Abstract
Aim
Injury to bone is a significant clinical challenge, due to its limited regenerative capacity. The current methods of repairing bone defect are surgical, highly invasive and not always successful. A systematic review and meta-analysis of preclinical studies involving large animals with bone defects were conducted to determine the treatment outcomes with stem cell therapies.
Methods
A random effects meta-analysis of the available studies was conducted to assess the treatment outcomes including the rate of new bone formation and new bone mineral density (BMD). Stratified analyses were also conducted by separating studies based on each characteristic independently.
Results
Pooled analysis of 20 preclinical studies showed a significant beneficial effect of stem cell therapy in increasing new bone formation (17.79%, 95% confidence interval [CI], 10.54, 25.03; P < 0.001) and BMD (276.94 mg cm−2, 95% CI, 62.71, 491.17; P < 0.001) for disease amelioration. Regarding new bone formation, a statistical improvement was similarly detected from randomized controlled trial groups (17.06%, 95% CI, 8.87, 25.24; P < 0.001) and cohort groups (17.43%, 95% CI, 10.79, 24.07; P < 0.001). Exploratory stratified analysis yielded significant predictors of new bone formation including cell number (<107 vs. ≥107; P = 0.048) and the route of cell delivery (combining with matrix scaffold showed more effect than direct cell injection, P = 0.041). The effect of stem cell therapy diminished after 12 weeks.
Conclusion
The study results suggest that stem cell therapy improves new bone formation and BMD in bone defect models. Future trials should focus on the transplantation of ≥107 stem cells, especially using slow release biodegradable scaffolds or repetitive cell injections.
Keywords: animal model, bone defect, meta-analysis, stem cell therapy
What is Already Known about this Subject
Studies from large animals present that stem cell therapy is a promising strategy for restoring bone damage.
What this Study Adds
The effects of stem cell therapy are likely dependent on both the transplanted cell number and cell transplantation mode.
Introduction
Human bone and articulation are vascular structures, which pose significant hurdles to repair or regeneration strategies during injury. Defective repair mechanisms result in pain, joint dysfunction, arthritis, degeneration and osteoarthritis. The damage to bone is a significant clinical problem, with huge health and socioeconomic impact.
The current methods of repairing bone defect are surgical, highly invasive and not always successful. Tissue engineering is a viable alternative with promising therapeutic advantage in restoring both the structure and function of damaged bone [1]. Although large animal studies have investigated the potential therapeutic effect of stem cell transplantation in repairing bone injuries, the results are conflicting, with some studies reporting bone regeneration when used alone [2,3] or in combination with scaffolds [4,5], while other studies failed to find significant differences [6,7]. The data on bone minerals are also uncertain [8–10]. Although these preclinical studies remain controversial, the results offer important clues to unanswered clinical issues which are critical to stem cell repair of the bone including safety, feasibility, efficacy, choice of cell type, cell number, method of delivery and follow-up. The present study involved a systematic review and meta-analysis to identify qualitative and quantitative data for stem cell transplantation as an alternative in bone defects. Such studies might help in the design of future clinical studies similar to the meta-analysis of bone stem cell trials in humans. A subgroup analysis was also performed to resolve the foregoing issues.
Methods
Literature search and eligibility criteria
A meta-analysis of available preclinical data was conducted on bone defects in accordance with the Cochrane Handbook guidelines [11]. The review is reported per the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines [12].
To identify preclinical studies investigating the use of stem cell therapy in bone defects, a literature search of PubMed and Embase was conducted for studies published until July 1 2013. The databases were searched using the following strategy: ‘(pig OR porcine OR swine OR canine OR dog OR sheep OR ovine OR rabbit) AND (stem cells OR progenitor cells OR bone marrow) AND (bone fracture OR bone repair OR bone defect OR bone injury)’. The inclusion criteria were as follows: (1) studies involving bone defects in large animal models, (2) randomized controlled trials (RCTs) and cohort studies investigating the effect of stem cell therapy on bone repair in terms of new bone formation and bone mineral density (BMD) and (3) articles written only in English. Trials that investigated only transfected or genetically engineered stem cells altering cell behaviour were excluded, but studies using reporter genes (solely for stem cell imaging purposes) were included. Reviews, editorials, comments, reports from scientific sessions and discussions were excluded. When two or more articles reported data from the same study, only the most recently updated data were included. References to the identified articles were also checked and principal investigators were asked whether they were aware of other trials.
Data extraction and quality assessment
Two reviewers independently screened titles and abstracts and then full-text articles. Discrepancies between the two reviewers were resolved by consensus or through discussion with a third reviewer. In the meta-analysis, effect sizes of increase in new bone formation and BMD improvement between stem cell treatment and control groups were calculated to assess the therapeutic effect. To this end, the total number of animals, mean and SEM or SD pertaining to new bone volume, and BMD were extracted from the studies. Other information regarding the types of animals, defect models, treatment dose, route of delivery, follow-up duration and comorbidity were incorporated in the database. In the case of missing data, corresponding authors were contacted. Five emails were sent and three authors responded.
Standard guidelines [13] for quality assessment of clinical trials were not universally applicable to these preclinical studies. Therefore, a modified Jadad scale criteria was used to assess selection, performance and detection bias: (1) randomization, (2) description of randomization, (3) adequate allocation, (4) blinding of the operator and (5) blinding of the outcome analysis. Trials scoring 1 point were deemed as low quality and 4–5 points as high quality.
Statistical analysis
Extracted data were entered into Review Manager version 5.0.2 database. The primary outcome was the difference in mean of the newly formed bone (%) between control and treated animals at follow-up. The secondary endpoint was the difference in BMD (reported as mg m−2). In the case of multiple measurements over time, data measured at the longest duration of follow-up were used for analysis. If multiple experimental groups were next to a single control group within one study, the number of animals in the control group was divided equally by the number of experimental groups. The meta-analysis was performed using weighted mean difference with random effects model to avoid heterogeneity [14]. Heterogeneity was considered significant at P < 0.10 [11]. Inconsistency was estimated using the I2 statistic. Values of 25, 50 and 75% were considered low, moderate and high inconsistency, respectively [15].
For a clinical perspective, a stratification analysis was also conducted to examine the impact of several factors, such as the type of animal (pig, dog, sheep, or rabbit), cell type [bone marrow mesenchymal stem cells (BMSCs), umbilical cord blood mesenchymal stem cells (UCB-MSCs), deciduous teeth stem cells, adipose stem cells (ASCs)], number of cells injected (<107 or ≥107), method of cell delivery (with scaffold, in situ injection, or intravenous administration) and follow-up after stem cell therapy (≤12 weeks, 12–24 weeks or >24 weeks).
To test the robustness of association and characterize possible sources of statistical heterogeneity, sensitivity analyses were carried out by excluding studies one by one and analyzing the homogeneity and effect size for all the remaining studies. Publication bias was assessed using the Begg adjusted rank correlation test and the Egger regression asymmetry test [16,17].
All analyses were performed using Review Manager version 5.0.2 and Stata version 11.0 (StataCorp, College Station, TX, USA).
Results
Search results and characteristics of studies included in the meta-analysis
The electronic database search identified 202 articles, of which 20 articles were eligible for review (10 RCTs and 10 cohort studies; Figure 1). Characteristics of the enrolled studies are depicted in Table 1. In 15 of the included studies, stem cells were seeded with matrix scaffolds, four studies with cells directly injected into the injury site and one administered by tail vein. Four different cell types were studied. All cases involved single frequency of stem cell therapy. Seventeen studies reported data on the rate of new bone formation and four studies were based on BMD outcomes. The rates of new bone formation were assessed by computed tomography (six studies), single-photon emission computed tomography (one study), histomorphology (nine studies) and X-ray (one study). No study used the animal model with comorbidity.
Figure 1.
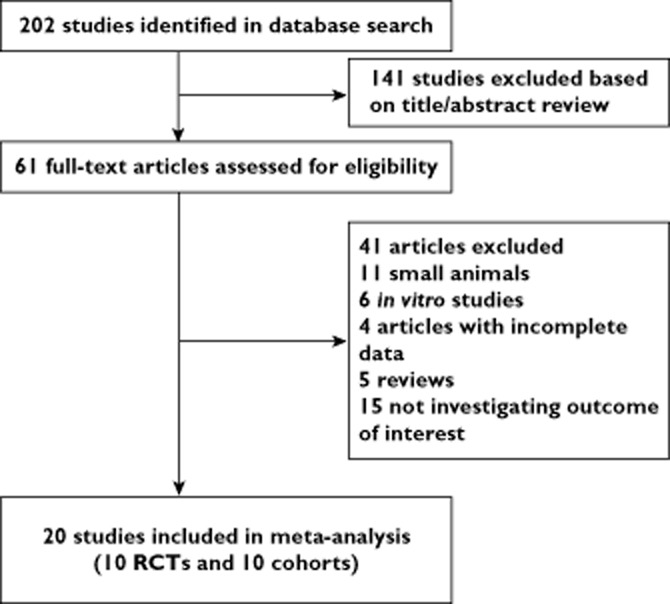
Study flow diagram
Table 1.
Study characteristics
| Author | n | Type of animal | Study design | Type of defect | Cell type | Number of cells | Route of delivery | Follow-up (weeks) |
|---|---|---|---|---|---|---|---|---|
| Aykan et al. [2] | 20 | Sheep | RCT | Mandibular | BMSCs | 8 × 106 | DI | 6 |
| Jang et al. [36] | 6 | Dog | RCT | Radiul | UCB-MSCs | 2 × 106 | CS | 12 |
| Lucarelli et al. [37] | 20 | Sheep | RCT | Metatarsal | BMSCs | 4 × 106 | CS | 16 |
| Field et al. [38] | 24 | Sheep | RCT | Tibial | BMSCs | 2.25 × 108 | CS | 36 |
| Liao et al. [39] | 8 | Dog | RCT | Mandibular | BMSCs | 1 × 108 | DI | 16 |
| Li et al. [6] | 12 | Dog | RCT | Ulna | ASCs | 2 × 107 | CS | 16 |
| Ai et al. [40] | 10 | Rabbit | Cohort | Tibial | BMSCs | 1 × 106 | CS | 8 |
| Peng et al. [41] | 12 | Dog | RCT | Femoral head | BMSCs | 1 × 107 | CS | 30 |
| Cui et al. [4] | 14 | Dog | Cohort | Parietal bones | ASCs | 2 × 107 | CS | 24 |
| Li et al. [42] | 16 | Sheep | RCT | Metatarsus | BMSCs | 2 × l08 | CS | 24 |
| Ren et al. [7] | 10 | Pig | Cohort | Ulna | ASCs | 1 × 106 | TVI | 12 |
| Niemeyer et al. [43] | 28 | Sheep | Cohort | Tibia | BMSCs | 2 × 107 | CS | 26 |
| Wang et al. [5] | 128 | Rabbit | RCT | Femurs | BMSCs | 5 × 106 | CS | 12 |
| Zheng et al. [44] | 7 | Pig | Cohort | Mandibular | SPDs | 2 × 107–4 × 108 | CS | 24 |
| Yan et al. [3] | 13 | Dog | Cohort | ONFH | BMSCs | 2 × 107 | DI | 12 |
| Yew et al. [18] | 15 | Rabbit | Cohort | Cranial bone | BMSCs | 1 × 106 | CS | 12 |
| Yamada et al. [45] | 12 | Dog | Cohort | Mandible | BMSCs | 1 × 107 | DI | 8 |
| Yuan et al. [8] | 11 | Dog | Cohort | Mandibular | BMSCs | 2 × 107 | CS | 32 |
| Yuan et al. [9] | 24 | Sheep | Cohort | Mandibular | BMSCs | 2 × 107 | CS | 32 |
| Zhou et al. [10] | 16 | Dog | RCT | Inferior orbital rim bone | BMSCs | 2 × 107 | CS | 12 |
ASCs, adipose stem cells; BMSCs, bone marrow mesenchymal stem cells; CS, cell-seeded scaffold; DI, directly inject into the defect; SPDs, stem cells of pig deciduous teeth; TVI, tail vein injection; UCB-MSCs, umbilical cord blood-derived MSCs.
Meta-analysis
The 20 identified studies involved 406 animals (211 control and 195 treated groups, respectively) to assess the effects of stem cell therapy on the rate of new bone formation and BMD. There was a significant beneficial effect of stem cell treatment on the new bone formation increase (17.79%, 95% CI 10.54, 25.03; P < 0.001), with significant heterogeneity (P < 0.001) and inconsistency (I2 99%; Figure 2). Regarding BMD, a statistical improvement by 276.94 mg cm−2 (95% CI 62.71, 491.17; P < 0.001) was similarly detected with significant heterogeneity (P < 0.001) and inconsistency (I2 98%; Figure 3).
Figure 2.
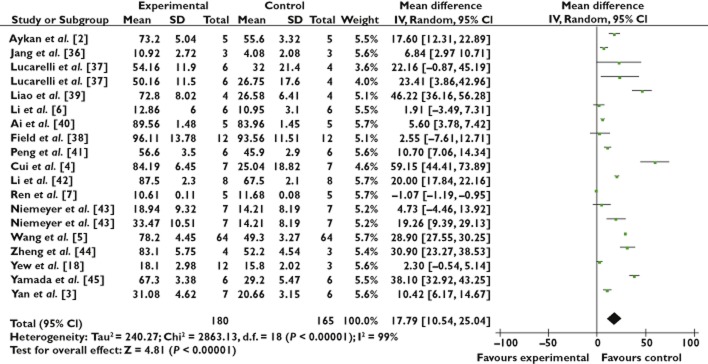
Forest plot showing the impact of stem cell therapy on new bone formation, compared with controls. 95% CI, 95% confidence interval
Figure 3.
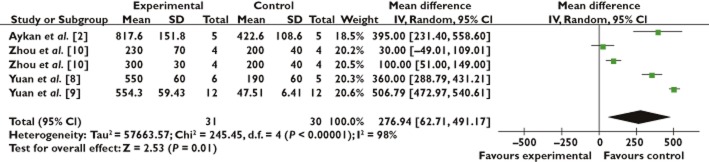
Forest plot showing the impact of stem cell therapy on histologic score improvement, compared with controls. 95% CI, 95% confidence interval
Stratified analysis
The stratified analysis showed that cell injection dose (P = 0.048) and route of cell delivery (P = 0.041) are the two significant predictors of enhanced new bone formation, although the heterogeneity among studies was significant (Table 2). No significant difference (P = 0.951) was observed regarding study design type: 17.06% in the RCT group (95% CI 8.87, 25.24; P < 0.001) vs. 17.43% in the cohorts group (95% CI 10.79, 24.07; P < 0.001). A similar effectiveness was observed between BMSCs and other stem cell types. During follow-up, the effect of stem cell therapy appeared to decline over time. Considering the sources of heterogeneity, we also separated the studies by animal type and found no difference (P = 0.814).
Table 2.
New bone formation rate: Stratified analysis of stem cell-treated vs. control
| Number of studies | Mean difference (IV, random, 95% CI) | I2 value (%) | P*h | P** | |
|---|---|---|---|---|---|
| Type of animal | |||||
| Dog | 78 | 23.52 (12.20, 34.84) | 97 | <0.001 | 0.8144 |
| Pig | 17 | 14.68 (−16.65, 46) | 75 | <0.001 | |
| Sheep | 88 | 14.24 (6.42, 22.06) | 99 | <0.001 | |
| Rabbit | 153 | 12.29 (−5.79, 30.37) | 96 | <0.001 | |
| Cell injection dose | |||||
| <107 | 249 | 12.37 (1.08, 23.67) | 97 | <0.001 | 0.048 |
| ≥107 | 146 | 21.35 (13.82, 28.88) | 95 | <0.001 | |
| Follow-up after cell therapy (weeeks) | |||||
| ≤12 | 194 | 28.64 (16.74, 40.55) | 98 | <0.001 | 0.129 |
| 12–24 | 77 | 12.95 (1.11, 24.79) | 94 | <0.001 | |
| >24 | 64 | 9.54 (3.66, 15.42) | 56 | 0.08 | |
| Type of cell | |||||
| BMSCs | 296 | 17.62 (10.67, 24.57) | 96 | <0.001 | 0.966 |
| Other types | 49 | 17.24 (5.87, 28.61) | 99 | <0.001 | |
| Route of delivery | |||||
| CS | 295 | 19.50 (15.74, 23.26) | 98 | <0.001 | 0.041 |
| DI | 95 | 12.22 (8.26, 16.149) | 97 | <0.001 | |
| Type of study | |||||
| RCT | 216 | 17.06 (8.87, 25.24) | 95 | <0.001 | 0.951 |
| Cohort | 129 | 17.43 (10.79, 24.07) | 98 | <0.001 | |
P value for heterogeneity within each subgroup.
P value for heterogeneity between subgroups with meta-regression analysis. BMSCs, bone marrow mesenchymal stem cells; CI, confidence interval; CS, cell seeded scaffold; DI, directly inject into the defect.
Data from BMD outcome were obtained only from four studies with a total of 61 animals, 30 of which were controls and 31 stem cell-treated animals (Figure 3). Therefore, the effect of stem cell therapy on BMD was not assessed in the stratified meta-analysis because of the limited data on each group.
Quality of the included trials
Methodological quality of the included trials was modest (2.6). Two trials were of low quality, with a score of 1 on the modified Jadad scale, four were judged high in quality with a score of 4. Blinded outcome analysis was performed on 13 studies. The operator was blinded in nine studies. No article reported the method of randomization. Further details on the scores for each trial are presented in Table 3.
Table 3.
Quality of the included trials
| Study ID | Modity Jadad scale | |||||
|---|---|---|---|---|---|---|
| a | b | c | d | e | Total | |
| Aykan et al. [2] | 1 | 0 | 1 | 1 | 0 | 3 |
| Jang et al. [36] | 1 | 0 | 1 | 0 | 0 | 2 |
| Lucarelli et al. [37] | 1 | 0 | 1 | 1 | 1 | 4 |
| Field et al. [38] | 1 | 0 | 1 | 1 | 1 | 4 |
| Liao et al. [39] | 1 | 0 | 1 | 0 | 0 | 2 |
| Li et al. [6] | 1 | 0 | 1 | 0 | 0 | 2 |
| Ai et al. [40] | 0 | 0 | 1 | 0 | 1 | 2 |
| Peng et al. [41] | 1 | 0 | 1 | 0 | 0 | 2 |
| Cui et al. [4] | 0 | 0 | 1 | 1 | 1 | 3 |
| Li et al. [42] | 1 | 0 | 1 | 0 | 1 | 3 |
| Ren et al. [7] | 0 | 0 | 1 | 1 | 1 | 3 |
| Niemeyer et al. [43] | 0 | 0 | 1 | 0 | 1 | 2 |
| Wang et al. [5] | 1 | 0 | 1 | 1 | 1 | 4 |
| Zheng et al. [44] | 0 | 0 | 1 | 0 | 0 | 1 |
| Yan et al.[3] | 0 | 0 | 1 | 0 | 0 | 1 |
| Yew et al. [18] | 0 | 0 | 1 | 1 | 1 | 3 |
| Yamada et al. [45] | 0 | 0 | 1 | 1 | 1 | 3 |
| Yuan et al. [8] | 0 | 0 | 1 | 0 | 1 | 2 |
| Yuan et al. [9] | 0 | 0 | 1 | 0 | 1 | 2 |
| Zhou et al. [10] | 1 | 0 | 1 | 1 | 1 | 4 |
Points were awarded as follows: a = the study was described as randomized, 1 point; b = the randomization scheme was described and appropriate, 1 point; c = adequate allocation, 1 point; d = blinding of the operator, 1 point; e = blinding of the outcome analysis, 1 point.
Sensitivity analysis and publication bias
In the sensitivity analysis of stem cell therapy on new bone formation outcomes, we sequentially removed one study at a time and re-analyzed the data. The 15 study-specific mean differences ranged from a low of 15.78 (95% CI 8.42, 23.14) after omitting the study by Cui et al. [4] to a high of 18.75 (95% CI 11.00, 26.50) after omitting the study by Yew et al. [18], but they were generally similar. For BMD outcomes, similar sensitivity analyses were carried out without a significant impact on the results (data not shown).
The funnel plots (Figure 4) revealed that no significant publication bias existed in new bone formation in the present analysis and Egger's test showed P = 0.838 and Begg's test showed P = 0.086.
Figure 4.
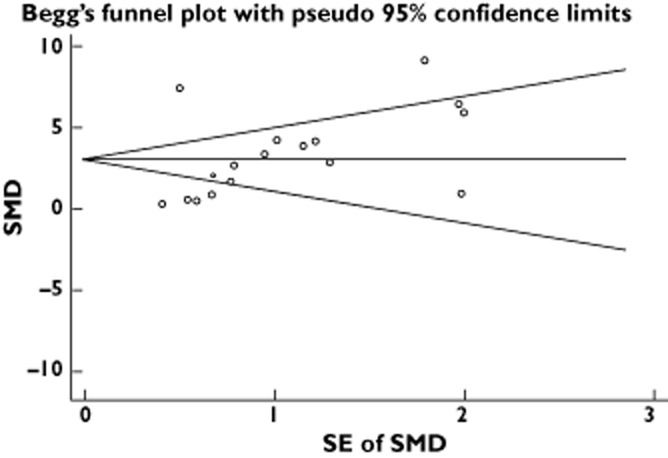
Funnel graph for the assessment of potential publication biasSMD, Standardized Mean Difference
Discussion
Twenty published preclinical studies involving large animals treated with stem cells were analyzed to investigate the treatment-related effects on bone injury. In brief, the study findings suggest that (1) stem cell therapy promoted new bone formation by 17.79% accompanied with BMD increase of 276.94 mg cm−2, (2) cell injection dose and the route of cell delivery were important predictors of new bone formation in the bone defect model, and (3) no differences in animal and stem cell types were found.
The meta-analysis results reinforced the evidence supporting stem cell therapy in experimental bone defects, especially in increasing the new bone formation. For BMD outcome, the present analysis showed consistent benefit of stem cell therapy, although the availability of limited data available and decreased number of experiments reporting this endpoint could render these findings less robust. Further evidence is required to assess BMD improvement in experimental bone injury models.
Transplantation of a higher number of cells (≥107) appeared to have a stronger impact on new bone formation. This could be due to the increasing stimulation of the endogenous regenerative capacity of the bone by release of growth factors, cytokines and other paracrine molecules from the transplanted and host cells, enhancing angiogenesis and reducing apoptosis [19–21]. Stratified analysis showed that combining stem cell therapy with matrix scaffold had significantly larger benefit compared with direct cell injection. The use of appropriate matrix scaffolds as stem cell delivery vehicles or as a three dimensional support for the repair tissue has advantages including enhanced osteogenic property, improved cell loading, prevention against leakage of transplanted cells, increased bone differentiation and tissue repair support [22,23]. The proposed design is an effective approach for future clinical trials.
In large animals, the effect of stem cell therapy disappears 12 weeks after cell injection, consistent with initial observations in patient studies [24]. This finding should motivate researchers to explore novel applications and strategies for stem cell therapy including slow release agents, genetic engineering of stem cells or repetitive injections over time.
Clinically, BMSCs are the most commonly used cell type in the autologous or allogeneic transplantation and can differentiate into various lineages including bone, cartilage, adipose, tendon, ligament, muscle and nerve cells in vivo and vitro [25–28]. The results from the present meta-analysis showed no added benefits with BMSCs compared with other stem cells on new bone formation. Compared with BMSCs, ASCs and UCB-MSCs have several advantages as new cell sources including ease of isolation, relative abundance, rapidity of expansion and multipotency that are independent of serum source and quality [29,30]. A recent study reported that human UCB-MSCs have a significantly stronger osteogenic potential but less capacity in adipogenic differentiation than BMSCs in vitro [31]. Further evidence is required to assess the effectiveness of different sources of stem cells on experimental bone injury models. Results concerning new bone formation from RCT (17.06%) and from cohort (17.43%) studies suggested that the effect of stem cell therapy on bone injury was consistent.
Recommendations
Meta-analyses of animal studies are not common, although still recommended in several settings [32] to guide research and clinical endeavours [33]. Meta-analyses of preclinical studies may also be attractive to evaluate the effect of other therapies and to design (pre-)clinical trials in the future. Over the next few years, adequately powered large animal studies and clinical trials should focus on the transplantation of ≥107 stem cells, with matrix scaffold or slow-release biodegradable scaffolds and repetitive cell injections.
Limitations
To the best of our knowledge, this analysis represents the first systematic review and meta-analysis assessing stem cell implantation in the treatment of bone defects, encompassing 20 studies and 406 large animals. Results of the meta-analysis revealed statistically significant heterogeneity for new bone formation and BMD. In the current work, the diversity in animal types, delivery methods, time to follow-up and number of cells may explain this heterogeneity and play a role in the observed outcomes. Heterogeneity may also be attributed to the extremely sensitive endpoints chosen. The risk of erroneous estimates could be minimized using random effects analysis.
Another limitation highlighted by this meta-analysis pertains to the lack of animal studies with comorbidities, unlike studies in humans [24,34,35]. Autologous stem cells extracted from large young animals are ‘fresh’, whereas cells from patients are ‘aged’. Further, animal studies offer a relatively short duration of follow-up. Despite these differences, the present analysis has shown that preclinical data are highly relevant to predict outcomes for clinical trials.
Conclusion
Based on the data included in this meta-analysis, stem cell therapy is associated with a 17.79% higher new bone formation and 276.94 mg cm−2 more BMD increase compared with control groups. The analysis demonstrated that large animal models were able to provide valid data to design or predict outcomes in clinical trials. In view of the limitations inherent in the design of a majority of the studies included in the meta-analysis, large, multicentre, well-designed RCTs with extensive follow-up are needed to validate these findings.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Source of funding
There was no specific funding source for this manuscript.
Data access and responsibility
Ming-Kang Zhong had full access to all the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis and acts as guarantor of the paper.
Authors' contributions
Study idea: Yun Liao, Xiao-Long Zhang
Study design: Yun Liao, Xiao-Long Zhang
Literature search: Yun Liao, Xiao-Long Zhang, Ling Li
Data collection: Yun Liao, Xiao-Long Zhang, Ling Li, Fu-Ming Shen, Ming-Kang Zhong
Statistical analysis: Yun Liao, Xiao-Long Zhang, Ling Li
Data interpretation: Yun Liao, Xiao-Long Zhang, Ling Li
First version of the manuscript: Yun Liao, Xiao-Long Zhang, Ling Li, Fu-Ming Shen, Ming-Kang Zhong
Critical revision for important intellectual content: Yun Liao, Xiao-Long Zhang, Ling Li, Fu-Ming Shen, Ming-Kang Zhong
Final approval of the version to be published: Yun Liao, Xiao-Long Zhang, Ling Li, Fu-Ming Shen, Ming-Kang Zhong
References
- 1.Khojasteh A, Behnia H, Dashti SG, Stevens M. Current trends in mesenchymal stem cell application in bone augmentation: a review of the literature. J Oral Maxillofac Surg. 2012;70:972–982. doi: 10.1016/j.joms.2011.02.133. [DOI] [PubMed] [Google Scholar]
- 2.Aykan A, Ozturk S, Sahin I, Gurses S, Ural AU, Oren NC, Isik S. Biomechanical analysis of the effect of mesenchymal stem cells on mandibular distraction osteogenesis. J Craniofac Surg. 2013;24:e169–175. doi: 10.1097/SCS.0b013e31827c8706. [DOI] [PubMed] [Google Scholar]
- 3.Yan Z, Hang D, Guo C, Chen Z. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res. 2009;27:442–446. doi: 10.1002/jor.20759. [DOI] [PubMed] [Google Scholar]
- 4.Cui L, Liu B, Liu G, Zhang W, Cen L, Sun J, Yin S, Liu W, Cao Y. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials. 2007;28:5477–5486. doi: 10.1016/j.biomaterials.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Fan H, Zhang ZY, Lou AJ, Pei GX, Jiang S, Mu TW, Qin JJ, Chen SY, Jin D. Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized β-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials. 2010;31:9452–9461. doi: 10.1016/j.biomaterials.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Dai K, Tang T, Zhang X, Yan M, Lou J. Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2. Biochem Biophys Res Commun. 2007;356:836–842. doi: 10.1016/j.bbrc.2007.02.165. [DOI] [PubMed] [Google Scholar]
- 7.Ren ML, Peng W, Yang ZL, Sun XJ, Zhang SC, Wang ZG, Zhang B. Allogeneic adipose-derived stem cells with low immunogenicity constructing tissue-engineered bone for repairing bone defects in pigs. Cell Transplant. 2012;21:2711–2721. doi: 10.3727/096368912X654966. [DOI] [PubMed] [Google Scholar]
- 8.Yuan J, Cui L, Zhang WJ, Liu W, Cao Y. Repair of canine mandibular bone defects with bone marrow stromal cells and porous beta-tricalcium phosphate. Biomaterials. 2007;28:1005–1013. doi: 10.1016/j.biomaterials.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Yuan J, Zhang WJ, Liu G, Wei M, Qi ZL, Liu W, Cui L, Cao YL. Repair of canine mandibular bone defects with bone marrow stromal cells and coral. Tissue Eng Part A. 2010;16:1385–1394. doi: 10.1089/ten.TEA.2009.0472. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Xiao C, Wang Y, Bi X, Ge S, Fan X. In vivo efficacy of bone marrow stromal cells coated with beta-tricalcium phosphate for the reconstruction of orbital defects in canines. Invest Ophthalmol Vis Sci. 2011;52:1735–1741. doi: 10.1167/iovs.10-5988. [DOI] [PubMed] [Google Scholar]
- 11.Green S, Higgins JP, Alderson P, Clarke M, Mulrow CD, Oxman AD. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Chichester, UK: John Wiley & Sons, Ltd; 2008. [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yew TL, Huang TF, Ma HL, Hsu YT, Tsai CC, Chiang CC, Chen WM, Hung SC. Scale-up of MSC under hypoxic conditions for allogeneic transplantation and enhancing bony regeneration in a rabbit calvarial defect model. J Orthop Res. 2012;30:1213–1220. doi: 10.1002/jor.22070. [DOI] [PubMed] [Google Scholar]
- 19.Vrijsen KR, Sluijter JP, Schuchardt MW, van Balkom BW, Noort WA, Chamuleau SA, Doevendans PA. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med. 2010;14:1064–1070. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mierisch CM, Wilson HA, Turner MA, Milbrandt TA, Berthoux L, Hammarskjöld ML, Rekosh D, Balian G, Diduch DR. Chondrocyte transplantation into articular cartilage defects with use of calcium alginate: the fate of the cells. J Bone Joint Surg Am. 2003;85-A:1757–1767. doi: 10.2106/00004623-200309000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Pavesio A, Abatangelo G, Borrione A, Brocchetta D, Hollander AP, Kon E, Torasso F, Zanasi S, Marcacci M. Hyaluronan-based scaffolds (Hyalograft C) in the treatment of knee cartilage defects: preliminary clinical findings. Novartis Found Symp. 2003;249:203–217. discussion 229–33, 234–208, 239–41. [PubMed] [Google Scholar]
- 24.Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplasty. 2012;27:679–686. doi: 10.1016/j.arth.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 27.Verfaillie CM, Schwartz R, Reyes M, Jiang Y. Unexpected potential of adult stem cells. Ann N Y Acad Sci. 2003;996:231–234. doi: 10.1111/j.1749-6632.2003.tb03251.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, Hedrick MH, Benhaim P. Bone induction by BMP-2 transduced stem cells derived from human fat. J. Orthop. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 30.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 31.Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, Hwang SM. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 2006;24:679–685. doi: 10.1634/stemcells.2004-0308. [DOI] [PubMed] [Google Scholar]
- 32.Sandercock P, Roberts I. Systematic reviews of animal experiments. Lancet. 2002;360:586. doi: 10.1016/S0140-6736(02)09812-4. [DOI] [PubMed] [Google Scholar]
- 33.Biondi-Zoccai GG, Abbate A, Parisi Q, Agostoni P, Burzotta F, Sandroni C, Zardini P, Biasucci LM. Is vasopressin superior to adrenaline or placebo in the management of cardiac arrest? A meta-analysis. Resuscitation. 2003;59:221–224. doi: 10.1016/s0300-9572(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 34.Yan ZQ, Chen YS, Li WJ, Yang Y, Huo JZ, Chen ZR, Shi JH, Ge JB. Treatment of osteonecrosis of the femoral head by percutaneous decompression and autologous bone marrow mononuclear cell infusion. Chin J Traumatol. 2006;9:3–7. [PubMed] [Google Scholar]
- 35.Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A:1153–1160. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Jang BJ, Byeon YE, Lim JH, Ryu HH, Kim WH, Koyama Y, Kikuchi M, Kang KS, Kweon OK. Implantation of canine umbilical cord blood-derived mesenchymal stem cells mixed with beta-tricalcium phosphate enhances osteogenesis in bone defect model dogs. J Vet Sci. 2008;9:387–393. doi: 10.4142/jvs.2008.9.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucarelli E, Fini M, Beccheroni A, Giavaresi G, Di Bella C. Stromal stem cells and platelet-rich plasma improve bone allograft integration. Clin Orthop Relat Res. 2005;435:62–68. doi: 10.1097/01.blo.0000165736.87628.12. [DOI] [PubMed] [Google Scholar]
- 38.Field JR, McGee M, Stanley R, Ruthenbeck G, Papadimitrakis T, Zannettino A, Gronthos S, Itescu S. The efficacy of allogeneic mesenchymal precursor cells for the repair of an ovine tibial segmental defect. Vet Comp Orthop Traumatol. 2011;24:113–121. doi: 10.3415/VCOT-10-03-0046. [DOI] [PubMed] [Google Scholar]
- 39.Liao HT, Chen CT, Chen CH, Chen JP, Tsai JC. Combination of guided osteogenesis with autologous platelet-rich fibrin glue and mesenchymal stem cell for mandibular reconstruction. J Trauma. 2011;70:228–237. doi: 10.1097/TA.0b013e3181e12b56. [DOI] [PubMed] [Google Scholar]
- 40.Ai J, Ebrahimi S, Khoshzaban A, Jafarzadeh Kashi TS, Mehrabani D. Tissue engineering using human mineralized bone xenograft and bone marrow mesenchymal stem cells allograft in healing of tibial fracture of experimental rabbit model. Iran Red Crescent Med J. 2012;14:96–103. [PMC free article] [PubMed] [Google Scholar]
- 41.Peng J, Wen C, Wang A, Wang Y, Xu W, Zhao B, Zhang L, Lu S, Qin L, Guo Q, Dong L, Tian J. Micro-CT-based bone ceramic scaffolding and its performance after seeding with mesenchymal stem cells for repair of load-bearing bone defect in canine femoral head. J Biomed Mater Res B Appl Biomater. 2011;96:316–325. doi: 10.1002/jbm.b.31770. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Yang Y, Wang C, Xia R, Zhang Y, Zhao Q, Liao W, Wang Y, Lu J. Repair of sheep metatarsus defects by using tissue-engineering technique. J Huazhong Univ Sci Technolog Med Sci. 2005;25:62–67. doi: 10.1007/BF02831389. [DOI] [PubMed] [Google Scholar]
- 43.Niemeyer P, Schönberger TS, Hahn J, Kasten P, Fellenberg J, Suedkamp N, Mehlhorn AT, Milz S, Pearce S. Xenogenic transplantation of human mesenchymal stem cells in a critical size defect of the sheep tibia for bone regeneration. Tissue Eng Part A. 2010;16:33–43. doi: 10.1089/ten.TEA.2009.0190. [DOI] [PubMed] [Google Scholar]
- 44.Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, Shi S, Le AD, Wang SL. Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res. 2009;88:249–254. doi: 10.1177/0022034509333804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 2004;10:955–964. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]


