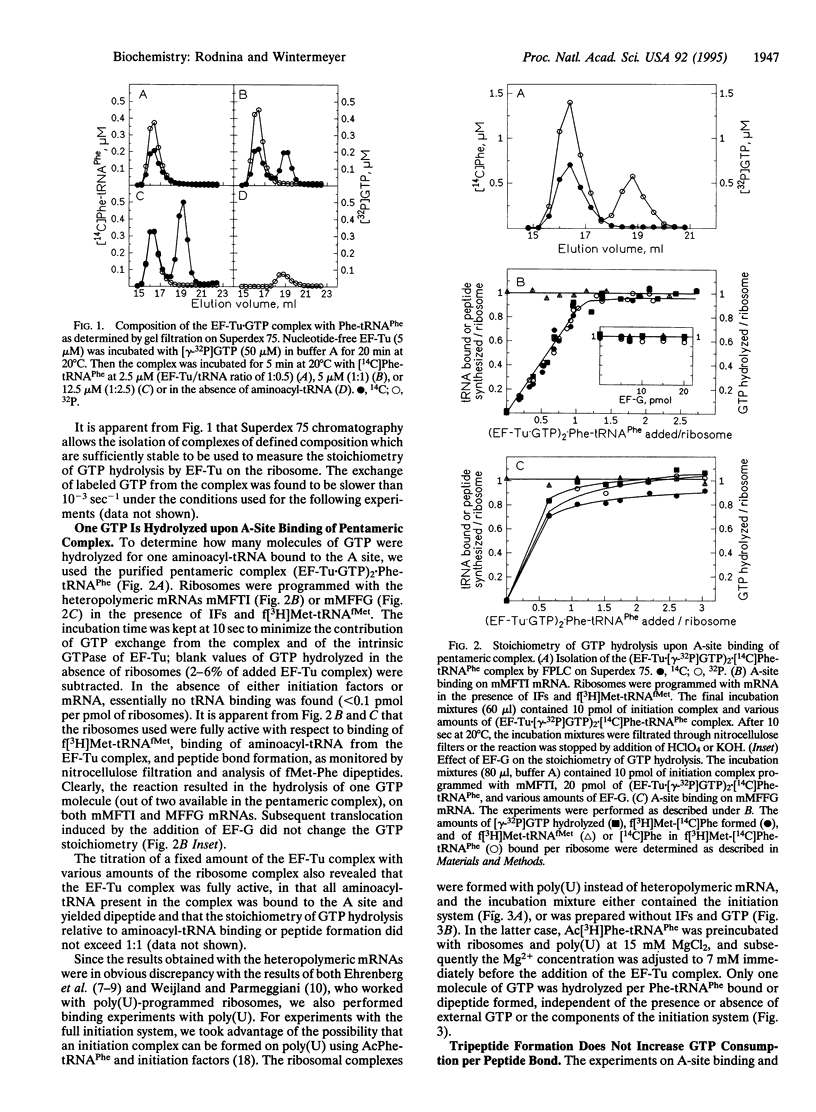
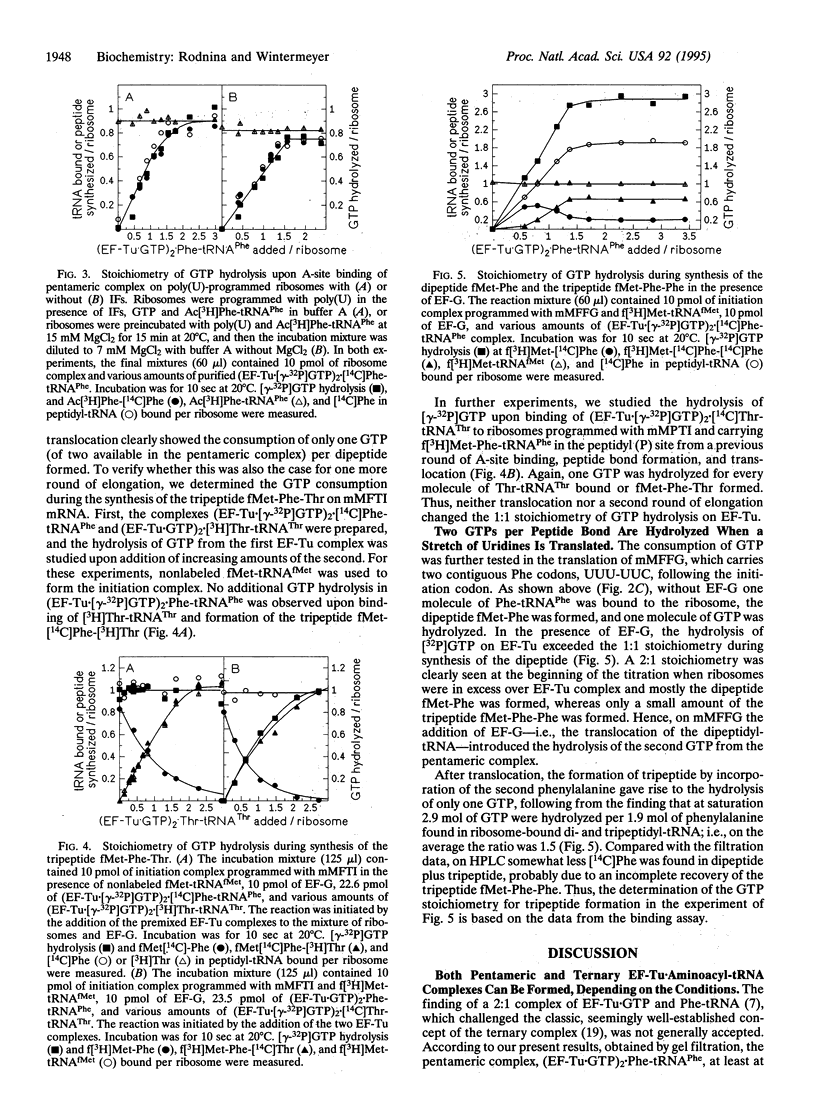
The stoichiometry of elongation factor Tu (EF-Tu) and GTP in the complex with aminoacyl-tRNA and the consumption of GTP during peptide bond formation on the ribosome were studied in the Escherichia coli system. The ribosomes were programmed either with two different heteropolymeric mRNAs coding for Met-Phe-Thr-Ile ... (mMFTI) or Met-Phe-Phe-Gly ... (mMFFG) or with poly(U). The composition of the complex of EF-Tu, GTP, and Phe-tRNA(Phe) was studied by gel chromatography. With equimolar amounts of factor and Phe-tRNA(Phe), a pentameric complex, (EF-Tu.GTP)2.Phe-tRNA(Phe), was observed, whereas the classical ternary complex, EF-Tu.GTP.Phe-tRNA(Phe), was found only when Phe-tRNA(Phe) was in excess. Upon binding of the purified pentameric complex to ribosomes carrying fMet-tRNA(fMet) in the peptidyl site and exposing a Phe codon in the aminoacyl site, only one out of two GTPs of the pentameric complex was hydrolyzed per Phe-tRNA bound and peptide bond formed, regardless of the mRNA used. In the presence of EF-G, the stoichiometry of one GTP hydrolyzed per peptide bond formed was found on mMFTI when one or two elongation cycles were completed. In contrast, on mMFFG, which contains two contiguous Phe codons, UUU-UUC, two GTP molecules of the pentameric complex were hydrolyzed per Phe incorporated into dipeptide, whereas the incorporation of the second Phe to form tripeptide consumed only one GTP. Thus, generally one GTP is hydrolyzed by EF-Tu per aminoacyl-tRNA bound and peptide bond formed, and more than one GTP is hydrolyzed only when a particular mRNA sequence, such as a homopolymeric stretch, is translated. The role of the additional GTP hydrolysis is not known; it may be related to frameshifting of peptidyl-tRNA during translocation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Weiss R. B., Thompson S., Gesteland R. F. Towards a genetic dissection of the basis of triplet decoding, and its natural subversion: programmed reading frame shifts and hops. Annu Rev Genet. 1991;25:201–228. doi: 10.1146/annurev.ge.25.120191.001221. [DOI] [PubMed] [Google Scholar]
- Bensch K., Pieper U., Ott G., Schirmer N., Sprinzl M., Pingoud A. How many EF-Tu molecules participate in aminoacyl-tRNA binding? Biochimie. 1991 Jul-Aug;73(7-8):1045–1050. doi: 10.1016/0300-9084(91)90146-r. [DOI] [PubMed] [Google Scholar]
- Bilgin N., Claesens F., Pahverk H., Ehrenberg M. Kinetic properties of Escherichia coli ribosomes with altered forms of S12. J Mol Biol. 1992 Apr 20;224(4):1011–1027. doi: 10.1016/0022-2836(92)90466-w. [DOI] [PubMed] [Google Scholar]
- Calogero R. A., Pon C. L., Canonaco M. A., Gualerzi C. O. Selection of the mRNA translation initiation region by Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6427–6431. doi: 10.1073/pnas.85.17.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg M., Rojas A. M., Weiser J., Kurland C. G. How many EF-Tu molecules participate in aminoacyl-tRNA binding and peptide bond formation in Escherichia coli translation? J Mol Biol. 1990 Feb 20;211(4):739–749. doi: 10.1016/0022-2836(90)90074-V. [DOI] [PubMed] [Google Scholar]
- Gordon J. Hydrolysis of guanosine 5'-triphosphate associated wh binding of aminoacyl transfer ribonucleic acid to ribosomes. J Biol Chem. 1969 Oct 25;244(20):5680–5686. [PubMed] [Google Scholar]
- Hughes D., Atkins J. F., Thompson S. Mutants of elongation factor Tu promote ribosomal frameshifting and nonsense readthrough. EMBO J. 1987 Dec 20;6(13):4235–4239. doi: 10.1002/j.1460-2075.1987.tb02772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakhniashvili D. G., Smailov S. K., Gavrilova L. P. The excess GTP hydrolyzed during mistranslation is expended at the stage of EF-Tu-promoted binding of non-cognate aminoacyl-tRNA. FEBS Lett. 1986 Feb 3;196(1):103–107. doi: 10.1016/0014-5793(86)80222-8. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A., Sander G. Properties and regulation of the GTPase activities of elongation factors Tu and G, and of initiation factor 2. Mol Cell Biochem. 1981 Mar 27;35(3):129–158. doi: 10.1007/BF02357085. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A., Swart G. W. Mechanism of action of kirromycin-like antibiotics. Annu Rev Microbiol. 1985;39:557–577. doi: 10.1146/annurev.mi.39.100185.003013. [DOI] [PubMed] [Google Scholar]
- Rodnina M. V., Fricke R., Wintermeyer W. Transient conformational states of aminoacyl-tRNA during ribosome binding catalyzed by elongation factor Tu. Biochemistry. 1994 Oct 11;33(40):12267–12275. doi: 10.1021/bi00206a033. [DOI] [PubMed] [Google Scholar]
- Rodnina M. V., Semenkov Y. P., Wintermeyer W. Purification of fMet-tRNA(fMet) by fast protein liquid chromatography. Anal Biochem. 1994 Jun;219(2):380–381. doi: 10.1006/abio.1994.1282. [DOI] [PubMed] [Google Scholar]
- Ruusala T., Ehrenberg M., Kurland C. G. Is there proofreading during polypeptide synthesis? EMBO J. 1982;1(6):741–745. doi: 10.1002/j.1460-2075.1982.tb01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Stone P. J. Proofreading of the codon-anticodon interaction on ribosomes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):198–202. doi: 10.1073/pnas.74.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgenboom E., Bosch L. Translational frameshifts induced by mutant species of the polypeptide chain elongation factor Tu of Escherichia coli. J Biol Chem. 1989 Aug 5;264(22):13012–13017. [PubMed] [Google Scholar]
- Weijland A., Parmeggiani A. Toward a model for the interaction between elongation factor Tu and the ribosome. Science. 1993 Feb 26;259(5099):1311–1314. doi: 10.1126/science.8446899. [DOI] [PubMed] [Google Scholar]
- Weijland A., Parmeggiani A. Why do two EF-Tu molecules act in the elongation cycle of protein biosynthesis? Trends Biochem Sci. 1994 May;19(5):188–193. doi: 10.1016/0968-0004(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Wintermeyer W., Gualerzi C. Effect of Escherichia coli initiation factors on the kinetics of N-Acphe-tRNAPhe binding to 30S ribosomal subunits. A fluorescence stopped-flow study. Biochemistry. 1983 Feb 1;22(3):690–694. doi: 10.1021/bi00272a025. [DOI] [PubMed] [Google Scholar]



