Abstract
Aims
While suboptimal adherence to statin medication has been quantified in real-world patient settings, a better understanding of its impact is needed, particularly with respect to distinct problems of medication taking. Our aim was to synthesize current evidence on the impacts of statin adherence, discontinuation and persistence on cardiovascular disease and mortality outcomes.
Methods
We conducted a systematic review of peer-reviewed studies using a mapped search of Medline, Embase and International Pharmaceutical Abstracts databases. Observational studies that met the following criteria were included: defined patient population; statin adherence exposure; defined study outcome [i.e. cardiovascular disease (CVD), mortality]; and reporting of statin-specific results.
Results
Overall, 28 studies were included, with 19 studies evaluating outcomes associated with statin adherence, six with statin discontinuation and three with statin persistence. Among adherence studies, the proportion of days covered was the most widely used measure, with the majority of studies reporting increased risk of CVD (statistically significant risk estimates ranging from 1.22 to 5.26) and mortality (statistically significant risk estimates ranging from 1.25 to 2.54) among non-adherent individuals. There was greater methodological variability in discontinuation and persistence studies. However, findings of increased CVD (statistically significant risk estimates ranging from 1.22 to 1.67) and mortality (statistically significant risk estimates ranging from 1.79 to 5.00) among nonpersistent individuals were also consistently reported.
Conclusions
Observational studies consistently report an increased risk of adverse outcomes associated with poor statin adherence. These findings have important implications for patients and physicians and emphasize the importance of monitoring and encouraging adherence to statin therapy.
Keywords: cardiovascular disease, hydroxymethylglutaryl-CoA reductase inhibitors, medication adherence, mortality, patient compliance, statins
Introduction
Statins are lipid-lowering agents that inhibit the rate-limiting step of cholesterol synthesis [1]. Their beneficial effects, including reduction of mortality and cardiovascular events, have been established in randomized clinical trials, including the West of Scotland Coronary Prevention Study in patients with hyperlipidaemia [2], the Scandinavian Simvastatin Survival Study of patients with angina pectoris or myocardial infarct [3] and the Heart Protection Study in coronary heart disease patients [4]. However, recent evidence has emerged that the effectiveness of statins in real-world settings is inferior to that seen in trials, and this has been attributed to poor medication adherence [5].
Medication adherence is a complex construct that encompasses the following distinct problems: (i) poor execution of the dosing regimen, such that doses are delayed or omitted, which may lead to transient interruptions in drug action; and (ii) discontinuation of the medication, which may lead to intermittent or permanent loss of drug effects [6,7]. There is great need for consistency and use of standardized terminology in the medication adherence literature [8]. The International Society of Pharmacoeconomics and Outcomes Research (ISPOR) Medication Compliance and Persistence Special Interest Group has proposed standardization of terms, with ‘adherence’ referring to conforming to recommendations with respect to timing, dosage and frequency of medication taking and ‘persistence’ referring to conforming to recommendations of continuing treatment for the prescribed duration [9]. We propose that ‘discontinuation’ be included as a separate term when evaluating the problem of stopping therapy, and not the period of medication use.
Poor statin adherence in terms of execution of dosing regimen has been reported in up to 50% of patients [10]. Statin discontinuation rates have been reported ranging from 15 [5] to 75% [11], with most studies reporting rates of ≥50% [12–16]. Long-term persistence is also suboptimal as it has been reported in a study that only 52% of patients remain on statin therapy after 5 years [17]. A comprehensive understanding of statin adherence involves not only quantifying the extent of the problem but also evaluating its impact on relevant patient outcomes. While prior articles have synthesized [18–20] and pooled data [21] on the impact of statin adherence on adverse outcomes, including cardiovascular disease (CVD) events and mortality, they have not distinguished impacts of specific adherence problems. With growing recognition that dynamics and processes involved in problems of medication adherence are different, it is important to evaluate the burden and impacts of statin adherence, discontinuation and persistence separately [7]. Prior articles have also combined clinical trials along with observational studies, which may be problematic as adherence rates reported in clinical trials may not reflect rates observed in real-world settings [5]. To address these issues and update the evidence, we conducted a systematic review of observational studies among real-world patient settings evaluating adverse outcomes associated with distinct problems of statin adherence, discontinuation and persistence.
Methods
Literature search strategy
In August 2013, we searched the databases Medline (1966 onwards), Embase (1980 onwards) and International Pharmaceutical Abstracts (1970 onwards), using terms that mapped to Medical Subject Headings in combination with keywords for nonmapping concepts (e.g. ‘discontinuation’, ‘persistence’; Table 1). We also conducted a hand search of bibliographies of articles retrieved from the electronic search.
Table 1.
Medical Subject Headings (MeSH) terms and keywords applied in electronic search strategy
| Concept | MeSH terms | Keywords |
|---|---|---|
| Statins | Antilipaemic agents, anticholesterolaemic agents, hydroxymethylglutaryl-CoA reductase inhibitors, lovastatin, simvastatin, pravastatin | Statins |
| Adherence | Health behaviour, patient compliance, medication adherence, quality of healthcare, guideline adherence | Patient compliance, compliance, adherence, persistence, discontinuation |
| Cardiovascular diseases | Cardiovascular diseases, heart diseases, coronary artery disease, myocardial infarction, vascular diseases | Acute myocardial infarction, cardiovascular disease(s) |
| Mortality | Mortality, cause of death, fatal outcome, hospital mortality, survival rate, death | Mortality, death |
Selection of studies
Titles and abstracts were reviewed for selection of publications meeting the following inclusion criteria: (i) prospective observational study design (e.g. cohort study, nested case–control study); (ii) population of adults (≥18 years of age); (iii) statin exposure as either execution of dosing (‘adherence’), stopping of medication (‘discontinuation’) or duration of therapy (‘persistence’); (iv) defined study outcome (e.g. CVD, mortality); and (v) reporting of statin-specific results. Two authors (MADeV and LCB) independently reviewed all titles, abstracts and articles for selection, quality assessment and data extraction. Discrepancies were resolved by consensus.
Data extraction and quality assessment
We extracted information on year of publication, country, study design, patient population and size, length of study follow-up and data source (e.g. administrative database, electronic pharmacy record). Of particular importance was information on the type of statin non-adherence, and we categorized studies as those evaluating the impact of the following: (i) poor execution of the dosing regimen (‘adherence’); (ii) stopping of medication (‘discontinuation’); or (iii) the duration of time patients remained on therapy (‘persistence’). Equally important as how the problem was operationalized was how it was measured, and we extracted information on measurement of the following factors: (i) adherence [e.g. proportion of days covered (PDC), medication possession ratio]; (ii) discontinuation (e.g. time point at which discontinuation status was defined); and (iii) persistence (e.g. number of months on statin treatment). We also assessed whether adherence was treated as a fixed-in-time or time-dependent variable, cut-off values to categorize subjects who were adherent and non-adherent, and the reference category assigned for statistical models. We also extracted information on study outcomes (e.g. CVD, mortality) and reported measures of association [e.g. odds ratio (OR), relative risk (RR), hazard ratio (HR)]. We plotted reported risk estimates (and corresponding lower and upper limits) for mortality (Figure 2) and CVD outcomes (Figure 3), according to adherence, discontinuation and persistence studies, to show the association between statin non-adherence and adverse outcome. That is, for studies that modelled non-adherent subjects as the reference group, we plotted the reported risk estimate and for studies that modelled adherent subjects as the reference group, we calculated the inverse of the reported risk estimate (i.e. 1/OR) and lower and upper limits.
Figure 2.
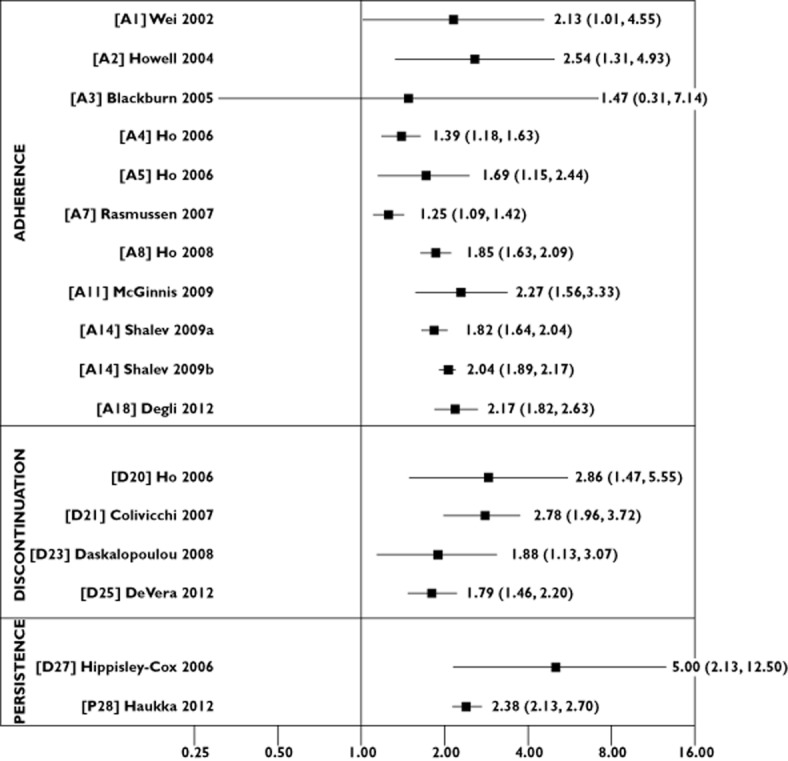
Risk estimates for the association between statin non-adherence and mortality outcomes according to adherence, discontinuation and persistence studies
Figure 3.
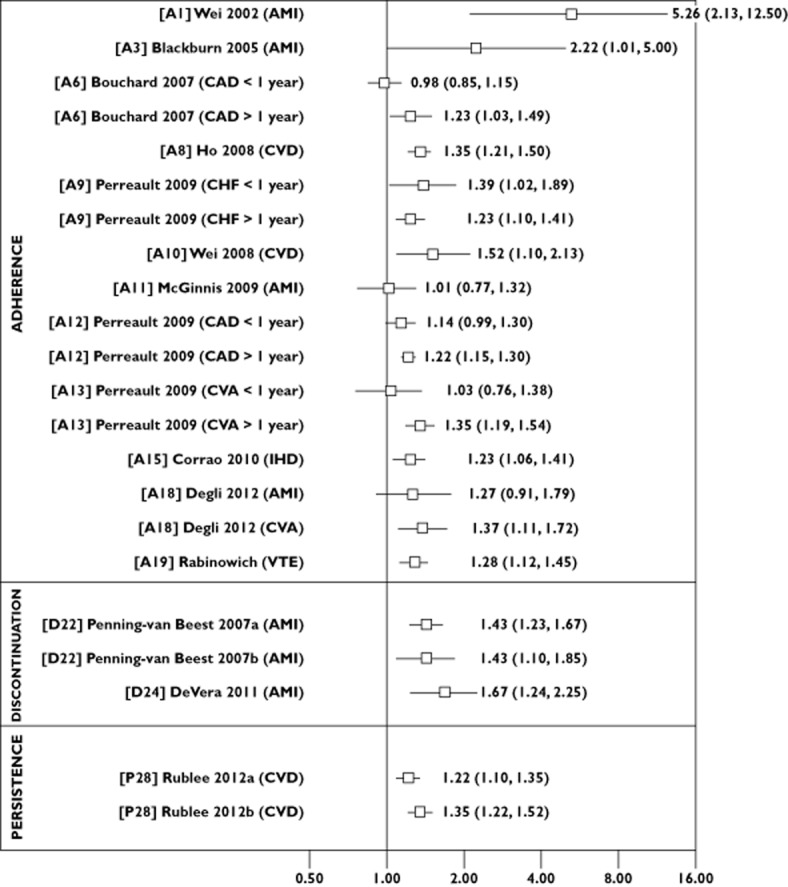
Risk estimates for the association between statin non-adherence and cardiovascular disease outcomes according to adherence, discontinuation and persistence studies. Abbreviations are as follows: AMI, acute myocardial infarction; CAD, coronary artery disease; CHF, chronic heart failure; CVA, cerebrovascular accident; CVD, cardiovascular disease; IHD, ischaemic heart disease; VTE, venous thromboembolism
We also extracted information on confounders included in multivariable analyses. We applied the World Health Organization's five dimensions (factors) of medication adherence as a framework and categorized confounders according to the following factors: (i) patient factors; (ii) condition factors; (iii) therapy factors; (iv) social/economic factors; and (v) healthcare system factors [22]. We further assigned subcategories; for example, demographic characteristics (e.g. age, sex) comprise a subcategory within patient factor, while comorbidities and medications are subcategories within condition factors.
We assessed studies by applying guidelines by ISPOR's Medication Compliance and Persistence Special Interest Group, which were developed to meet the need for improved consistency and quality among studies evaluating problems of medication taking, by establishing standards for data sources, operational definitions, measurement of medication adherence and reporting of results [23]. We condensed the guidelines into a 20-item checklist, which included items on appropriate description of data sources, explicit definition and calculation of adherence exposures, and explicit definition of outcome, to yield a score (0–20) based on the sum of items. Due to the heterogeneity in patient populations and exposure definitions of statin adherence across studies, a meta-analysis was not conducted.
Results
Literature search
The electronic search strategy identified 2615 articles, with 31 articles included after hand searching references (Figure 1). Abstract review led to the exclusion of 142 articles due to lack of outcome (n = 33), exposure (n = 22) or both (n = 28), irrelevant study type (n = 56) and lack of assessment of impact of exposure on outcome (n = 3). After review of 36 studies, failure to report statin-specific results led to further exclusion of eight studies. Overall, 28 studies were included; 19 studies evaluated outcomes associated with statin adherence, six with statin discontinuation and three with statin persistence. As described earlier in the Introduction, while discontinuation and persistence represent opposite concepts and could be classified together, we separated studies into respective types to distinguish between those that evaluated the problem of stopping therapy and those that evaluated the period of continuous use.
Figure 1.
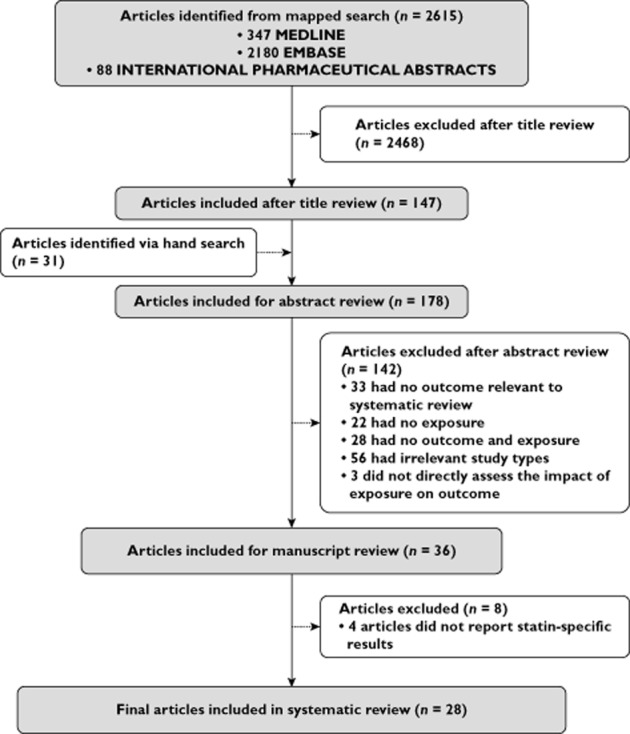
Systematic review study flow diagram
Study characteristics, including design, patient population, sample size, follow-up period and assessment score, are summarized in Table 2. Scores ranged from 15 to 19, suggesting that most articles included key recommended items. Table 3 summarizes definition and measurement of statin adherence, outcomes evaluated and main results. In addition, the final column in Table 3 shows confounders included in reported multivariable models grouped according to the World Health Organization's five dimensions of medication adherence, i.e. patient (P), condition (C), therapy (T), social/economic (S) and healthcare system (H) factors. Table S1 provides detailed, item-by-item information on these confounders. Figures 2 and 3 summarize the main results by showing risk estimates for the association between non-adherence and mortality and CVD outcomes, respectively.
Table 2.
Characteristics of studies included in the systematic review
| Study ID | Author | Country | Setting | Study design | Patient population | Sample size | Follow-up (years) | Quality score |
|---|---|---|---|---|---|---|---|---|
| Statin adherence studies | ||||||||
| A1 | Wei et al. (2002) [32] | Scotland | Population based | Cohort | AMI | 5590 | 2.4 | 17 |
| A2 | Howell et al. (2004) [40] | UK | General practitioner practice | Cohort | Primary care | 1010 | 2.6 | 15 |
| A3 | Blackburn et al. (2005) [34] | Canada | Population based | Cohort | CAD | 1221 | 3.2 | 18 |
| A4 | Ho et al. (2006) [42] | USA | Health maintenance organization | Cohort | Diabetes | 11 532 | 1.3 | 17 |
| A5 | Ho et al. (2006) [36] | USA | Health maintenance organization | Cohort | Diabetes with IHD | 3696 | 1 | 19 |
| A6 | Bouchard et al. (2007) [24] | Canada | Population based | Nested case–control | General population | 20 543 | 1.6 | 18 |
| A7 | Rasmussen et al. (2007) [35] | Canada | Population based | Cohort | AMI | 31 455 | 2.4 | 18 |
| A8 | Ho et al. (2008) [37] | USA | Health maintenance organization | Cohort | CAD | 13 596 | 4.1 | 19 |
| A9 | Perreault et al. (2008) [25] | Canada | Population based | Nested case–control | General population | 111 481 | – | 19 |
| A10 | Wei et al. (2008) [33] | Scotland | Population based | Cohort | CVD | 3472 | – | 17 |
| A11 | McGinnis et al. (2009) [38] | USA | Health maintenance organization | Cohort | AMI | 2201 | – | 15 |
| A12 | Perreault et al. (2009) [26] | Canada | Population based | Nested case–control | General population | 115 290 | – | 19 |
| A13 | Perreault et al. (2009) [27] | Canada | Population based | Nested case–control | General population | 112 092 | 2.95 | 19 |
| A14 | Shalev et al. (2009) [41] | Israel | Health maintenance organization | Cohort | (i) General population (ii) CHD |
229 918 | (i) 4 (ii) 5 |
18 |
| A15 | Corrao et al. (2010) [29] | Italy | Population based | Cohort | General population | 90 832 | 4.25 | 19 |
| A16 | Tuppin et al. (2010) [39] | France | National health insurance (∼70%) | Cohort | AMI | 11 604 | 2.5 | 17 |
| A17 | Dragomir et al. (2010) [28] | Canada | Population based | Cohort | General population | 55 134 | 3 | 18 |
| A18 | Degli Esposti et al. (2012) [30] | Italy | Local health unit | Cohort | General population | 19 232 | 1.9 | 17 |
| A19 | Rabinowich et al. (2012) [31] | Israel | Health maintenance organization | Cohort | General population | 127 822 | 4.65 | 18 |
| Statin discontinuation studies | ||||||||
| D20 | Ho et al. (2006) [43] | USA | Hospital | Cohort | AMI | 1521 | 1 | 16 |
| D21 | Colivicchi et al. (2007) [44] | Italy | Hospital | Cohort | CVA | 631 | 1 | 16 |
| D22 | Penning-van Beest et al. (2007) [46] | The Netherlands | Population based | Cohort | (i) General population (ii) CVD |
(i) 46 332 (ii) 12 762 |
– | 17 |
| D23 | Daskalopoulou et al. (2008) [45] | UK | Population based | Cohort | AMI | 9939 | 1 | 18 |
| D24 | De Vera et al. (2011) [47] | Canada | Population based | Cohort | Rheumatoid arthritis | 4102 | 3.5 | 19 |
| D25 | De Vera et al. (2012) [48] | Canada | Population based | Cohort | Rheumatoid arthritis | 4102 | 3.6 | 20 |
| Statin persistence studies | ||||||||
| P26 | Hippisley-Cox & Coupland (2006) [49] | UK | Population based | Nested case–control | IHD | 13 029 | 1.6 | 19 |
| P27 | Haukka et al. (2012) [50] | Finland | Population based | Cohort | General population | 336 618 | 4.4 | 18 |
| P28 | Rublee et al. (2012) [51] | USA | Commercial insurance members | Cohort | (i) General population (ii) CHD |
(i) 79 010 (ii) 15 277 |
1 | 16 |
Abbreviations are as follows: AMI, acute myocardial infarction; CAD, coronary artery disease; CHD, coronary heart disease; CVA, cerebrovascular accident; CVD, cardiovascular disease; IHD, ischaemic heart disease.
Table 3.
Statin adherence exposure definitions, outcomes, results and confounders considered in multivariable models of studies included in the systematic review
| Study ID | Author | Patient population | Exposure definition | Type of variable | Variable categories and reference group | Outcomes | Main results | Confounders according to WHO dimensions | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statin adherence studies | ||||||||||||
| A1 | Wei et al. (2002) [32] | AMI | PDC statin initiation to outcome/end of study | Fixed in time | ≥0.80 0.40–070 <0.39 0* (NA) |
(i) AMI | (i) RR 0.19 (0.08–0.47) | P | C | T | S | H |
| (ii) Mortality | (ii) RR 0.47 (0.22–0.99) | |||||||||||
| A2 | Howell et al. (2004) [40] | Primary care | PDC statin from first to last statin prescription | Fixed in time | ≥0.80* <0.80 |
(i) AMI | (i) Not reported | P | C | – | – | – |
| (ii) Mortality | (ii) HR 2.54 (1.31–4.93) | |||||||||||
| A3 | Blackburn et al. (2005) [34] | CAD | Statin fill frequency | Fixed in time | ≥80% ≤60%* (NA) |
(i) AMI | HR 0.45 (0.20–0.99) | P | C | T | – | H |
| (ii) Mortality | HR 0.68 (0.14–3.26) | |||||||||||
| A4 | Ho et al. (2006) [42] | Diabetes | 1 year PDC from statin initiation | Fixed in time | ≥0.80* (A) <0.80 |
Mortality | OR 1.39 (1.18–1.63) | P | C | – | – | – |
| A5 | Ho et al. (2006) [36] | Diabetes with IHD | 1 year PDC from statin initiation | Fixed in time | ≥0.80 <0.80* (NA) |
Mortality | OR 0.59 (0.41–0.87) | P | C | – | – | – |
| A6 | Bouchard et al. (2007) [24] | General population | PDC statin initiation to outcome/end of study | Fixed in time | ≥0.90 <0.90* (NA) |
(i) CAD <1 year | (i) OR 1.02 (0.87–1.18) | P | C | T | S | – |
| (ii) CAD >1 year | (ii) OR 0.81 (0.67–0.97) | |||||||||||
| A7 | Rasmussen et al. (2007) [35] | AMI | 1 year PDC from statin initiation | Fixed in time | ≥0.80* (A) 0.40–0.80 <0.40 |
Mortality | RR 1.25 (1.09–1.42) | P | C | – | S | H |
| A8 | Ho et al. (2008) [37] | CAD | PDC over 180 day time intervals | Time dependent | ≥0.80* (A) <0.80 |
(i) Mortality | (i) HR 1.85 (1.63–2.09) | P | C | – | – | – |
| (ii) CVD mortality | (ii) HR 1.62 (1.24–2.13) | |||||||||||
| (iii) CVD | (iii) HR 1.35 (1.21–1.50) | |||||||||||
| A9 | Perreault et al. (2008) [25] | General population | MPR statin initiation to outcome/end of study | Fixed in time | ≥80% 60–79% 40–59% 20–39% 1–19%* (NA) |
(i) CHF <1 year | (i) OR 0.72 (0.53–0.98) | P | C | T | S | – |
| (ii) CHF >1 year | (ii) OR 0.81 (0.71–0.91) | |||||||||||
| A10 | Wei et al. (2008) [33] | CVD | PDC statin initiation to outcome/end of study | Fixed in time | ≥0.80 <0.80* (NA) |
CVD | HR 0.66 (0.47–0.91) | P | C | – | S | – |
| A11 | McGinnis et al. (2009) [38] | AMI | PDC statin initiation to outcome/end of study | Fixed in time | ≥0.80 <0.80* (NA) |
– | (i) HR 0.44 (0.30–0.64) | P | C | – | – | – |
| (ii) HR 0.99 (0.76–1.30) | ||||||||||||
| A12 | Perreault et al. (2009) [26] | General population | MPR statin initiation to outcome/end of study | Fixed in time | ≥80% 60–79% 40–59% 20–39% 1–19%* (NA) |
(i) CAD <1 year | (i) OR 0.88 (0.77–1.01) | P | C | T | S | – |
| (ii) CAD >1 year | (ii) OR 0.82 (0.77–0.87) | |||||||||||
| A13 | Perreault et al. (2009) [27] | General population | MPR statin initiation to outcome/end of study | Fixed in time | ≥80% 60–79% 40–59% 20–39% 1–19%* (NA) |
(i) CVA <1 year | (i) OR 1.03 (0.76–1.38) | P | C | T | S | – |
| (ii) CVA >1 year | (ii) OR 0.74 (0.65–0.84) | |||||||||||
| A14 | Shalev et al. (2009) [41] | (i) General population (ii) CHD |
PDC statin initiation to outcome/end of study | Fixed in time | PDC ≥ 0.90 PDC < 0.90* (NA) |
Mortality | (i) HR 0.55 (0.49–0.61) | P | C | T | S | H |
| (ii) HR 0.49 (0.46–0.53) | ||||||||||||
| A15 | Corrao et al. (2010) [29] | General population | PDC statin initiation to outcome/end of study | Time dependent | >75% 51–75% 26–50% ≤25%* (NA) |
IHD | HR 0.81 (0.71–0.94) | P | C | T | – | – |
| A16 | Tuppin et al. (2010) [39] | AMI | PDC statin initiation to outcome/end of study | Fixed in time | >0.80* (A) ≤0.80 |
Mortality or ACS | HR 1.58 (1.37–1.81) | P | C | – | S | H |
| A17 | Dragomir et al. (2010) [28] | General population | MPR statin initiation to outcome/end of study | Fixed in time | ≥0.80* (A) <0.80 |
(i) CAD | (i) HR 1.07 (1.01–1.13) | P | C | – | S | H |
| (ii) CVA | (ii) HR 1.13 (1.01–1.25) | |||||||||||
| (iii) CHF | (iii) HR 1.13 (1.01–1.15) | |||||||||||
| A18 | Degli Esposti et al. (2012) [30] | General population | PDC statin initiation to outcome/end of study | Fixed in time | >80% 61–80% 41–60% 21–40%* (NA) |
(i) Mortality | (i) HR 0.46 (0.38–0.55) | P | C | – | – | – |
| (ii) AMI | (ii) HR 0.79 (0.56–1.10) | |||||||||||
| (iii) CVA | (iii) HR 0.73 (0.58–0.90) | |||||||||||
| A19 | Rabinowich et al. (2012) [31] | General population | PDC statin initiation to outcome/end of study | Fixed in time | ≥66% 33–66% <33%* (NA) |
VTE | HR 0.78 (0.69–0.89) | P | C | – | – | – |
| Statin discontinuation studies | ||||||||||||
| D20 | Ho et al. (2006) [43] | AMI | Statin discontinuation at 1 month postdischarge | Fixed in time | Nondiscontinuer* (A) Discontinuer |
Mortality | HR 2.86 (1.47–5.55) | P | C | – | S | H |
| D21 | Colivicchi et al. (2007) [44] | CVA | Statin discontinuation at 1, 6 and 12 months after discharge | Time dependent | Nondiscontinuer* (A) Discontinuer |
Mortality | HR 2.78 (1.96–3.72) | P | C | – | – | – |
| D22 | Penning-van Beest et al. (2007) [46] | (i) General population (ii) CVD |
Continuous statin use in the first 2 years of treatment | Fixed in time | 2 years continous use 18 months–2 years continuous use <18 months continous use* (NA) |
AMI | (i) RR 0.70 (0.60–0.81) | P | C | S | – | – |
| (ii) RR 0.70 (0.54–0.91) | ||||||||||||
| D23 | Daskalopoulou et al. (2008) [45] | AMI | Statin discontinuation in the first 90 days post-AMI | Fixed in time | Non-user* (NU) Users Starters Stoppers |
Mortality | RR 1.88 (1.13–3.07) | P | C | – | – | H |
| D24 | De Vera et al. (2011) [47] | Rheumatoid arthritis | Statin discontinuation status in month before outcome | Time dependent | Nondiscontinuer* (A) Discontinuer |
AMI | HR 1.67 (1.24–2.25) | P | C | T | – | H |
| D25 | De Vera et al. (2012) [48] | Rheumatoid arthritis | Statin discontinuation status in month before outcome | Time dependent | Nondiscontinuer* (A) Discontinuer |
(i) CVD mortality | (i) HR 1.60 (1.15–2.23) | P | C | T | – | H |
| (ii) Mortality | (ii) HR 1.79 (1.46–2.20) | |||||||||||
| Statin persistence studies | ||||||||||||
| P26 | Hippisley-Cox & Coupland (2006) [49] | IHD | Persistence of statin use (months) | Time dependent | >60 months No statin use* (NU) |
Mortality | HR 0.20 (0.08–0.47) | P | C | – | S | – |
| P27 | Haukka et al. (2012) [50] | General population | Statin use as function of time | Time dependent | Persistent Nonpersistent* (NA) |
Mortality | HR 0.39 (0.37–0.40) | P | C | – | — | – |
| P28 | Rublee et al. (2012) [51] | (i) General population (ii) CHD |
Persistence of statin use using anniversary method | Time dependent | Persistent Nonpersistent* (NA) |
(i) CVD (ii) CVD |
(i) HR 0.82 (0.74–0.91) | P | C | – | S | H |
| (ii) HR 0.74 (0.66–0.82) | ||||||||||||
Abbreviations are as follows: ACS, acute coronary syndrome; AMI, acute myocardial infarction; CAD, coronary artery disease; CHF, chronic heart failure; CVA, cerebrovascular accident; CVD, cardiovascular disease; HR, hazard ratio; MPR, medication possession ratio; OR, odds ratio; PDC, proportion of days covered; RR, relative risk; VTE, venous thromboembolism. Abbreviations for confounders considered according to the World Health Organization's five dimensions (factors) of medication non-adherence are as follows: C, condition factors; H, healthcare system factors; P, patient factors; S, social/economic factors; T, therapy factors.
Abbreviations for referent categories are as follows: A, adherent group is referent category; NA, non-adherent group is referent category; NU, non-user group is referent category.
Statin adherence studies
Most of the studies included (n = 19; study IDs A1–A19) evaluated outcomes associated with statin adherence. Eight studies investigated statin adherence for primary prevention in general populations (A6, A9, A12, A13, A15, A17–A19), eight for secondary prevention among patients with prior CVD conditions [A1, A3, A5, A7, A8, A10, A11, A16], and three studies investigated both primary and secondary prevention [A2, A4, A14].
Primary prevention
Five Canadian studies evaluated the impact of statin adherence in the general population on various CVD outcomes using administrative data from Quebec. Bouchard et al. [24] [A6] used a cohort design and defined a population of individuals aged 50–64 years without CVD and newly treated with statins. They calculated PDC as the number of days of statin medication dispensed divided by the number of days over which the prescriptions were used and applied a cut-off of ≥0.90 to define good adherence. Altogether, authors reported a significant association between good adherence and lower risk of coronary artery disease [CAD; OR, 0.81; 95% confidence interval (CI), 0.67–0.97] after the first year of follow-up [24]. Applying a nested case–control design, three subsequent studies by Perreault et al. used the same cohort of individuals aged 45–85 years without CVD and newly treated with statins to evaluate the following unique outcomes of: (i) chronic heart failure [25] [A9]; (ii) CAD [26] [A12]; and (iii) cerebrovascular accidents (CVA) [27] [A13]. Across all studies, the medication possession ratio, representing the percentage of days exposed to statins in a given follow-up period, was calculated from the start of statin prescription until the date of the outcome for cases and date of selection for controls and categorized as ≥80, 60–79, 40–59, 20–39 and 1–19% (reference group) [25–27]. Authors reported similar associations with the highest adherence category and chronic heart failure occurring in the first year of follow-up (OR, 0.72; 95% CI, 0.53–0.98) and after 1 year of follow-up (OR, 081; 95% CI, 0.71–0.91) [25] [A9] and significant associations for CAD (OR, 0.82; 95% CI, 0.77–0.87) [26] [A12] and CVA (OR, 0.74; 95% CI, 0.65–0.84) [27] [A13] after 1 year of follow-up. Of note, while the same base cohort was applied, each study yielded unique samples of cases representing each outcome and corresponding controls {coronary heart disease (n = 4309 cases; n = 45 707 controls) [A9]; CAD (n = 15 268 cases; n = 227 646 controls) [A12]; and CVA (n = 3959 cases; n = 58 972 controls) [A13]} suggesting minimal to no overlap across. Finally, Dragomir et al. [28] [A17] also used a cohort of new statin users and the medication possession ratio with a cut-off of 80% to evaluate the impact of adherence on healthcare services and costs. While not the primary outcomes, they additionally reported significant associations with CAD (OR, 1.07; 95% CI, 1.01–1.13), CVA (OR, 1.13; 95% CI, 1.01–1.25) and chronic heart failure (OR, 1.13; 95% CI, 1.01–1.15) [28] [A17].
Two studies [A15, A18] from Italy evaluated statin adherence in the general population. Corrao et al. [29] [A15] used population-based data from Italy's National Health Service to identify individuals with first statin prescriptions. Statin adherence was assessed using PDC (the number of days for which medication was dispensed divided by the number of follow-up days) categorized as very low (≤25%), low (26–50%), intermediate (51–75%) and high (>75%) coverage. Compared with individuals in the very low coverage group, those with high coverage had lower risk of ischaemic heart disease (HR, 0.81; 95% CI, 0.71–0.94) [29]. Degli Esposti et al. [30] [A18] used local health unit databases from Florence in their study of incident statin users, with PDC from the first prescription to the study outcome calculated, and categorized as low (21–40%), intermediate-low (41–60%), intermediate-high (61–80%) and high (>80%; individuals with PDC ≤20% were excluded). Compared with individuals with low adherence, those with high (HR, 0.46; 95% CI, 0.37–0.55), intermediate-high (HR, 0.53; 95% CI, 0.43–0.66) and intermediate-low adherence (HR, 0.81; 95% CI, 0.66–0.99) had reduced mortality [30].
Finally, the study by Rabinowich et al. [31] [A19] using health maintenance organization data in Israel was the only one evaluating venous thromboembolism outcomes. The PDC was calculated from the first prescription to the end of the study and divided into three groups (<33, 33–66 and ≥66%). Individuals with intermediate (HR, 0.81; 95% CI, 0.70–0.93) and high adherence (HR, 0.78; 95% CI, 0.69–0.89) had lower risk of venous thromboembolism compared with those who had low adherence [31].
Secondary prevention
The majority of studies of statin adherence were conducted among patients with prior CVD [A1, A3, A5, A7, A8, A10, A11, A16]. Using population-based data from the Medicine Monitoring Unit's database in Tayside, UK, the study by Wei et al. [32] [A1] in post-acute myocardial infarction (AMI) patients used PDC calculated as the number of days of statin supply divided by the number of days from the first prescription to the end of the study, grouped into four categories (0, <0.39, 0.40–0.70 and ≥0.80). Compared with the zero adherence group, those in the highest adherence category had a lower risk of AMI recurrence (HR, 0.19; 95% CI, 0.08–0.47) and mortality (HR, 0.47; 95% CI, 0.22–0.99) [32]. Using the same database, Wei et al. [33] [A10] focused on individuals with prior CVD, defined as hospitalization due to angina, AMI, heart failure, CVA or peripheral vascular disease. The PDC was calculated in a similar manner but dichotomized using a cut-off value of ≥0.80 to define good adherence. A protective effect of good adherence on recurrent CVD outcomes was reported (HR, 0.66; 95% CI, 0.47–0.91) [33].
Two Canadian studies [A3, A7] used population-based provincial administrative health databases. Blackburn et al. [34] [A3] studied individuals with prior CAD in Saskatchewan, defining adherence using statin fill frequency or the number of prescription fills divided by the months of observation [34]. Cut-off values were ≤60% (non-adherent) and ≥80% (adherent). Compared with the non-adherent group, those in the adherent group had significantly lower risk of AMI (HR, 0.45; 95% CI, 0.20–0.99) [34]. In Ontario, Rasmussen et al. [35] [A7] evaluated the impact of adherence to cardioprotective medications, including statins, in AMI patients. One year PDC was calculated and categorized as high (≥0.80), intermediate (0.40–0.79) and low adherence (<0.40). Compared with patients in the high adherence group, those in the low adherence group had a higher risk of mortality (HR, 1.25; 95% CI, 1.09–1.42) [35].
Three secondary prevention studies of statin adherence were based on data from a health maintenance organization in the USA [A5, A8, A11]. Ho et al. [36] [A5] evaluated diabetes patients with ischaemic heart disease, using 1 year PDC, calculated as the number of days of prescription divided by 365 [36]. Compared with patients in the non-adherent group (<0.80), patients in the adherent group had a lower risk of mortality (OR, 0.59; 95% CI, 0.41–0.87) [36]. Another study by Ho et al. [37] [A8] evaluated the impact of adherence to cardioprotective medications among individuals with prior CAD. The PDC was calculated over 180 day intervals from initiation of medication until the end of follow-up, and modelled as a time-dependent variable. The authors reported that statin non-adherence (<0.80) was associated with increased overall mortality (HR, 1.85; 95% CI, 1.63–2.09), CVD mortality (HR, 1.62; 95% CI 1.24–2.13) and CVD (HR, 1.35; 95% CI 1.21–1.50) [37]. McGinnis et al. [38] [A11] evaluated the impact of statin adherence on mortality and AMI recurrence in patients with prior AMI, using PDC categorized at the cut-off value of ≥0.80. Good statin adherence was significantly associated with a decreased mortality (HR, 0.44; 95% CI, 0.30–0.64), but not AMI (HR, 0.99; 95% CI, 0.76–1.30) [38].
Finally, using national health insurance data covering ∼70% of the population of France, Tuppin et al. [39] [A16] evaluated statin adherence following hospital admission for AMI. Adherence was defined using PDC calculated from statin initiation until the end of the study and a cut-off value of 0.80. Non-adherence was significantly associated with higher risk of the combined study outcome of mortality or hospitalization for acute coronary syndrome (HR 1.58; 95% CI, 1.37–1.81); however, separate risk estimates for each outcome were not reported [39].
Mixed primary and secondary prevention
In the UK, the study by Howell et al. [40] [A2] of individuals in primary care with and without a history of coronary heart disease used electronic medical record data from a group of general practitioners in Liverpool. The PDC was calculated as the number of days of statin prescription dispensed divided by the number of days between the first and last prescriptions, and dichotomized using a cut-off value of 0.80. A 2.5-fold increased risk of mortality was reported in the non-adherent group compared with the adherent group (HR, 2.54; 95% CI, 1.31–4.93) [40]. A study by Shalev et al. [41] [A14] used health maintenance organization data from Israel to evaluate the impact of statin adherence on mortality in adults from the general population prescribed statins and in coronary heart disease patients. The PDC was calculated from the date of first statin prescription to the end of follow-up, and subjects were categorized as adherent or non-adherent based on a cut-off value of 0.90. Authors reported similar protective effects of statin adherence on mortality in both the general (HR, 0.55; 95% CI, 0.49–0.61) and coronary heart disease patient populations (HR, 0.49; 95% CI, 0.46–0.53) [41]. Finally, a study by Ho et al. [42] [A4] evaluated the impact of medication adherence on mortality in a community cohort of patients with diabetes, regardless of baseline CVD risk, using US health maintenance organization data. Medications evaluated included oral hypoglycaemic agents, antihypertensives and statins, and for each medication category the PDC in the first year of therapy was calculated as the number of days of prescription dispensed divided by 365, and dichotomized using a 0.80 cut-off value. The authors reported an increased risk of mortality with non-adherence to statins (OR, 1.39; 95% CI, 1.18–1.63) and cardioprotective medications overall (HR, 1.77; 95% CI, 1.45–2.15) [42].
Statin discontinuation studies
A smaller number of statin discontinuation studies were identified (n = 6; study IDs D20–D25). Three studies investigated statin use for secondary prevention [D20, D21, D23] and three studies investigated both primary and secondary prevention [D22, D24, D25].
Secondary prevention
Using data from the registry data from 19 US hospitals, Ho et al. [43] [D20] evaluated mortality associated with discontinuation of statins among patients following AMI hospitalization. Discontinuation of medication was based on patient reports during telephone interviews 1 month after discharge. Statin discontinuation was associated with a 2.86-fold increased risk of mortality (HR, 2.86; 95% CI, 1.47–5.55) over the 1 year study follow-up [43]. In Italy, Colivicchi et al. [44] [D21] assessed the impact of statin discontinuation on mortality among patients discharged from hospital following an acute ischaemic stroke. Discontinuation was assessed by telephone interview at 1, 6 and 12 months, and a time-dependent explanatory variable was used in Cox proportional hazards models to account for changes in statin use status over follow-up. Authors reported a 2.78-fold increased risk of death associated with statin discontinuation (HR, 2.78; 95% CI, 1.96–3.72) [44]. Finally, using data from the UK General Research Practice Database (GPRD), Daskalopoulou et al. [45] [D23] evaluated the extent to which different patterns of statin use before and after an index AMI event were associated with mortality. Patients were classified into the following four groups based on statin use before the index AMI and during the 90 days following the AMI: non-users (never on statins); users (statins before and post-AMI); starters (no statin before and started statins post-AMI); and stoppers (statins before and stopped statins post-AMI). Authors used ‘non-users’ as the reference group and reported an 88% increased risk of mortality in statin stoppers relative to individuals who never used a statin [45]. Authors did not report results comparing statin stoppers vs. continuous users.
Mixed primary and secondary prevention
In The Netherlands, Penning-van Beest et al. [46] [D22] used administrative pharmacy records to evaluate the impact of statin discontinuation on AMI outcomes in low-risk (general population) and high-risk (prior CVD) populations. The definition of statin exposure was based on the number of days of continuous statin use in the first 2 years of treatment, and patients were categorized as follows: (i) 2 years of continuous use; (ii) 18 months of continuous use; and (iii) <18 months of continuous use (noncontinuous). Authors reported that compared with noncontinuous users, 2 years continuous statin users had a lower risk of AMI in both the primary (HR, 0.70; 95% CI, 0.60–0.81) and secondary (HR, 0.70; 95% CI, 0.54–0.91) prevention groups [46].
Using administrative databases in British Columbia, Canada, De Vera et al. studied incident statin users from a population-based cohort of rheumatoid arthritis patients, with and without prior CVD, to evaluate risk of AMI [47] [D24] and mortality [48] [D25]. Statin discontinuation was defined as no statin prescription for 3 months or more at any time during therapy course, and Cox proportional hazards models were used to model statin discontinuation as a time-dependent variable over the follow-up. Statin discontinuation was associated with an increased risk of AMI in patients with no prior AMI (HR, 1.61; 95% CI, 1.16–2.22) as well as in patients with prior AMI (HR, 1.55; 95% CI, 1.07–3.36) [47]. In the latter study, statin discontinuation was also associated with an increased risk of CVD mortality (HR, 1.79; 95% CI, 1.46–2.20) and of all-cause mortality (HR, 1.60; 95% CI, 1.15–2.23) [48].
Statin persistence studies
Three studies evaluated statin persistence; one for primary prevention [P26], one for secondary prevention [P27] and one for both [P28]. Hippisley-Cox & Coupland [49] [P26] studied individuals with ischaemic heart disease using the UK QRESEARCH database. Persistence of statin use was measured in months of use, and the authors found that compared with nonstatin users, those with the longest persistence (>60 months duration of use) had the lowest risk of mortality (OR, 0.20; 95% CI, 0.08–0.47) [49]. Using nationwide healthcare databases in Finland, Haukka et al. [50] [P27] assessed statin persistence as a function of time remaining on statin therapy since the beginning of the first statin prescription. When using a cut-off value of ≥80%, high persistence was associated with reduced mortality (HR, 0.39; 95% CI, 0.37–0.40) [50]. Using US databases for commercially insured individuals, Rublee et al. [51] [P28] applied an anniversary model [52] of a 90 day period to define atorvastatin persistence in individuals without and with prior coronary heart disease. Compared with nonpersistent users, those with persistent use had lower risk of cardiovascular events in both primary (HR, 0.82; 95% CI, 0.74–0.91) and secondary (HR, 0.74; 95% CI, 0.66–0.82) prevention groups [51].
Discussion
The objective of this systematic review was to synthesize evidence on the adverse outcomes associated with distinct problems of statin adherence, discontinuation and persistence in real-world patient settings. Altogether, the included studies consistently reported increased risks of CVD and mortality associated with poor adherence with respect to both execution of regimen and stopping of therapy. It is important to note that the majority of included studies were published in the last 5 years (17 of 28; study IDs A9–A19, D23–D25, P27–P28), suggesting an increased recognition of the importance of assessing statin adherence and its adverse impact.
Of interest in this systematic review were methodological considerations, particularly operationalization and measurement of medication adherence, which lacked consistency across studies. All studies evaluating statin adherence used electronic data sources (e.g. administrative data, pharmacy records) [A1–A19] and applied measures of medication availability, such as the PDC or medication possession ratio. Yet despite use of PDC in 14 of 19 studies, there was variability in the method to calculate values, with some studies using an interval-based PDC calculated over 1 year, others using a prescription-based PDC calculated between first and last statin prescriptions and still others using a combination of interval and prescription-based PDC calculated from the first statin prescription to the study outcome or end of follow-up. Each approach provides advantages, and it is unclear which is preferable to use. Calculating PDC over 1 year allows a fixed interval, which may facilitate comparison across studies. However, an interval-based measure using the first and last statin prescriptions may better reflect ‘true’ adherence by eliminating aspects of persistence that may be incorporated into the measure. Calculating PDC from the first statin prescription to study outcome, as done in some studies, has the advantage of evaluating longer-term effects of adherence, but also poses the problem of capturing both persistence and adherence in a single variable, especially if the outcome occurs long after the last prescription. Aside from differences in calculation of the adherence measure, cut-off values used to define good adherence also varied across studies. While the majority of studies used a cut-off value of ≥0.80 to dichotomize adherence, there were studies that divided their adherence measure into more than two categories, again with varying cut-off values.
Statin discontinuation studies reflected even greater variability across data sources and measures used. Electronic data sources were used in four studies, while self-reported data on medication use were used in two studies. With these data sources, a challenge in evaluating outcomes associated with statin discontinuation is that subjects must first be followed for a sufficient time to allow discontinuation and then be followed for a sufficient time to develop the outcome of interest. An additional complexity is that use may vary over time, including intermittently stopping and then resuming statins. Therefore, the use of a fixed time point to define statin discontinuation employed in some studies may potentially lead to inaccurate assessment of true exposure status. For example, Ho et al. [43] [D20] defined statin discontinuation exposure based on patient self-reports of medication use 1 month after hospital discharge. Subjects reporting ‘discontinuation’ may have filled their statin prescription after 1 month and remained continuous users for the duration of follow-up or, conversely, subjects reporting continuous use at 1 month may have subsequently discontinued. Penning-van Beest et al. [46] [D22] used a similar approach of defining statin discontinuation over a fixed period of 2 years, which may be less problematic as stabilization of drug use patterns would be more likely to have occurred over this period. Three studies [D21, D24–25] modelled statin discontinuation as a time-dependent variable in their analyses. By modelling ‘actual’ statin discontinuation exposure over the entire duration of follow-up and efficiently using both exposed and non-exposed periods for all subjects, time-dependent approaches provide the ability to capture real-life patterns of drug use, where people might stop and resume drugs over time, and to evaluate their long-term effects.
Given our focus on observational studies, confounders included in multivariable models or biases accounted for are also important methodological considerations, because many of the electronic databases used in included studies may not capture important clinical and lifestyle factors associated with statin adherence and outcomes of interest. Applying the World Health Organization's five dimensions of medication adherence [22] as a framework provided a systematic assessment of whether studies considered patient, condition, therapy, social/economic and healthcare system factors. As shown in Table 3 (and Table S1), only patient factors (particularly age and sex) and condition factors (particularly comorbidities and use of other medications) were considered across all studies, probably because the databases used are comprised of coded healthcare visits that allow for assignment of comorbid diagnoses as well as prescription events that allow capture of comedications. Only two studies considered all five dimensions (A2, A14), 13 studies considered four (A3, A6, A7, A9, A12, A13, A16, A17, D20, D23–D25, P28), four studies considered three dimensions (A10, A15, D22, P26) and nine studies considered only patient and condition factors (A2, A4, A5, A8, A11, A18, A19, D21, D27). Along with these dimensions, also important are variables that may be associated with statin adherence and outcomes of interest that are often not captured in these databases, including smoking and lifestyle factors. Along with being confounders, lifestyle factors may also contribute to a potential ‘healthy adherer’ effect, whereby individuals who tend to follow prescribed medication regimens closely are also those who exhibit healthier behaviours, such as better diet, more physical activity and less smoking. The majority of studies included in our systematic review address the possibility that this ‘healthy adherer’ bias may limit their findings, including 13 adherence studies (A4–A7, A9, A10, A12–19), three discontinuation studies (D21, D24, D25) and one persistence study (P28). Only one study by Wei et al. (A10) directly evaluated the ‘healthy adherer’ effect by studying patients with CVD and comparing the impact of statin adherence and aspirin adherence [33]. The authors reported that in patients taking both drugs, adherence to statins but not aspirin was associated with a lower risk of CVD recurrence, but the same was not observed with adherence to aspirin but not statins, suggesting that healthy behaviour alone may not fully explain adverse outcomes in poorly adherent patients [33].
Our systematic review highlights the need for consistency in the medication adherence literature, which has been advocated in recent papers, including those by ISPOR's Medication Compliance and Persistence Special Interest Group for adherence studies in general [9] and by Hess et al. for studies using pharmacy administrative databases [8]. We observed inconsistencies in our systematic review, including the interchangeable use of terminology; for example, among the articles that evaluated statin adherence, some used the term ‘compliance’ in their title and one article used the term ‘continuation’. Of note, the three studies that evaluated statin persistence did not indicate the term in the title, such that it was only at the data abstraction stage that the persistence exposure was confirmed. Overall, this emphasizes the importance of using standard terminology in future studies evaluating medication adherence and also highlights the importance of applying a comprehensive search strategy in systematic reviews of medication adherence, because some articles might not have been found had our search strategy not accounted for inconsistent terminology use.
Our systematic review builds on recent literature that has synthesized the impact of poor statin adherence, including previous review articles by Liberopoulous et al. [18] and Simpson et al. [19]. However, as these were based on single PUBMED searches, the inclusion of EMBASE and International Pharmaceutical Abstracts database searches as well as a more recent search (conducted in 2013) in our systematic review provides a more comprehensive and updated capture of studies. Our systematic review also builds on a previous systematic review by Gomez Sandoval et al. [20] and a recent meta-analysis Chowdhury et al. [21]. However, unlike prior works, we evaluated the impacts of distinct adherence problems, in line with recognition that differences in dynamics and economics of adherence problems warrant separate assessments [7]. Previous articles have allowed the inclusion of clinical trials in which the impact of adherence was assessed as a secondary objective, whereas our systematic review focused solely on observational studies, better to reflect statin use in real-world patient populations [5]. As described in the Methods, because patient populations, follow-up periods and, in particular, exposure definitions for the different types of adherence were heterogeneous across included studies, we did not conduct meta-analysis. For example, of 19 studies evaluating the impact of execution of dosing (‘adherence’), 14 were based on PDC calculated across various time periods (e.g. 1 year PDC from statin initiation to 365 days, PDC over 180 day intervals, PDC from initiation to end of study/outcome, PDC from statin initiation to last statin prescription) and using varying adherence categories (e.g. binary cut-off at 0.80, at least two categories with varying cut-offs). Although it may be possible to pool studies across exposures that could be harmonized (for example, PDCs or medication possession ratios that were calculated across similar time periods), this would have been possible for only a small subset of included studies (e.g. three studies used 1 year PDCs which may be pooled), and this would not have been possible for discontinuation and persistence studies.
Overall, our systematic review identified a number of studies that consistently reported the association between statin adherence, across problems of execution and discontinuation, and the risk of CVD and mortality. These data expand upon previous reports quantifying the magnitude of statin adherence in real-world patient settings, by focusing on impacts on adverse outcomes. The findings have important implications for people taking statins, healthcare providers who prescribe them and other healthcare professionals involved in pharmacological care by emphasizing the importance of monitoring and discussing adherence to therapy.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
This research was supported from an operating grant from the Canadian Institutes of Health Research (CIHR grant number MOP 77605). Dr Mary De Vera is a recipient of The Arthritis Society Network Scholar Award. Dr Vidula Bhole received postdoctoral training support from the Canadian Arthritis Network/The Arthritis Society. Dr Diane Lacaille holds the Mary Pack Chair in Rheumatology from the University of British Columbia and The Arthritis Society.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Confounders evaluated in multivariable models for included studies according to the World Health Organization's five dimensions of medication adherence
References
- 1.Gaw A, Packard C, Shepherd J. Statins The HMG CoA Reductase Inhibitors in Perspective. London: Martin Dunitz Ltd; 2000. [Google Scholar]
- 2.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 3.4S-Investigators. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 4.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 5.Andrade SE, Walker AM, Gottlieb LK, Hollenberg NK, Testa MA, Saperia GM, Platt R. Discontinuation of antihyperlipidemic drugs–do rates reported in clinical trials reflect rates in primary care settings? N Engl J Med. 1995;332:1125–1131. doi: 10.1056/NEJM199504273321703. [DOI] [PubMed] [Google Scholar]
- 6.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 7.Urquhart J, Vrijens B. New findings about ptaient adherence to prescribed drug dosing regimens: an introduction to pharmionics. Eur J Hosp Pharm Sci. 2005;11:103–106. [Google Scholar]
- 8.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 9.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 10.Blackburn DF, Dobson RT, Blackburn JL, Wilson TW, Stang MR, Semchuk W. Adherence to statins, beta-blockers and angiotensin-converting enzyme inhibitors following a first cardiovascular event: a retrospective cohort study. Can J Cardiol. 2005;21:485–488. [PubMed] [Google Scholar]
- 11.Chodick G, Shalev V, Gerber Y, Heymann AD, Silber H, Simah V, Kokia E. Long-term persistence with statin treatment in a not-for-profit health maintenance organization: a population-based retrospective cohort study in Israel. Clin Ther. 1996;30:2167–2179. doi: 10.1016/j.clinthera.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Simons LA, Levis G, Simons J. Apparent discontinuation rates in patients prescribed lipid-lowering drugs. Med J Aust. 1996;164:208–211. doi: 10.5694/j.1326-5377.1996.tb94138.x. [DOI] [PubMed] [Google Scholar]
- 13.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med. 2004;19:638–645. doi: 10.1111/j.1525-1497.2004.30516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deambrosis P, Saramin C, Terrazzani G, Scaldaferri L, Debetto P, Giusti P, Chinellato A. Evaluation of the prescription and utilization patterns of statins in an Italian local health unit during the period 1994–2003. Eur J Clin Pharmacol. 2007;63:197–203. doi: 10.1007/s00228-006-0239-3. [DOI] [PubMed] [Google Scholar]
- 15.Foody JM, Joyce AT, Rudolph AE, Liu LZ, Benner JS. Persistence of atorvastatin and simvastatin among patients with and without prior cardiovascular diseases: a US managed care study. Curr Med Res Opin. 2008;24:1987–2000. doi: 10.1185/03007990802203279. [DOI] [PubMed] [Google Scholar]
- 16.Vinker S, Shani M, Baevsky T, Elhayany A. Adherence with statins over 8 years in a usual care setting. Am J Manag Care. 2008;14:388–392. [PubMed] [Google Scholar]
- 17.Avorn J, Monette J, Lacour A, Bohn RL, Monane M, Mogun H, LeLorier J. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279:1458–1462. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 18.Liberopoulos EN, Florentin M, Mikhailidis DP, Elisaf MS. Compliance with lipid-lowering therapy and its impact on cardiovascular morbidity and mortality. Expert Opin Drug Saf. 2008;7:717–725. doi: 10.1517/14740330802396984. [DOI] [PubMed] [Google Scholar]
- 19.Simpson RJ, Jr, Mendys P. The effects of adherence and persistence on clinical outcomes in patients treated with statins: a systematic review. J Clin Lipidol. 2010;4:462–471. doi: 10.1016/j.jacl.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Gomez Sandoval YH, Braganza MV, Daskalopoulou SS. Statin discontinuation in high-risk patients: a systematic review of the evidence. Curr Pharm Des. 2011;17:3669–3689. doi: 10.2174/138161211798220891. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J, Franco OH. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 22.Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 23.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 24.Bouchard MH, Dragomir A, Blais L, Bérard A, Pilon D, Perreault S. Impact of adherence to statins on coronary artery disease in primary prevention. Br J Clin Pharmacol. 2007;63:698–708. doi: 10.1111/j.1365-2125.2006.02828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perreault S, Dragomir A, Blais L, Bérard A, Lalonde L, White M. Impact of adherence to statins on chronic heart failure in primary prevention. Br J Clin Pharmacol. 2008;66:706–716. doi: 10.1111/j.1365-2125.2008.03269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perreault S, Dragomir A, Blais L, Bérard A, Lalonde L, White M, Pilon D. Impact of better adherence to statin agents in the primary prevention of coronary artery disease. Eur J Clin Pharmacol. 2009;65:1013–1024. doi: 10.1007/s00228-009-0673-0. [DOI] [PubMed] [Google Scholar]
- 27.Perreault S, Ellia L, Dragomir A, Côté R, Blais L, Bérard A, Lalonde L. Effect of statin adherence on cerebrovascular disease in primary prevention. Am J Med. 2009;122:647–655. doi: 10.1016/j.amjmed.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Dragomir A, Cote R, White M, Lalonde L, Blais L, Bérard A, Perreault S. Relationship between adherence level to statins, clinical issues and health-care costs in real-life clinical setting. Value Health. 2010;13:87–94. doi: 10.1111/j.1524-4733.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 29.Corrao G, Conti V, Merlino L, Catapano AL, Mancia G. Results of a retrospective database analysis of adherence to statin therapy and risk of nonfatal ischemic heart disease in daily clinical practice in Italy. Clin Ther. 2010;32:300–310. doi: 10.1016/j.clinthera.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Degli Esposti L, Saragoni S, Batacchi P, Benemei S, Geppetti P, Sturani A, Buda S, Degli Esposti E. Adherence to statin treatment and health outcomes in an Italian cohort of newly treated patients: results from an administrative database analysis. Clin Ther. 2012;34:190–199. doi: 10.1016/j.clinthera.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Rabinowich L, Steinvil A, Leshem-Rubinow E, Berliner S, Zeltser D, Rogowski O, Shalev V, Raz R, Chodick G. Adherence to statins is associated with reduced incidence of idiopathic venous thromboembolism: real-life data from a large healthcare maintenance organisation. Heart. 2012;98:1817–1821. doi: 10.1136/heartjnl-2012-302906. [DOI] [PubMed] [Google Scholar]
- 32.Wei L, Wang J, Thompson P, Wong S, Struthers AD, MacDonald TM. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow up study. Heart. 2002;88:229–233. doi: 10.1136/heart.88.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei L, Fahey T, MacDonald TM. Adherence to statin or aspirin or both in patients with established cardiovascular disease: exploring healthy behaviour vs drug effects and 10-year follow-up of outcome. Br J Clin Pharmacol. 2008;66:110–116. doi: 10.1111/j.1365-2125.2008.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blackburn DF, Dobson RT, Blackburn JL, Wilson TW. Cardiovascular morbidity associated with nonadherence to statin therapy. Pharmacotherapy. 2005;25:1035–1043. doi: 10.1592/phco.2005.25.8.1035. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 36.Ho PM, Magid DJ, Masoudi FA, McClure D, Rumsfeld J. Adherence to cardioprotective medications and mortality among patients with diabetes and ischemic heart disease. BMC Cardiovasc Disord. 2006;6:48–56. doi: 10.1186/1471-2261-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, Masoudi FA, Rumsfeld J. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155:772–779. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 38.McGinnis BD, Olson KL, Delate TM, Stolcpart RS. Statin adherence and mortality in patients enrolled in a secondary prevention program. Am J Manag Care. 2009;15:689–695. [PubMed] [Google Scholar]
- 39.Tuppin P, Neumann A, Danchin N, de Peretti C, Weill A, Ricordeau P, Allemand H. Evidence-based pharmacotherapy after myocardial infarction in France: adherence-associated factors and relationship with 30-month mortality and rehospitalization. Arch Cardiovasc Dis. 2010;103:363–375. doi: 10.1016/j.acvd.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Howell N, Trotter R, Mottram D, Rowe P. Compliance with statins in primary care. Pharm J. 2004;272:23–26. [Google Scholar]
- 41.Shalev V, Chodick G, Silber H, Kokia E, Jan J, Heymann AD. Continuation of statin treatment and all-cause mortality: a population-based cohort study. Arch Intern Med. 2009;169:260–268. doi: 10.1001/archinternmed.2008.552. [DOI] [PubMed] [Google Scholar]
- 42.Ho PM, Rumsfeld JS, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, Krumholz HM, Rumsfeld JS. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 43.Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, Krumholz HM, Rumsfeld JS. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 44.Colivicchi F, Bassi A, Santini M, Caltagirone C. Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke. 2007;38:2652–2657. doi: 10.1161/STROKEAHA.107.487017. [DOI] [PubMed] [Google Scholar]
- 45.Daskalopoulou SS, Delaney JA, Filion KB, Brophy JM, Mayo NE, Suissa S. Discontinuation of statin therapy following an acute myocardial infarction: a population-based study. Eur Heart J. 2008;29:2083–2091. doi: 10.1093/eurheartj/ehn346. [DOI] [PubMed] [Google Scholar]
- 46.Penning-van Beest FJ, Termorshuizen F, Goettsch WG, Klungel OH, Kastelein JJ, Herings RM. Adherence to evidence-based statin guidelines reduces the risk of hospitalizations for acute myocardial infarction by 40%: a cohort study. Eur Heart J. 2007;28:154–159. doi: 10.1093/eurheartj/ehl391. [DOI] [PubMed] [Google Scholar]
- 47.De Vera MA, Choi H, Abrahamowicz M, Kopec J, Goycochea-Robles M, Lacaille D. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2011;70:1020–1024. doi: 10.1136/ard.2010.142455. [DOI] [PubMed] [Google Scholar]
- 48.De Vera MA, Choi H, Abrahamowicz M, Kopec J, Lacaille D. Impact of statin discontinuation on mortality in patients with rheumatoid arthritis: a population-based study. Arthritis Care Res. 2012;64:809–816. doi: 10.1002/acr.21643. [DOI] [PubMed] [Google Scholar]
- 49.Hippisley-Cox J, Coupland C. Effect of statins on the mortality of patients with ischaemic heart disease: population based cohort study with nested case-control analysis. Heart. 2006;92:752–758. doi: 10.1136/hrt.2005.061523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haukka J, Niskanen L, Partonen T, Lönnqvist J, Tiihonen J. Statin usage and all-cause and disease-specific mortality in a nationwide study. Pharmacoepidemiol Drug Saf. 21:61–69. doi: 10.1002/pds.2255. [DOI] [PubMed] [Google Scholar]
- 51.Rublee DA, Chen SY, Mardekian J, Wu N, Rao P, Boulanger L. Evaluation of cardiovascular morbidity associated with adherence to atorvastatin therapy. Am J Ther. 2012;19:24–32. doi: 10.1097/MJT.0b013e3181ee707e. 2012. [DOI] [PubMed] [Google Scholar]
- 52.Caetano PA, Lam JM, Morgan SG. Toward a standard definition and measurement of persistence with drug therapy: examples from research on statin and antihypertensive utilization. Clin Ther. 2006;28:1411–1424. doi: 10.1016/j.clinthera.2006.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Confounders evaluated in multivariable models for included studies according to the World Health Organization's five dimensions of medication adherence


