Abstract
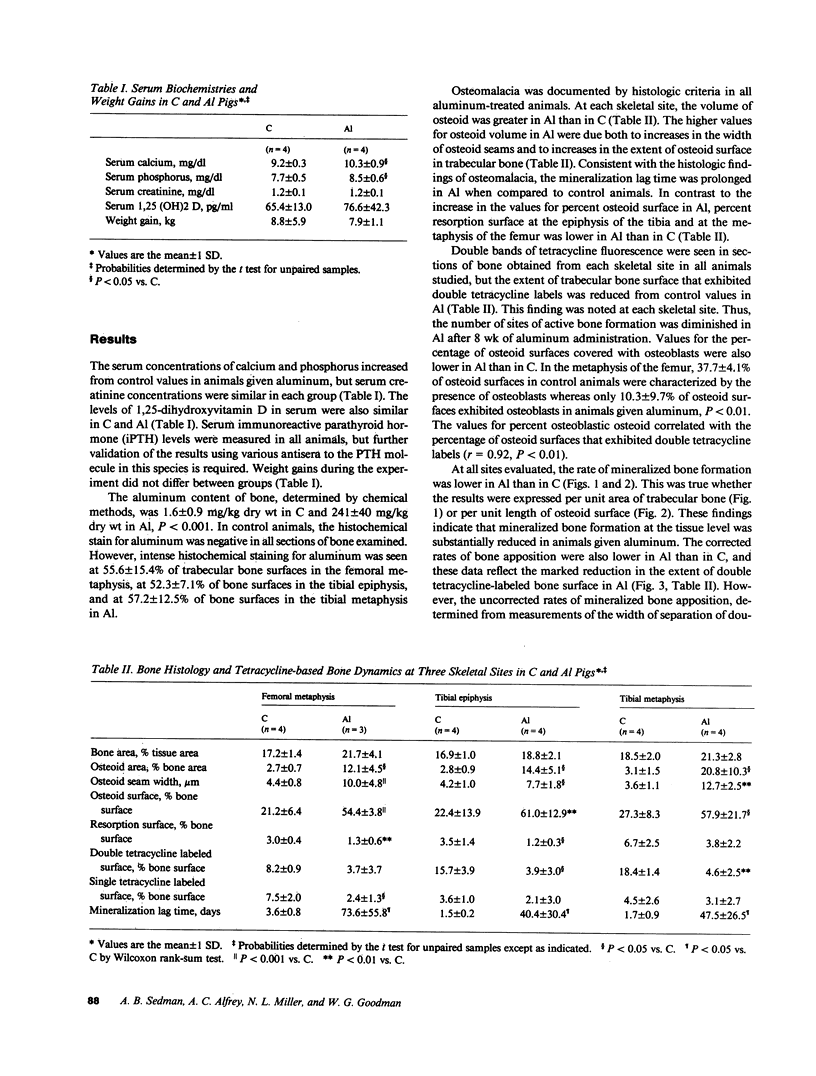
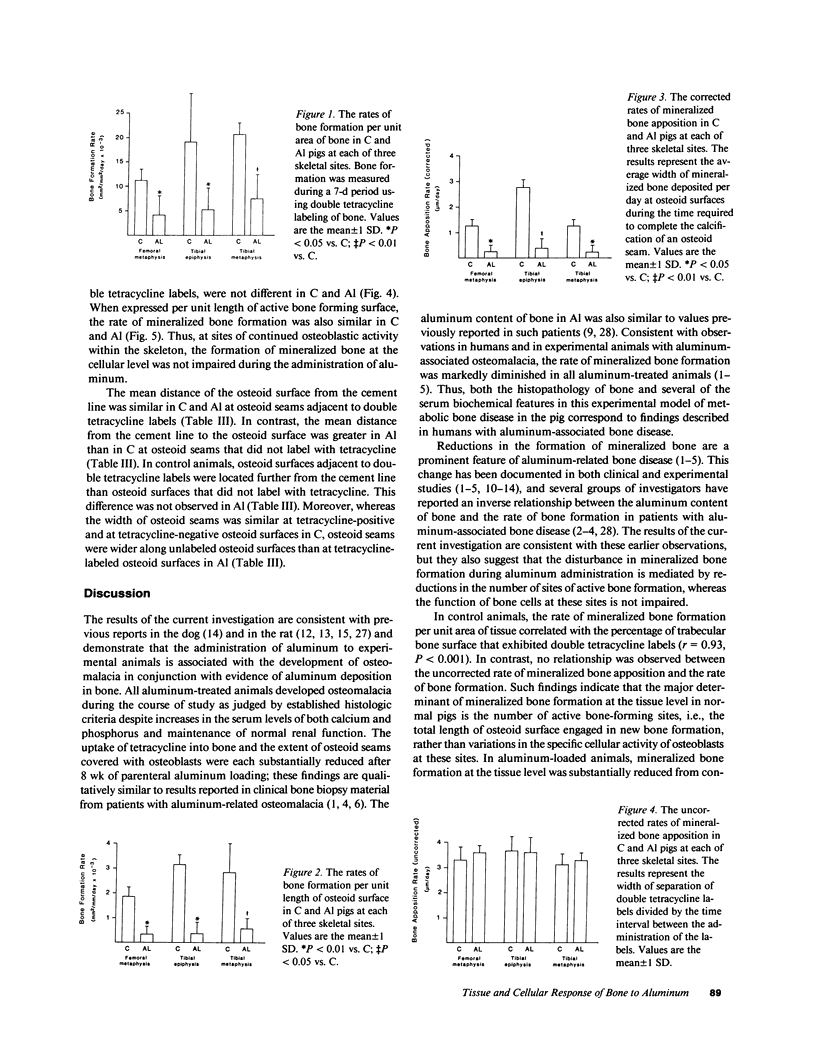
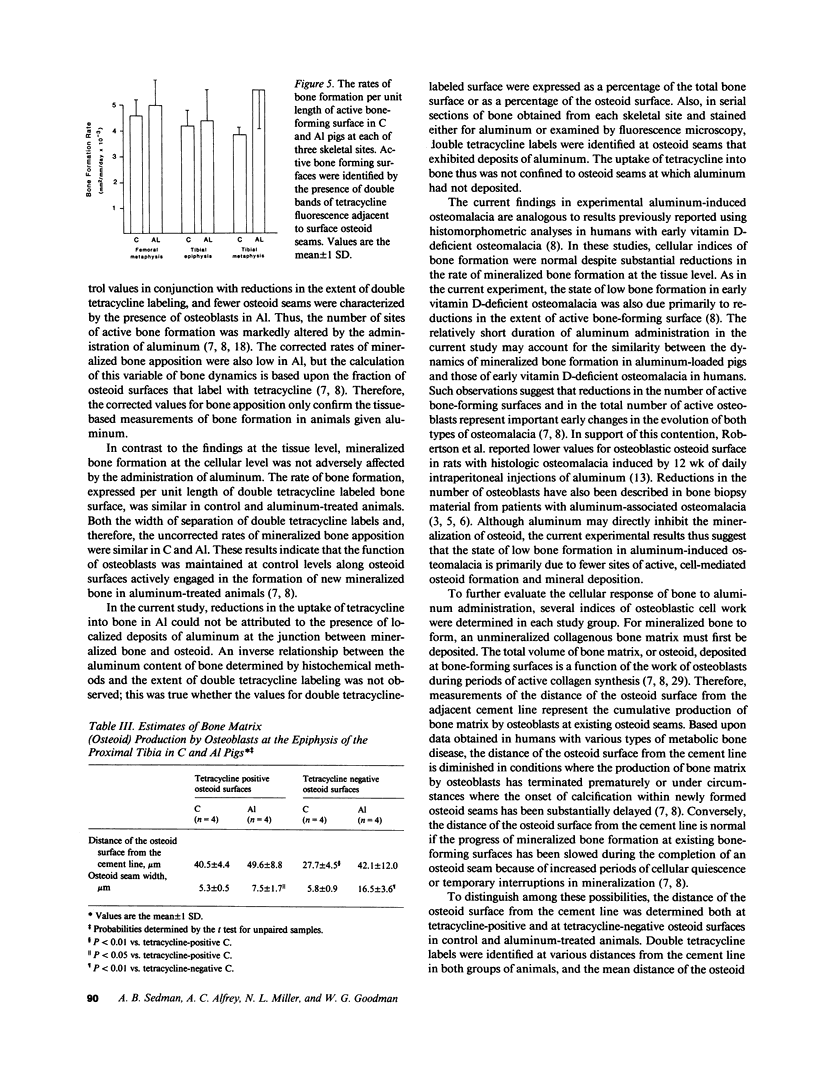
Bone formation is impaired in aluminum-associated bone disease. Reductions in the number of osteoblasts or in the function of individual osteoblasts could account for this finding. Thus, quantitative bone histology and measurements of bone formation were done at three skeletal sites in piglets given aluminum (Al) parenterally, 1.5 mg/kg per d, for 8 wk (Al, n = 4) and in control animals (C, n = 4). Bone Al was 241 +/- 40 mg/kg per dry weight in Al and 1.6 +/- 0.9 in C, P less than 0.001. All Al-treated animals developed osteomalacia with increases in osteoid seam width, osteoid volume, and mineralization lag time at each skeletal site, P less than 0.05 vs. C for all values. Mineralized bone formation at the tissue level was lower in Al than in C, P less than 0.05 for each skeletal site, due to reductions in active bone forming surface. Bone formation at the cellular level was similar in each group, however, and total osteoid production by osteoblasts did not differ in C and Al. Aluminum impairs the formation of mineralized bone in vivo by decreasing the number of active osteoblasts, and this change can be distinguished from the effect of aluminum to inhibit, either directly or indirectly, the calcification of osteoid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfrey A. C., LeGendre G. R., Kaehny W. D. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N Engl J Med. 1976 Jan 22;294(4):184–188. doi: 10.1056/NEJM197601222940402. [DOI] [PubMed] [Google Scholar]
- Andress D., Felsenfeld A. J., Voigts A., Llach F. Parathyroid hormone response to hypocalcemia in hemodialysis patients with osteomalacia. Kidney Int. 1983 Sep;24(3):364–370. doi: 10.1038/ki.1983.168. [DOI] [PubMed] [Google Scholar]
- Baylink D., Stauffer M., Wergedal J., Rich C. Formation, mineralization, and resorption of bone in vitamin D-deficient rats. J Clin Invest. 1970 Jun;49(6):1122–1134. doi: 10.1172/JCI106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. L., Alfrey A. C., Posen S., Lissner D., Hills E., Dunstan C. R., Evans R. A. Effect of aluminum on normal and uremic rats: tissue distribution, vitamin D metabolites, and quantitative bone histology. Calcif Tissue Int. 1983 May;35(3):344–351. doi: 10.1007/BF02405056. [DOI] [PubMed] [Google Scholar]
- Charhon S. A., Chavassieux P. M., Chapuy M. C., Boivin G. Y., Meunier P. J. Low rate of bone formation with or without histologic appearance of osteomalacia in patients with aluminum intoxication. J Lab Clin Med. 1985 Aug;106(2):123–131. [PubMed] [Google Scholar]
- Ellis H. A., McCarthy J. H., Herrington J. Bone aluminium in haemodialysed patients and in rats injected with aluminium chloride: relationship to impaired bone mineralisation. J Clin Pathol. 1979 Aug;32(8):832–844. doi: 10.1136/jcp.32.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame B., Parfitt A. M. Osteomalacia: current concepts. Ann Intern Med. 1978 Dec;89(6):966–982. doi: 10.7326/0003-4819-89-6-966. [DOI] [PubMed] [Google Scholar]
- Goodman W. G., Gilligan J., Horst R. Short-term aluminum administration in the rat. Effects on bone formation and relationship to renal osteomalacia. J Clin Invest. 1984 Jan;73(1):171–181. doi: 10.1172/JCI111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman W. G., Henry D. A., Horst R., Nudelman R. K., Alfrey A. C., Coburn J. W. Parenteral aluminum administration in the dog: II. Induction of osteomalacia and effect on vitamin D metabolism. Kidney Int. 1984 Feb;25(2):370–375. doi: 10.1038/ki.1984.26. [DOI] [PubMed] [Google Scholar]
- Goodman W. G. Short-term aluminum administration in the rat: reductions in bone formation without osteomalacia. J Lab Clin Med. 1984 May;103(5):749–757. [PubMed] [Google Scholar]
- Goodman W. G. The differential response of cortical and trabecular bone to aluminum administration in the rat. Proc Soc Exp Biol Med. 1985 Sep;179(4):509–516. doi: 10.3181/00379727-179-42131. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Tiderström G. Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta. 1973 Feb 12;43(3):305–310. doi: 10.1016/0009-8981(73)90466-x. [DOI] [PubMed] [Google Scholar]
- Hodsman A. B., Sherrard D. J., Alfrey A. C., Ott S., Brickman A. S., Miller N. L., Maloney N. A., Coburn J. W. Bone aluminum and histomorphometric features of renal osteodystrophy. J Clin Endocrinol Metab. 1982 Mar;54(3):539–546. doi: 10.1210/jcem-54-3-539. [DOI] [PubMed] [Google Scholar]
- Hodsman A. B., Sherrard D. J., Wong E. G., Brickman A. S., Lee D. B., Alfrey A. C., Singer F. R., Norman A. W., Coburn J. W. Vitamin-D-resistant osteomalacia in hemodialysis patients lacking secondary hyperparathyroidism. Ann Intern Med. 1981 May;94(5):629–637. doi: 10.7326/0003-4819-94-5-629. [DOI] [PubMed] [Google Scholar]
- Horst R. L., Littledike E. T. Comparison of plasma concentrations of vitamin D and its metabolites in young and aged domestic animals. Comp Biochem Physiol B. 1982;73(3):485–489. doi: 10.1016/0305-0491(82)90064-5. [DOI] [PubMed] [Google Scholar]
- Horst R. L., Littledike E. T., Riley J. L., Napoli J. L. Quantitation of vitamin D and its metabolites and their plasma concentrations in five species of animals. Anal Biochem. 1981 Sep 1;116(1):189–203. doi: 10.1016/0003-2697(81)90344-4. [DOI] [PubMed] [Google Scholar]
- Kahn A. J., Stewart C. C., Teitelbaum S. L. Contact-mediated bone resorption by human monocytes in vitro. Science. 1978 Mar 3;199(4332):988–990. doi: 10.1126/science.622581. [DOI] [PubMed] [Google Scholar]
- Kraut J. A., Shinaberger J. H., Singer F. R., Sherrard D. J., Saxton J., Miller J. H., Kurokawa K., Coburn J. W. Parathyroid gland responsiveness to acute hypocalcemia in dialysis osteomalacia. Kidney Int. 1983 May;23(5):725–730. doi: 10.1038/ki.1983.85. [DOI] [PubMed] [Google Scholar]
- Lustgarten J. A., Wenk R. E. Simple, rapid, kinetic method for serum creatinine measurement. Clin Chem. 1972 Nov;18(11):1419–1422. [PubMed] [Google Scholar]
- Maloney N. A., Ott S. M., Alfrey A. C., Miller N. L., Coburn J. W., Sherrard D. J. Histological quantitation of aluminum in iliac bone from patients with renal failure. J Lab Clin Med. 1982 Feb;99(2):206–216. [PubMed] [Google Scholar]
- Morrissey J., Rothstein M., Mayor G., Slatopolsky E. Suppression of parathyroid hormone secretion by aluminum. Kidney Int. 1983 May;23(5):699–704. doi: 10.1038/ki.1983.81. [DOI] [PubMed] [Google Scholar]
- Mundy G. R. Monocyte-macrophage system and bone resorption. Lab Invest. 1983 Aug;49(2):119–121. [PubMed] [Google Scholar]
- Ott S. M., Maloney N. A., Coburn J. W., Alfrey A. C., Sherrard D. J. The prevalence of bone aluminum deposition in renal osteodystrophy and its relation to the response to calcitriol therapy. N Engl J Med. 1982 Sep 16;307(12):709–713. doi: 10.1056/NEJM198209163071202. [DOI] [PubMed] [Google Scholar]
- Ott S. M., Maloney N. A., Klein G. L., Alfrey A. C., Ament M. E., Coburn J. W., Sherrard D. J. Aluminum is associated with low bone formation in patients receiving chronic parenteral nutrition. Ann Intern Med. 1983 Jun;98(6):910–914. doi: 10.7326/0003-4819-98-6-910. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part I of IV parts: mechanisms of calcium transfer between blood and bone and their cellular basis: morphological and kinetic approaches to bone turnover. Metabolism. 1976 Jul;25(7):809–844. doi: 10.1016/0026-0495(76)90151-7. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part III of IV parts; PTH and osteoblasts, the relationship between bone turnover and bone loss, and the state of the bones in primary hyperparathyroidism. Metabolism. 1976 Sep;25(9):1033–1069. doi: 10.1016/0026-0495(76)90133-5. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone diseases. II. PTH and bone cells: bone turnover and plasma calcium regulation. Metabolism. 1976 Aug;25(8):909–955. doi: 10.1016/0026-0495(76)90124-4. [DOI] [PubMed] [Google Scholar]
- Quarles L. D., Dennis V. W., Gitelman H. J., Harrelson J. M., Drezner M. K. Aluminum deposition at the osteoid-bone interface. An epiphenomenon of the osteomalacic state in vitamin D-deficient dogs. J Clin Invest. 1985 May;75(5):1441–1447. doi: 10.1172/JCI111846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt T. A., Horst R. L., Orf J. W., Hollis B. W. A microassay for 1,25-dihydroxyvitamin D not requiring high performance liquid chromatography: application to clinical studies. J Clin Endocrinol Metab. 1984 Jan;58(1):91–98. doi: 10.1210/jcem-58-1-91. [DOI] [PubMed] [Google Scholar]
- Robertson J. A., Felsenfeld A. J., Haygood C. C., Wilson P., Clarke C., Llach F. Animal model of aluminum-induced osteomalacia: role of chronic renal failure. Kidney Int. 1983 Feb;23(2):327–335. doi: 10.1038/ki.1983.23. [DOI] [PubMed] [Google Scholar]
- Sherrard D. J., Baylink D. J., Wergedal J. E., Maloney N. A. Quantitative histological studies on the pathogenesis of uremic bone disease. J Clin Endocrinol Metab. 1974 Jul;39(1):119–135. doi: 10.1210/jcem-39-1-119. [DOI] [PubMed] [Google Scholar]
- Stauffer M., Baylink D., Wergedal J., Rich C. Decreased bone formation, mineralization, and enhanced resorption in calcium-deficient rats. Am J Physiol. 1973 Aug;225(2):269–276. doi: 10.1152/ajplegacy.1973.225.2.269. [DOI] [PubMed] [Google Scholar]



