Abstract
Aims
There are concerns regarding increased risk of acute coronary syndrome with dabigatran. We aimed to assess whether alternative treatment options such as rivaroxaban or apixaban carry a similar risk as compared with dabigatran.
Methods
We searched MEDLINE and EMBASE for randomized controlled trials of apixaban, dabigatran or rivaroxaban against control (placebo, heparin or vitamin K antagonist). We pooled odds ratios (OR) for adverse coronary events (acute coronary syndrome or myocardial infarction) using fixed effect meta-analysis and assessed heterogeneity with I2. We conducted adjusted indirect comparisons to compare risk of adverse coronary events with apixaban or rivaroxaban vs. dabigatran.
Results
Twenty-seven randomized controlled trials met the inclusion criteria. Dabigatran was associated with a significantly increased risk of adverse coronary events in pooled analysis of nine trials (OR 1.45, 95% CI 1.14, 1.86). There was no signal for coronary risk with apixaban from nine trials (pooled OR 0.89, 95% CI 0.78, 1.03) or rivaroxaban from nine trials (pooled OR 0.81, 95% CI 0.72, 0.93). Overall, adjusted indirect comparison suggested that both apixaban (OR 0.61, 95% CI 0.44, 0.85) and rivaroxaban (OR 0.54; 95% CI 0.39, 0.76) were associated with lower coronary risk than dabigatran.
Restricting the indirect comparison to a vitamin K antagonist as a common control, yielded similar findings, OR 0.57 (95% CI 0.39, 0.85) for apixaban vs. dabigatran and 0.53 (95% CI 0.37, 0.77) for rivaroxaban vs. dabigatran.
Conclusions
There are significant differences in the comparative safety of apixaban, rivaroxaban and dabigatran with regards to acute coronary adverse events.
Keywords: acute coronary syndrome, apixaban, dabigatran, rivaroxaban
Introduction
A number of new oral anticoagulants have been developed that could be used in place of low molecular weight heparins or oral vitamin K antagonists to prevent arterial and venous thrombotic events. These new agents include direct thrombin inhibitors (i.e. dabigatran) and factor Xa inhibitors (i.e. rivaroxaban and apixaban). However, the absence of direct head to head trials makes it difficult to quantify the comparative safety of these three agents, which we believe is an important consideration for patients and clinicians who are trying to decide on the preferred option. A number of researchers have used the technique of adjusted indirect comparison (AIC) to compare these agents. In an earlier meta-analysis, we used AIC to demonstrate that rivaroxaban was associated with relatively higher efficacy and slightly increased risk of haemorrhage compared with dabigatran in thromboprophylaxis in hip and knee surgery [1]. Since then, other systematic reviews of the new anticoagulants (including apixaban) in atrial fibrillation or thromboprophylaxis have not identified any major differences in efficacy [2,3].
The potential cardiac harm of dabigatran was raised by Uchino & Hernandez's systematic review demonstrating a significant association between dabigatran and risk of myocardial infarction (MI) or acute coronary syndrome (ACS) [4]. A more recent review (limited only to trials that recruited >1000 participants) also indicated that dabigatran use was associated with increased risk of coronary events, but no similar signal was detected with rivaroxaban, apixaban and ximelagatran [5]. Heterogeneity in trial populations and control interventions were important limitations of both meta-analyses. One crucial point in this debate is whether warfarin (used as the control intervention) provides a cardioprotective role, instead of dabigatran itself being harmful [6]. If that hypothesis were true, then we might also expect to see similar cardiovascular benefits with warfarin in trials against other new oral anticoagulants.
Given the availability of alternative drugs of comparable efficacy (such as apixaban and rivaroxaban), clinicians and patients who are concerned about the threat of acute coronary events with dabigatran would be greatly aided by information on the presence or absence of any similar signal with apixaban or rivaroxaban. Despite the absence of head to head trials, we are able to use adjusted indirect comparisons based on common control agents (such as warfarin) to assess quantitatively the relative likelihood of acute coronary events with apixaban or rivaroxban as compared with dabigatran. Hence, we conducted a meta-analysis of randomized trials and multiple treatments comparison of acute coronary events with the new oral anticoagulants.
Methods
Eligibility criteria
We considered parallel group randomized controlled trials of apixaban, dabigatran and rivaroxaban for prophylaxis/treatment of venous thromboembolism, ACS or atrial fibrillation. Comparator arms could consist of placebo, other anticoagulant or antiplatelet agents (examples include low molecular weight heparin, vitamin K antagonist, aspirin or clopidogrel).
We excluded trials where the duration of intervention was less than 1 week, as well as those where the planned duration of intervention differed between the two arms.
Search strategy
We searched MEDLINE and EMBASE in April 2012 using the search strategy shown in Appendix S1. We regularly updated the search through PubMed on a monthly basis (most recent December 2013) based on automated electronic notifications for any new articles on PubMed. We also checked the bibliographies of included trials for any relevant studies.
Study screening and data abstraction
Pairs of reviewers (CSK and SP) or (CSK and JKY) independently and in duplicate assessed the eligibility and extracted numerical outcomes data from the included studies. The data extracted were then checked by a senior reviewer (YKL). The team obtained full consensus on inclusion of the studies and data extraction after resolving any discrepancies though discussion.
The primary outcome of interest was ACS adverse events (encompassing non-ST segment elevation MI as well as ST segment MI and unstable angina). Where ACS outcomes were not explicitly reported, we extracted data on MI as reported by the trial authors.
Study characteristics and quality assessment
We recorded the dose and duration of interventions and comparators, follow-up, as well as the indications for therapy.
YKL and CSK independently assessed study validity. This included consideration of randomization sequence generation, allocation concealment, blinding, outcome ascertainment of acute coronary events (including whether pre-specified and independently adjudicated) and the possibility of selective reporting or missing data [7].
Quantitative data synthesis
We used RevMan 5.2 (Nordic Cochrane Center, Kobenhavn) to conduct a fixed effect meta-analysis for dichotomous outcomes, and generated pooled odds ratios (OR), with 95% confidence intervals (CI). The main analysis was on an intention to treat basis. We used the inverse variance method to pool odds ratios obtained from different subgroups of trials.
We assessed statistical heterogeneity using the I2 statistic, with I2 values of 30–60% representing a moderate level of heterogeneity [8]. If there was substantial heterogeneity (I2 >60%), we planned to use random effects meta-analysis and to explore potential sources of heterogeneity.
We performed AIC with Bucher's method [9] using ITC software (Canadian Agency for Drugs and Technologies in Health, Ottawa, Canada) [10]. Here, pooled ORs from the separate dabigatran, rivaroxaban and apixaban meta-analyses were indirectly compared using common controls. As there may have been different indications for therapy, we also performed the indirect comparisons based on groups of patients with similar disease conditions.
We estimated the number needed to treat (NNT) using Visual Rx 3.0 (http://www.nntonline.net/visualrx/).
Results
Study selection, design and methodology
The process of study selection is shown in Figure 1. Twenty-seven trials met the inclusion criteria (nine dabigatran, nine rivaroxaban and nine apixaban) [11–36]. The control groups varied with a variety of agents such as enoxaparin (nine trials), warfarin (five trials), low molecular weight heparin and then warfarin (five trials), placebo (five trials), aspirin (two trials) and low molecular weight heparin initially before placebo (one trial). There were 18 trials in prevention or treatment of venous thromboembolism in different settings, five trials in ACS and four trials in atrial fibrillation. The follow-up ranged from 40 days to 36 months. These results along with the dosages and regimes of treatment groups, time of follow up and follow-up are shown in Appendix S2.
Figure 1.
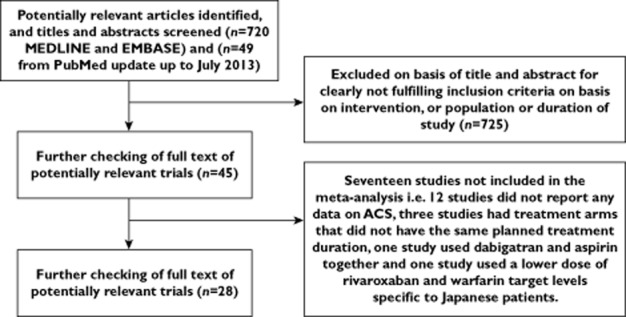
Flow chart describing study selection of randomized controlled trials of dabigatran, rivaroxaban or apixaban with myocardial infarction events
Quality assessment
The quality of studies is shown in Appendix S3. Most trials had some degree of lost to follow-up which ranged from 1 to 323 patients who were excluded from safety analysis, withdrew consent or discontinued treatment. All the studies had adequate randomization sequence generation, allocation concealment and reporting of outcomes. Aside from two studies which were unclear, the cardiac outcomes reported for all studies were pre-specified [16,31] and the majority (24/27 trials) conducted independent adjudication.
Pooled analysis of ACS adverse events
The results for individual outcomes for each trial are shown in Appendix S4.
Pooled estimates for each oral anticoagulant are presented according to control intervention (Figures 2, 3 and 4).
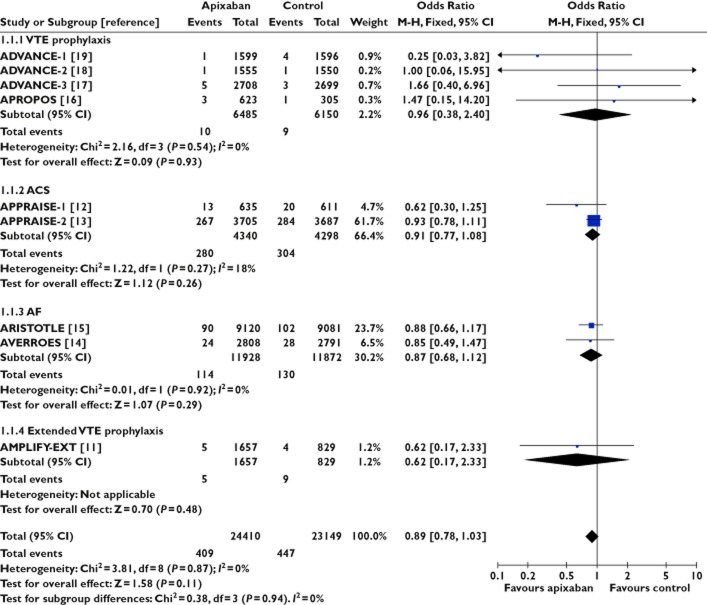
Figure 2.
Apixaban and risk of acute coronary syndrome
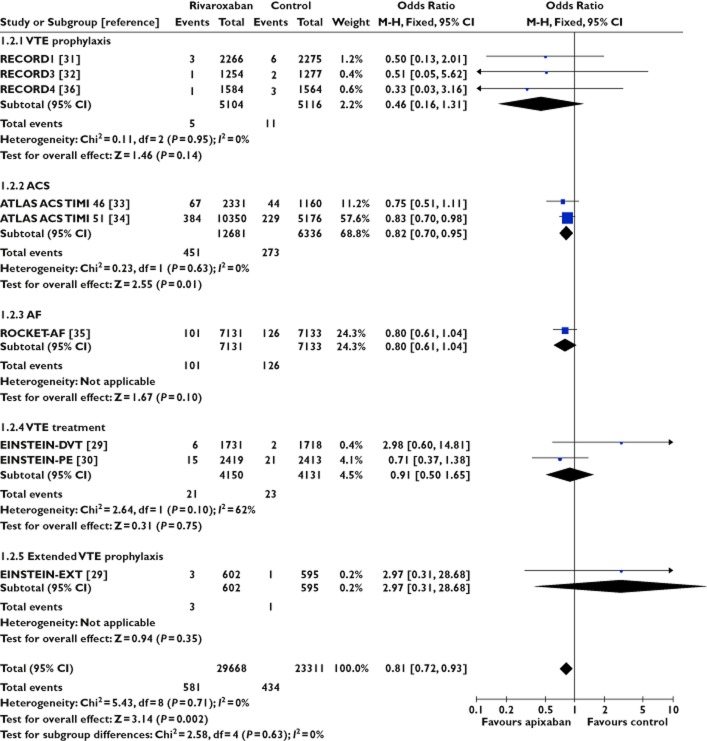
Figure 3.
Rivaroxaban and risk of acute coronary syndrome
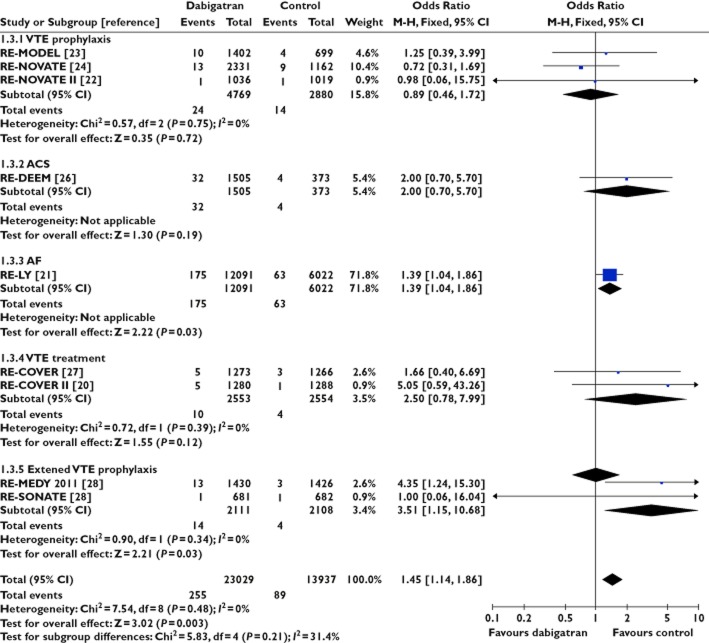
Figure 4.
Dabigatran and risk of acute coronary syndrome
There was no signal of increased risk of ACS with apixaban from nine trials involving 47 559 participants (OR 0.89, 95% CI 0.78, 1.03) or rivaroxaban from nine trials involving 52 979 participants (OR 0.81, 95% CI 0.72, 0.93). We did not detect any evidence for statistical heterogeneity in either meta-analysis (I2 = 0%). In contrast, dabigatran appeared to be associated with ACS in data from nine trials involving 36 966 patients (OR 1.45, 95% CI 1.14, 1.86, I2 = 0%). Here we emphasize that statistically significant estimates with dabigatran were demonstrated, not only in patients with atrial fibrillation [21], but also in another trial focused on extended prophylaxis for venous thromboembolism [28].
Statistical testing between subgroups of trials according to anticoagulant showed that the pooled risk of ACS was significantly different between apixaban and dabigatran (P = 0.0007) or between rivaroxaban and dabigatran (P = 0.0001). No difference was observed between the subgroups of trials involving apixaban and rivaroxaban (P = 0.33).
Overall, the adjusted indirect comparison yielded an OR of 0.61 (95% CI 0.44, 0.85) for apixaban vs. dabigatran and an OR of 0.54 (95% CI 0.39, 0.76) for rivaroxaban vs. dabigatran with regard to acute coronary adverse events.
Adjusted indirect comparison based on common controls
As the indirect comparison may have been affected by differences in choice of pharmacological agent in the control arms, we performed an analysis based solely on trials that used vitamin K antagonists as the comparator against the new oral anticoagulant. This demonstrated an OR of 0.57 (95% CI 0.42, 0.84) for apixaban vs. dabigatran and an OR of 0.51 (95% CI 0.37, 0.72) for rivaroxaban, using vitamin K antagonists as the common control. (Table 1 and Figure 5).
Table 1.
Indirect comparison of oral anticoagulants for risk of acute coronary syndrome, stratified according to common control intervention
| Vitamin K antagonist as common control | Studies | Participants | Risk of ACS odds ratio (95% CI) |
|---|---|---|---|
| Apixaban vs. vitamin K antagonist | 1 | 18 201 | 0.88 (0.66, 1.17) |
| Rivaroxaban vs. vitamin K antagonist | 3 | 22 545 | 0.82 (0.64, 1.04) |
| Dabigatran vs. vitamin K antagonist | 4 | 26 076 | 1.54 (1.17, 2.02) |
| AIC via vitamin K antagonist | Apixaban vs. dabigatran | 0.57 (0.39, 0.85) | |
| Rivaroxaban vs. dabigatran | 0.53 (0.37, 0.77) | ||
| Placebo as common control | |||
| Apixaban vs. placebo | 3 | 11 124 | 0.90 (0.76, 1.07) |
| Rivaroxaban vs. placebo | 3 | 20 754 | 0.83 (0.71, 0.96) |
| Dabigatran vs. placebo | 2 | 32 41 | 1.87 (0.71, 4.91) |
| AIC via placebo | Apixaban vs. dabigatran | 0.48 (0.18, 1.29) | |
| Rivaroxaban vs. dabigatran | 0.44 (0.17, 1.18) | ||
| Enoxaparin as control | |||
| Apixaban vs. enoxaparin | 4 | 12 635 | 0.96 (0.38, 2.40) |
| Rivaroxaban vs. enoxaparin | 3 | 10 220 | 0.46 (0.16, 1.31) |
| Dabigatran vs. enoxaparin | 3 | 7649 | 0.89 (0.46, 1.72) |
| AIC via enoxaparin | Apixaban vs. dabigatran | 1.08 (0.35, 3.35) | |
| Rivaroxaban vs. dabigatran | 0.52 (0.15, 1.79) | ||
Figure 5.
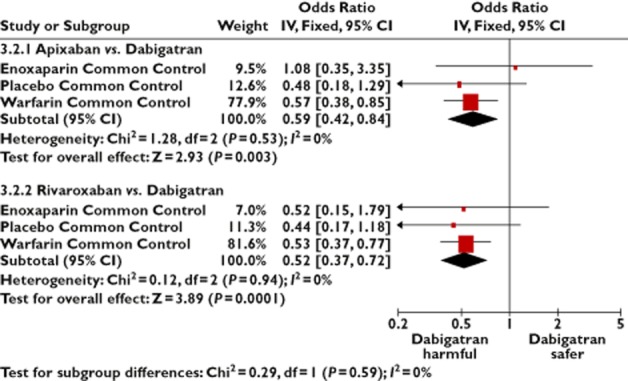
Adjusted indirect comparisons of oral anticoagulants, stratified according to common control intervention
Adjusted indirect comparisons stratified according to clinical indication
As the indirect comparison may have been affected by differences in the disease state between participants in the trials, we compared the effects of the anticoagulants in a subgroup of trials covering similar indications. (Figure 6) There was evidence across a number of clinical settings demonstrating a lower risk of acute coronary events with apixaban when compared with dabigatran. Adjusted indirect comparison with rivaroxaban against dabigatran also yielded similar findings of lower risk of acute coronary events with rivaroxaban across all clinical settings. The Forest plot illustrates that the direction of effect in the indirect comparisons were generally consistent, with no significant statistical heterogeneity.
Figure 6.
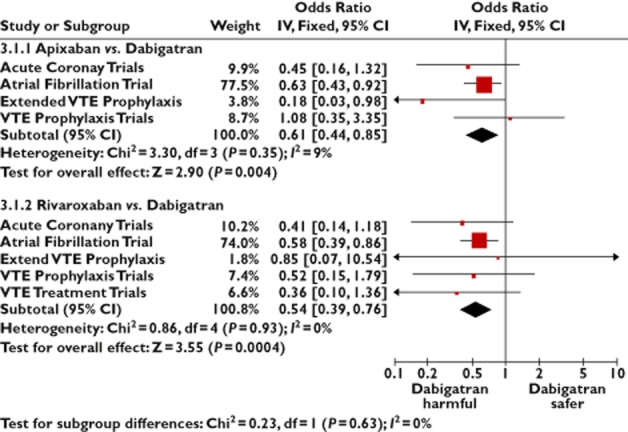
Adjusted indirect comparison of oral anticoagulants, stratified according to indication for treatment
Even if we excluded studies of new oral anticoagulants in patients presenting with ACS itself (Acute Coronary Trials in Figure 6), the AIC OR was 0.61 (95% CI 0.43, 0.87) for apixaban vs. dabigatran and 0.56 (95% CI 0.40, 0.79) for rivaroxaban vs. dabigatran with regards to acute coronary events.
Sensitivity analysis
Most of the weight in the dabigatran pooled analysis comes from an open label trial in atrial fibrillation [21]. Even if this trial were excluded, the elevated risk of acute coronary events with dabigatran remained significant, with a pooled OR of 1.62 (95% CI 1.03, 2.54).
More recently, the data on myocardial infarction in this trial were subjected to further post hoc analysis with a revised number of MIs in both the dabigatran and warfarin arms [37]. Inclusion of this post hoc evaluation data in our meta-analysis did not lead to any major change in our pooled estimate of acute coronary events with dabigatran, OR of 1.38 (95% CI 1.10, 1.74).
Number needed to treat
We used the acute coronary event rate of 1.31% (over a median of 2 years) from a large clinical trial (RELY-AF) [21], and applied the odds ratios from the AIC in estimating the absolute effects of using apixaban or rivaroxaban rather than dabigatran. If apixaban were given to this group of patients instead of dabigatran, there would be five fewer acute coronary events per 1000 patients treated, and an NNT of 198 (95% CI 143, 407) for this beneficial effect.
Similarly, if rivaroxaban were given to the group of patients instead of dabigatran, there would be six fewer acute coronary events per 1000 patients treated and a NNT of 175 (95% CI 133, 297) for this beneficial effect.
Selective outcome reporting, dissemination bias and missing data
There were a number of trials with missing outcome data in the journal manuscript where we were unable to obtain the data from the authors or the clinical trials registry (Appendix S5). We also provide a list of studies where suitable data were available but the trial was excluded due to other reasons (Appendix S6).
Discussion
Our meta-analysis of randomized controlled trials (involving more than 38 000 participants) clearly demonstrates a signal of increased coronary risk with dabigatran, whereas no such signal was seen in meta-analyses of trials that used apixaban (with >45 000 participants) or rivaroxaban (>50 000 participants) in patients with similar conditions. This signal was not completely eliminated even if we used post hoc re-adjudicated data from a large trial of dabigatran, or if we removed that trial altogether. In contrast, the relative lack of cardiac risk with apixaban or rivaroxaban was demonstrated through adjusted indirect comparison, stratified either according to common clinical indication or control therapy, against dabigatran.
We are conscious that dabigatran therapy can have beneficial effects on stroke prevention and we do not aim, in this meta-analysis, to make isolated judgments on whether the benefits of dabigatran outweigh any possible harm. Instead, our primary focus is on the comparative safety of dabigatran relative to other oral anticoagulants that are available as alternative agents for atrial fibrillation, or in patients with venous thromboembolism. Recent systematic reviews have concluded that there are no consistent differences in comparative efficacy of the three agents in atrial fibrillation [38], and that rivaroxaban has similar efficacy to dabigatran in patients with venous thromboembolism [39]. In situations where the available drug therapies are similarly efficacious, we strongly believe that patients and physicians involved in making treatment choices should be fully informed on any potential differences in harm, particularly if there is a signal of coronary risk with one agent but not the alternative agents. Moreover, neither rivaroxaban nor apixaban appear to be associated with any significantly greater risk of bleeding than dabigatran [38,39]. While the Canadian Cardiovascular Society have cautioned against dabigatran in patients with atrial fibrillation who are at high risk of coronary events, we are not aware of similar advice from other expert or regulatory bodies [40].
Eikelboom et al. have made a number of observations regarding the associated coronary risk with dabigatran [6]. One possibility is that dabigatran causes acute coronary events while the other is that warfarin carries greater efficacy in preventing such events. However, our analysis did not find any inherent superiority of warfarin in reducing acute coronary events when compared with rivaroxaban or apixaban. Conversely, when we restricted our analysis to trials with warfarin as the common control intervention, dabigatran was found to increase significantly coronary events in a pooled analysis of four trials, and to have greater associated cardiac risk in the adjusted indirect comparison against rivaroxaban or apixaban. While Eikelboom et al. suggest that placebo controlled trials are the optimal method of examining risk of coronary events with dabigatran, we identified only two large placebo controlled trials available, with one demonstrating a non-significantly increased rate of coronary events in the dabigatran group [26]. The question surrounding placebo controlled trials is scientifically interesting but perhaps somewhat moot, given that clinicians and patients have the choice of apixaban and rivaroxaban. Here, we believe the most clinically relevant question is whether dabigatran might be any more harmful than other available agents that have been tested in similar settings against common control interventions.
Our findings are consistent with other meta-analyses that have hypothesized increased ACS risk with dabigatran [4,5]. Uchino & Hernandez's meta-analysis was the first review to demonstrate the association between dabigatran and increased risk of MI or ACS across different controls and indications for anticoagulant use [4], while Mak's meta-analysis assessed the risk associated with other oral anticoagulants including apixaban, rivaroxaban and ximelagatran and suggested the possibility of differential risk [5]. A recent meta-analysis of five RCTs also reported a statistically significant 48% relative increase in MI with dabigatran [41]. The main weaknesses of these existing reviews is the pooling of data from patients with different conditions, as well as variation in control interventions, and we have aimed to overcome these limitations by conducting an analysis stratified by indication of treatment as well as the type of control. Moreover, none of the previous reviews has evaluated comparative cardiac safety through the use of adjusted indirect comparison.
There is as yet no clear pharmacological mechanism to account for any potential cardiovascular risk with dabigatran, although long term use of a related agent (ximelagatran) has been linked with pro-inflammatory effects in coronary ischaemia [42]. It has been suggested that dabigatran may also give rise to these unfavourable inflammatory effects which may increase atherosclerotic thrombotic events [4]. We have previously postulated that the disparity in efficacy between dabigatran (a direct thrombin inhibitor) and other new oral anticoagulants (direct factor Xa inhibitors) may be related to site of action on the clotting cascade [1].
Our review has several strengths. All the studies included were mainly high quality randomized controlled trials. We excluded trials which could have biased the results such as those with unequal treatment duration for oral anticoagulant and control arms. For our analysis, we were able to stratify by clinical indication as well as control arms and we were also able to use adjusted indirect comparison to directly compare different oral anticoagulants using a common control group. Our results have several limitations. There were 12 trials which we identified which did not report ACS/MI results. We attempted to contact the authors for additional data but we did not receive any response. Differences in trial methods, patient characteristics and outcome measures can affect the validity of adjusted indirect comparisons and we have attempted to tackle this by matching the trials more closely through a number of sensitivity analyses stratifying by common clinical indication or common control.
Our quantitative evaluation has identified significant differences in the comparative coronary risks of apixaban, dabigatran and rivaroxaban. Although we appreciate that head to head RCTs would be preferable, the absence of such RCTs means that our adjusted indirect comparison represents the next best option in helping inform the treatment decision for patients and physicians who are concerned about coronary adverse events with oral anticoagulants.
Competing Interests
C.S. Kwok, S. Pradhan, J.K. Yeong and Y.K. Loke have no competing interests to declare. All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work.
Contributors: YKL and CSK conceptualized the review, developed the protocol, analyzed the data and wrote the manuscript. CSK, SP and JKY abstracted the data which was checked by YKL who conducted the data analysis. YKL is the Principal Investigator and will act as the guarantor for the paper.
Funding: None
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Appendix S1
Search strategy
Appendix S2
Study design, indications for therapy and intervention arms in the randomized control trials
Appendix S3
Study quality assessment for included randomized controlled trials
Appendix S4
Table of main outcomes from individual trials
Appendix S5
Potentially eligible studies that could not be included in meta-analysis because of missing or unreported coronary events data
Appendix S6
Acute coronary syndrome data were reported but the trials were excluded for other reasons
PRISMA statement
References
- 1.Loke YK, Kwok CS. Dabigatran and rivaroxaban for prevention of venous thromboembolism – Systematic review and adjusted indirect comparison. J Clin Pharm Ther. 2011;36:111–124. doi: 10.1111/j.1365-2710.2010.01162.x. [DOI] [PubMed] [Google Scholar]
- 2.Gomez-Outes A, Terleira-Fernandez AI, Suarez-Gea ML, Vargas-Castrillon E. Dabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: systematic review, meta-analysis, and indirect treatment comparisons. BMJ. 2012;344:e3675. doi: 10.1136/bmj.e3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lip GY, Larsen TB, Skjoth F, Rasmussen LH. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2012;60:738–746. doi: 10.1016/j.jacc.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med. 2012;172:397–402. doi: 10.1001/archinternmed.2011.1666. [DOI] [PubMed] [Google Scholar]
- 5.Mak KH. Coronary and mortality risk of novel oral antithrombotic agents: a meta-analysis of large randomised trials. BMJ Open. 2012;2:e001592. doi: 10.1136/bmjopen-2012-001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eikelboom JW, Anticoagulation WJI. therapy. Dabigatran and risk of myocardial infarction. Nat Rev Cardiol. 2012;9:260–262. doi: 10.1038/nrcardio.2012.34. [DOI] [PubMed] [Google Scholar]
- 7.Loke YK, Price D, Herxheimer A. Chapter 14: adverse effects. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons; 2008. pp. 243–296. eds. In. [Google Scholar]
- 8.Higgins JPT, Deeks JJ, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons; 2008. pp. 433–448. eds. In. [Google Scholar]
- 9.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 10.Wells GA, Sultan SA, Chen M, Khan D. Indirect Treatment Comparison. 1.0 Edition. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- 11.Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JI. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708. doi: 10.1056/NEJMoa1207541. [DOI] [PubMed] [Google Scholar]
- 12.Alexander JH, Becker RC, Bhatt DL, Cools F, Crea F, Dellborg M, Fox KA, Goodman SG, Harrington RA, Huber K, Husted S, Lewis BS, Lopez-Sendon J, Mohan P, Montalescot G, Ruda M, Ruzyllo W, Verheugt F, Wallentin L. Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial. Circulation. 2009;119:2877–2885. doi: 10.1161/CIRCULATIONAHA.108.832139. [DOI] [PubMed] [Google Scholar]
- 13.Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman S, Verheugt FW, Flather M, Huber K, Liaw D, Husted SE, Lopez-Sendon J, De Caterina R, Jansky P, Darius H, Vinereanu D, Cornel JH, Cools F, Atar D, Leiva-Pons JL, Keltai M, Ogawa H, Pais P, Parkhomenko A, Ruzyllo W, Diaz R, White H, Ruda M, Geraldes M, Lawrence J, Harrington RA, Wallentin L. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699–708. doi: 10.1056/NEJMoa1105819. [DOI] [PubMed] [Google Scholar]
- 14.Connolly SJ, Eikelboom J, Joyner C, Diener H-C, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim K-H, Lewis BS, Van Mieghem W, Lip GYH, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O'Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 15.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FWA, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 16.Lassen MR, Davidson BL, Gallus A, Pineo G, Ansell J, Deitchman D. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haem. 2007;5:2368–2375. doi: 10.1111/j.1538-7836.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- 17.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM. Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–2498. doi: 10.1056/NEJMoa1006885. For the ADVANCE-3. [DOI] [PubMed] [Google Scholar]
- 18.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P. for the ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807–815. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 19.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- 20.Boehringer-Ingelheim-Pharmaceuticals. 2013. Phase III Study Testing Efficacy & Safety of Oral Dabigatran Etexilate vs Warfarin for 6 m Treatment for Acute Symp Venous Thromboembolism (VTE) NCT00680186. Available at http://clinicaltrials.gov/ct2/show/results/NCT00680186?sect=Xed3015#outcome9 (last accessed 24 July 2013)
- 21.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener H-C, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, Schnee JM, Friedman RJ. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II): A randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011;105:721–729. doi: 10.1160/TH10-10-0679. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Kalebo P, Christiansen AV, Hantel S, Hettiarachchi R, Schnee J, Buller HR. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haem. 2007;5:2178–2185. doi: 10.1111/j.1538-7836.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Buller HR. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–956. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 25.Ginsberg JS, Davidson BL, Comp PC, Francis CW, Friedman RJ, Huo MH, Lieberman JR, Muntz JE, Raskob GE, Clements ML, Hantel S, Schnee JM, Caprini JA. Oral thrombin inhibitor dabigatran etexilate vs. North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24:1–9. doi: 10.1016/j.arth.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 26.Oldgren J, Budaj A, Granger CB, Khder Y, Roberts J, Siegbahn A, Tijssen JGP, Van de Werf F, Wallentin L. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J. 2011;32:2781–2789. doi: 10.1093/eurheartj/ehr113. [DOI] [PubMed] [Google Scholar]
- 27.Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 28.Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, Kvamme AM, Friedman J, Mismetti P, Goldhaber SZ. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–718. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 29.Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 30.Buller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J, Verhamme P, Wells P, Agnelli G, Cohen A, Berkowitz SD, Bounameaux H, Davidson BL, Misselwitz F, Gallus AS, Raskob GE, Schellong S, Segers A. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W, Group RS. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 32.Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AGG. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–2786. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 33.Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, Hricak V, Barnathan ES, Bordes P, Witkowski A, Markov V, Oppenheimer L, Gibson CM. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29–38. doi: 10.1016/S0140-6736(09)60738-8. [DOI] [PubMed] [Google Scholar]
- 34.Mega JL, Braunwald E, Wiviott SD, Bassand J-P, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KAA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FWA, Gibson CM. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 35.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 36.Turpie AGG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, Benson A, Misselwitz F, Fisher WD. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673–1680. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]
- 37.Hohnloser SH, Oldgren J, Yang S, Wallentin L, Ezekowitz M, Reilly P, Eikelboom J, Brueckmann M, Yusuf S, Connolly SJ. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation. 2012;125:669–676. doi: 10.1161/CIRCULATIONAHA.111.055970. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen LH, Larsen TB, Graungaard T, Skjoth F, Lip GY. Primary and secondary prevention with new oral anticoagulant drugs for stroke prevention in atrial fibrillation: indirect comparison analysis. BMJ. 2012;345:e7097. doi: 10.1136/bmj.e7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox BD, Kahn SR, Langleben D, Eisenberg MJ, Shimony A. Efficacy and safety of novel oral anticoagulants for treatment of acute venous thromboembolism: direct and adjusted indirect meta-analysis of randomised controlled trials. BMJ. 2012;345:e7498. doi: 10.1136/bmj.e7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M Committee CCSAFG. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic thromboembolism in atrial fibrillation and flutter. Can J Cardiol. 2011;27:74–90. doi: 10.1016/j.cjca.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Sipahi I, Celik S, Akyol A. Dabigatran's ‘real-world’ data about risk of myocardial infarction and gastrointestinal bleeding contradicts with randomized trials. J Am Coll Cardiol. 2013;62:945–946. doi: 10.1016/j.jacc.2013.05.066. [DOI] [PubMed] [Google Scholar]
- 42.Christersson C, Oldgren J, Wallentin L, Siegbahn A. Treatment with an oral direct thrombin inhibitor decreases platelet activity but increases markers of inflammation in patients with myocardial infarction. J Intern Med. 2011;270:215–223. doi: 10.1111/j.1365-2796.2011.02354.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Search strategy
Appendix S2
Study design, indications for therapy and intervention arms in the randomized control trials
Appendix S3
Study quality assessment for included randomized controlled trials
Appendix S4
Table of main outcomes from individual trials
Appendix S5
Potentially eligible studies that could not be included in meta-analysis because of missing or unreported coronary events data
Appendix S6
Acute coronary syndrome data were reported but the trials were excluded for other reasons
PRISMA statement