Abstract
Inappropriate use of medication is widespread, especially in older people, and is associated with risks, including adverse drug reactions, hospitalization and increased mortality. Optimization of appropriate medication use to minimize these harms is an ongoing challenge in healthcare. The term ‘deprescribing’ has been used to describe the complex process that is required for safe and effective cessation of medication. Patients play an important role in their own health and, while they may complain about the number of medications they have to take, they may also be reluctant to cease a medication when given the opportunity to do so. A review of previously proposed deprescribing processes and relevant literature was used to develop the patient-centred deprescribing process, which is a five-step cycle that encompasses gaining a comprehensive medication history, identifying potentially inappropriate medications, determining whether the potentially inappropriate medication can be ceased, planning the withdrawal regimen (e.g. tapering where necessary) and provision of monitoring, support and documentation. This is the first deprescribing process developed using knowledge of the patients' views of medication cessation; it focuses on engaging patients throughout the process, with the aim of improving long-term health outcomes. Despite a comprehensive review of the literature, there is still a lack in the evidence base on which to conduct deprescribing. The next step in broadening the evidence to support deprescribing will be to test the developed process to determine feasibility in the clinical setting.
Keywords: deprescribing, deprescribing process, elderly, inappropriate medication use, medication withdrawal, polypharmacy
Introduction
Inappropriate medication use (IMU) is common in older people with polypharmacy [1,2]. Owing to the increased risk of adverse drug reactions (ADRs), hospitalization and mortality [3–5], much research has focused on methods to reduce the prevalence of IMU [6–8]. While interventions targeted at reducing IMU, such as education and medication reviews, have shown some effectiveness, data on clinical outcomes (i.e. hospitalization and mortality) are lacking, and there are concerns about their long-term sustainability [8,9]. A missing element of these interventions is an evidence-based process for withdrawal of the identified inappropriate medication, i.e. a deprescribing process.
The need for a focus on the process required to stop inappropriate medications safely and effectively is highlighted not only by the prevalence of IMU, but also by quantitative and qualitative evidence indicating that the processes and resources currently in use are insufficient [10].
Cessation of a medication is the most common recommendation following a formal medication review, yet it is the least likely to be enacted [11,12]. General practitioners (GPs) report finding it difficult to conduct deprescribing in regular practice, mostly due to time restraints, and feel that there should be systematic processes to follow [13,14]. Despite the attention paid to the development of guidelines on how to initiate medications, there are very few examples that detail how to cease medications from either a medical or a holistic patient point of view [15–17]. Even when inappropriate medications are ceased, insufficient processes result in as many as 27% being restarted in the following 6 months [18–20], and a small but not insignificant proportion of restarted medications result in an ADR [21].
In 2003, Woodward proposed the following five principles of deprescribing: review all current medications; identify medications to be targeted for cessation; plan a deprescribing regimen; plan in partnership with patients and carers; and frequent review and support [22]. While interest in the area of deprescribing is increasing [with 10 of the 18 articles yielded on PubMed using the search term ‘deprescribing’ published in the past year (February 2013–2014)], the evidence underpinning the currently proposed deprescribing processes is sparse, lacking altogether or based on expert opinion. For this reason, we conducted a critical review of deprescribing processes and the evidence surrounding optimization of medication use in older people to develop an evidence-based, patient-centred deprescribing process that can be applied to individual patients in a one-to-one manner.
Development of the patient-centred deprescribing process
Why patient-centred?
In a group of primarily older people, >90% report being hypothetically willing to trial deprescribing; however, there are some major barriers to accepting medication cessation, including fear of consequences and disagreeing that the medication should be ceased [23,24]. Patient-centred care has been shown to improve patient satisfaction, adherence, quality of life and overall health outcomes [25–27]. Qualitative research has revealed that medical practitioners recognize the need for shared decision making (a fundamental aspect of patient-centred care [28]) when considering medication cessation [17,29], and the majority of patients want to be involved in the decision-making process, even if they prefer to leave the final decision up to their primary care practitioner (GP) [25,30].
The patient is an irreplaceable source of information, not only regarding their medical history, but also for establishing care goals. Most importantly, a review of approaches to cessation of IMU found that patient-mediated interventions (e.g. patient-directed educational interventions) were among the most effective [31].
In light of the above evidence, deprescribing should involve patient engagement throughout. Elements of patient-centred care include shared decision making, viewing the person as a whole and fostering a positive doctor–patient relationship [32–34]. The judge of whether a process is truly patient-centred must be the patient themselves, because this has been shown to be the best predictor of health outcomes and efficiency of healthcare [35]. Ultimately, true patient-centred care cannot be a one-model-fits-all approach.
Involvement and engagement of the GP is also required for successful deprescribing [31]. The GP has detailed knowledge of the patient's past medical history (including diagnoses and investigations) and their current condition. Difficulties in accessing this information have been identified as a barrier to the implementation of non-GP medication review services [11]. The quality of the doctor–patient relationship, specifically the trust that the patient has in his/her GP, has been established as an influence on patient willingness to cease medications [23]. Ferguson [36] described it best: ‘the withdrawal program should be actively managed by the doctor, but remain the property of the patient’.
Literature review
Articles were included for review if they proposed either potential elements or steps of the process of medication withdrawal. The term ‘deprescribing’ was not considered essential; however, the included articles had to discuss how to conduct cessation of medications rather than only how to identify inappropriate ones. The literature review was conducted in June 2013. The initial search strategy, using the search term ‘deprescribing process’, did not yield any results in PubMed. Use of broader terms, including ‘medication withdrawal/discontinuation’ and ‘process/algorithm/method’ yielded >30 000 results. Review of a random sample of these results showed that they were almost universally irrelevant and, as such, this was considered an inappropriate search strategy. Therefore, a ‘snowballing’ approach was used by hand-searching reference lists of key articles identified, which in turn were reviewed and reference lists of these searched. A citation search was also conducted for those articles which fulfilled the eligibility criteria. Initial key articles included the following: (i) all articles using the term ‘deprescribing’ (yielded by PubMed, Medline and Google Scholar); (ii) all articles citing Woodward's 2003 article [22]; and (iii) recent systematic reviews in the area of medication optimization in older people with polypharmacy. To determine the evidence base underpinning the recommendations, references provided in the included articles and additional literature on IMU were reviewed.
Process development
Starting with Woodward's five steps [22], we grouped the principles/elements recommended in each of the included articles according to similarity. The evidence base behind each of the recommendations (i.e. references provided within the articles) and additional relevant literature were reviewed to clarify each of the five steps. The resultant deprescribing process was then compared with deprescribing intervention studies that had been identified through the previously described search strategy. Intervention studies were considered to employ a deprescribing process if they used a systematic method for identifying the medication suitable for withdrawal, if there was some form of patient consent for withdrawal and if tapering and/or monitoring were conducted. Only studies conducted in community patients were considered.
Review of proposed deprescribing processes
Ten articles were identified; five reported a deprescribing process [15,22,37–39], while the other five reported potential/critical elements required for deprescribing (i.e. the process was not the main focus of the article) [10,40–43]; (Supporting Information Table S1). Of the five which reported a process, very little detail was provided on how this process was developed. Bain [15] reported expanding the previously developed and used ‘medication use process’, and Scott et al. reported that their framework was developed via relevant literature [39] and was validated as a tool to enable medical practitioners to identify more medications for cessation when applied to a hypothetical case [44]. There was no information provided in these or the other three studies reporting deprescribing processes as to how the relevant literature was identified or synthesized. This is, however, not surprising given the limitation of using the term ‘deprescribing’ and the difficulties in identifying the relevant articles yielded when using more general terms (i.e. withdrawal/cessation) as discussed above (see ‘Literature review’). Limited references were provided for the specific steps/elements considered to be essential in the identified studies (discussed further with regard to each of the steps below); however, two articles highlighted the limited evidence available to guide recommendations [10,37].
Critical review and amalgamation of the recommendations in the identified articles resulted in a five-step deprescribing process, depicted as a cycle in Figure 1. Of the 10 articles reviewed, only four included all five steps of the patient-centred deprescribing process (Supporting Information Table S1), highlighting the diverse opinions and lack of research available to guide deprescribing.
Figure 1.
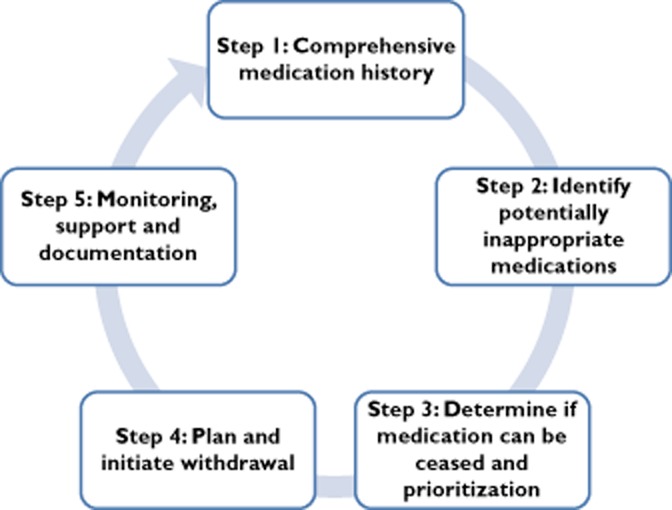
The five-step patient-centred deprescribing process
The patient-centred deprescribing process
The deprescribing process should ideally start when the medication is first prescribed, with the patient being informed of the likely duration of treatment and the need for ongoing review of appropriateness [24,45]. This could be considered step 0; however, as it may not be possible to know what the duration of treatment will be at initiation or it may not be remembered by the patient, it is relevant to consider the deprescribing process as a standalone event, to be initiated at any time in the patient's care.
Step 1: complete a comprehensive medication history
Obtaining a comprehensive medication history is the first step of the patient-centred deprescribing process and is fundamental for any medication-optimizing activity [29,46]. Six of the included articles report this as an element of the deprescribing process, with only two of these citing relevant research as to why this is required [22,39].
Undertaking a comprehensive medication history involves gaining a complete list of all the medications that the patient takes regularly, ‘as required’ and intermittently, including all prescription and nonprescription medications. Each listed medication should include the dose, frequency, formulation, route of administration, duration of use and patient-reported indication; any previous medication allergies, intolerances and ADRs should be documented [47,48].
General practitioner and hospital admission medication lists are often inaccurate, with up to 96% having at least one discrepancy compared with what the patient is actually taking [49,50], and 24–59% of these errors have the potential for harm [50]. A pharmacist-completed comprehensive medication history on admission to hospital has been associated with reduced medication costs, length of stay, medication errors, ADRs and mortality [51,52].
As previously discussed, the patient is central to the deprescribing process and should therefore be engaged throughout. Patients should be aware of the reason(s) for considering a reduction in the overall number of medications, and their willingness to engage in the process should be ascertained. When discussing that the medication review is being undertaken with the aim to determine whether all their medications are still appropriate, patients are able to highlight what medications they value, which ones they feel they may no longer need and if any are causing an ADR [24].
Step 2: identify potentially inappropriate medications
Different approaches to identification of potentially inappropriate medications were recommended in nine of the reviewed articles (Supporting Information Table S1). Ample evidence was provided, discussing why and how to identify medications for cessation. Most commonly discussed were identification of high-risk medications, including benzodiazepines and anticholinergics as well as medications without an indication. The foundation of all recommendations regarding identification of potentially inappropriate medications was whether the medication was providing more potential harm than benefit. Quantifying these risks and benefits in the individual, however, is not always straightforward, and many factors require consideration [53,54]. Of the 10 steps in the quality use of medicines framework described by Scott et al. [39], eight focus on how to identify medications for deprescribing. This illustrates not only the importance of this step, but also its complicated nature.
Evidence supporting the benefits and risks of medications are usually established via randomized controlled trials in relatively young patients with a single morbidity. These results cannot be applied reliably to older people and those with multiple morbidities [55,56]. Additionally, medication appropriateness surpasses the pharmacological benefits and risks, to include patient and cost considerations [55]. Some of the factors that should be considered when determining medication appropriateness in older people are necessity, benefit, contributing to or causing an ADR, future risk of ADRs, potential drug–drug or drug–disease interactions, adherence, patient preferences, care goals and life expectancy [9,53,57,58]. Application and consideration of these principles will enable the identification of medications that were once appropriate, but with time (i.e. the ageing process, addition of new medications and new medical conditions) the risks may have increased and/or the benefits may have decreased, resulting in it now being inappropriate to continue. In addition to these factors, it may be helpful to review why the medication was originally prescribed and its therapeutic intent (i.e. primary or secondary prevention, or symptom relief), and whether there was a planned duration of use at initiation [58,59].
Many tools have been developed to identify potentially inappropriate medications in older people, some of which may be useful in the deprescribing process. Implicit tools for identification of potentially inappropriate medications (e.g. Medication Appropriateness Index) may be used to standardize the process, while explicit tools (e.g. Beers Criteria) or medication class targeting may be useful to identify patients most likely to benefit from deprescribing [1,22,42,55,59,60]. Regardless of the method used, identification of potentially inappropriate medications suitable for withdrawal will require clinical knowledge and judgement, patient input and time.
Step 3: determine whether medication can be ceased and prioritization
When identifying a medication as ‘potentially inappropriate’, it must be remembered that this does not mean that it is possible to withdraw it at the time it is identified, if at all. In the absence of strong clinical evidence on the risks and benefits of medications, which is often the case in older people, it may be almost impossible to identify undoubtedly inappropriate medications [55,61,62]. It is more likely that ‘potentially’ or ‘probably’ inappropriate medications will be identified, and it is through the later steps of the deprescribing process (particularly monitoring and follow-up) that the benefit (and perhaps to a lesser extent the risks) and therefore appropriateness of the medication in the individual can be established. Determining the ability to trial withdrawal will involve patient consent, appropriate timing of withdrawal and consideration of whether or not withdrawal has been attempted previously.
While patient willingness to cease a medication is hypothetically high [23], genuine willingness may be much lower [63,64]. Patient preferences and care goals are taken into account when determining appropriateness from a medical point of view, but this does not mean that the patient will agree with cessation. Approaches to optimizing patient willingness to deprescribe include the following: (i) introducing deprescribing in a way that does not evoke fear or stress, and does not impair the relationship that the patient has with the prescriber; (ii) making it clear that the recommendations are being made to achieve therapeutic goals and not because the patient is ‘not worth treating’; (iii) discussion of the lack of benefits/necessity of the medication and the potential risks associated with its use (e.g. ADRs and cost); and (iv) discussion of the steps that will be taken to minimize the risks of deprescribing, and confirmation that deprescribing is a ‘trial’ [24]. It is important that the discussion is tailored to the individual, because patients have reported that when deciding on treatment options they want to know what is most appropriate for them personally, rather than what is appropriate for patients with their medical condition in general [65]. The deprescribing process may be enhanced by considering it a positive intervention, with an emphasis on the potential benefits to the patient.
The patient's choice needs to be respected. A person may choose to continue a medication if they have a firm belief in its benefit despite there being little evidence to support this, or may even cease a medication which they feel is responsible for an ADR (even if there is no objective evidence to support this). Balancing this patient choice against the pharmacological evidence will be a challenge for prescribers, and research into and acknowledgement of the ‘why’ behind patient decisions may help enable this process further. The need to consider patient preferences was discussed by all included articles; however, only one provided a reference of why this is important for medication cessation [10,31].
In some circumstances, despite a medication being identified as potentially inappropriate, it may not be suitable to cease immediately [41,66]. Patients should be medically stable so that any withdrawal reactions or return of symptoms can be attributed to the medication being withdrawn in order that appropriate measures can be taken. It is also best to trial cessation at a time that, if the condition was to return, it would not have a significant impact on the person's quality of life [17,67]. If the medication is going to be replaced by nonpharmacological management (e.g. physiotherapy or psychology), medication cessation may need to be postponed until these services have been organized and initiated.
Furthermore, it should be determined whether cessation has been trialled previously and failed. If there was an identifiable reason for the failure (e.g. the medication was not tapered), it may be possible to trial deprescribing again. Additionally, for some medications where dependency (physical or psychological) is an issue (e.g. benzodiazepines) previous failure to deprescribe will not exclude future attempts [36,68]. In other patients, a return of symptoms will confirm the necessity of the medication [e.g. return of reflux symptoms upon tapered withdrawal of a proton pump inhibitor (PPI) [69]].
If more than one potentially inappropriate medication is identified for deprescribing, it is best that they are withdrawn sequentially [15,22,37,70,71]. This will allow for identification of the responsible medication if a withdrawal reaction or return of condition occurs (so that the necessary corrective action can be taken) and to maximize patient comprehension and ability to follow the tapering regimen. Deciding which medication to cease first will not always be clear. Patient and GP priorities may differ; the patient may elect to cease the most expensive medication first, while the medical practitioner may prioritize cessation of a medication that is involved in a drug–drug interaction [22]. It would be sensible to discuss these different priorities so that mutually agreed goals can be developed.
Step 4: plan and initiate medication withdrawal
Once it has been decided that a medication discontinuation can be attempted, it needs to be determined how to proceed with this. Specifically, does the medication need to be tapered and, if so, how should it be tapered? There are three main reasons why a medication should be tapered prior to cessation: to prevent adverse drug withdrawal reactions; to detect return of condition early; and to increase patient comfort.
When ceasing a medication used for a symptomatic disease, there may also need to be a symptom action plan that the patient can self-initiate if they experience a return of symptoms. Having a plan for withdrawal will be critical for patient agreement with cessation [24].
Nine of the articles discussed considering tapering prior to cessation, with the evidence surrounding the potential for adverse drug withdrawal events provided as the main reason for this step. Abrupt cessation of many medications can cause symptoms due to a physiological withdrawal reaction [15]. Prevention of withdrawal symptoms is important not only from a patient comfort point of view, but also because some withdrawal symptoms mimic the original condition. If symptoms occur despite tapering, it can be concluded that they are most likely to be a return of the medical condition (tapering does not completely prevent withdrawal reactions) [72].
It may be considered appropriate to trial discontinuation of medication to determine whether a medication is still providing a benefit to a patient [15,73]. However, in the situation where the medication was beneficial, if it is stopped abruptly this may result in a rapid return of symptoms, significantly impacting the patient, their disease management and the patient–provider relationship. If the medication cannot be ceased due to return of the condition, tapering will also identify the minimal effective dose, reducing their overall ADR risk [74,75].
Finally, the thought of medication discontinuation can evoke fear in some patients, even in relationship to medications that are not commonly associated with withdrawal reactions [24]. Patients report feeling more comfortable with cessation (and more willing to trial cessation) if the medication is reduced gradually [76].
Despite the knowledge that tapering helps to prevent withdrawal symptoms, the most effective regimens (i.e. how quickly to reduce the dose and by how much it should be reduced at a time) are mostly unknown [15,16].
Step 5: monitoring, support and documentation
Seven of the included articles reported the need for monitoring during and after medication withdrawal; two of these supplied references concerning the need for this, but the rationale for monitoring was to maximize patient adherence [39,43].
A limitation of some interventions to reduce inappropriate medication use is the lack of sustainability. Reductions in polypharmacy and inappropriate medication use achieved during the intervention period may be lost in the following months [7]. Although provision of monitoring (i.e. follow-up), support and documentation will be crucial for the long-term success of deprescribing, very little evidence exists to guide how they should be conducted [77].
What monitoring is required, how often and how long the monitoring should continue for will depend on the medication but should be tailored to the individual patient. Symptom monitoring may be conducted over the telephone; however, most patients prefer a face-to-face consultation for at least the first follow-up after cessation [23]. There are circumstances, for example if the medication being withdrawn was involved in a drug–drug interaction, where additional monitoring may be required [10,78].
Support, while intertwined with monitoring, is mentioned separately because it is highly valued by patients for the process of medication withdrawal [79]. Feeling supported may result from time spent with the healthcare professional. Other kinds of support may involve providing education on lifestyle measures (e.g. foods to avoid in order to reduce the need for proton pump inhibitor therapy in gastro-oesophageal reflux disease [80]), advice on coping strategies (to reduce reliance on benzodiazepines [68]) or referral to counselling services.
At the conclusion of the deprescribing process, the outcome should be documented, including whether the medication was ceased or the dose reduced (and if it was not ceased, why it was not ceased) as well as the process that was undertaken that led to this result. This documentation should minimize both medication errors and reinitiation of previously ceased medications [18,19,21,81,82]. Two of the included articles discussed communication with other healthcare professionals [15,43], while one specifically mentioned documentation in the form of updating the patient's medication list [41]. Where the documentation should occur (i.e. who is the keeper of the information) will be a challenge and may need to be tailored for different national contexts.
Previous ‘deprescribing’ trials
Four studies have been identified which could be considered as employing and testing a deprescribing process to determine outcomes, although only one of these identified itself as such [64,83–85]. Details of these studies and how their methods compare with the patient-centred deprescribing process are shown in Supporting Information Table S2.
The results of these studies provide further support to the recommendations contained within the patient-centred deprescribing process. Firstly, the patient and their GP should be engaged throughout deprescribing. These elements were contained in the two studies with the highest medication cessation rates [84,85], and lack of these elements was specifically identified by Williams et al. [64] as a major contributor to their limited success.
Patient acceptance of deprescribing between these four studies cannot be compared truly, because there were considerable differences in how this element was reported. The earliest two studies reported vastly different acceptance rates of 33% [64] and 82% [84], which was, in fact, combined patient and GP acceptance. The fourth study had the highest participant acceptance rate (94%), although this may not be a true representation of the total population because invitation to attend the clinic specifically to review use of the target medication was likely to have self-selected patients who were more willing to trial deprescribing [85]. In addition, different medication classes were targeted, which may have influenced acceptance rates (Supporting Information Table S2). Differences in patient acceptance of medication cessation may also relate to the country in which the study was conducted, because medication-taking behaviour as well as beliefs about medications can vary depending on cultural background as well as country characteristics [86,87].
While the study by van Duijn et al. [85] had the greatest patient willingness to cease a medication, it also had the highest rate of reinitiation of medication, with 41% of ceased medications being restarted within 6 months. A much lower reinitiation rate of 2% was found in the study by Garfinkel and Mangin [84]. Different medications were ceased in the two studies, with one study involving the cessation of any medication determined to be inappropriate [84] and the other only antihypertensives and cholesterol-lowering medications [85]. It was reported that the reinitiation of antihypertensives and cholesterol-lowering medications was due to increases in blood pressure or cholesterol readings, but not necessarily to a level that warranted medication use [85]. They could not fully explore the reasons for this (hence the high reinitiation rate), but it may relate to difficulties in changing prescribing behaviour or the compulsion for GPs to ‘act’, with prescribing being a familiar and comfortable action [68,88,89].
Future directions
Despite a comprehensive review of the literature, there is still a lack of an evidence base on which to conduct deprescribing. There is currently minimal intervention-type research to support the recommendations made, in particular those that relate to how to employ tapering and how monitoring and support should be provided. The next step in broadening the evidence to support deprescribing will be to test the developed patient-centred process to determine feasibility in a clinical setting.
Deprescribing has the potential to improve clinical outcomes, but whether there is a real benefit (and what the risks are) can be determined only after development and testing of a systematic deprescribing process. Determination of the benefits in terms of mortality and morbidity will require large randomized controlled trials, requiring hundreds or even thousands of participants in each arm, so the conduct of these trials may, unfortunately, not be achievable.
Consideration should be given to how a deprescribing process can be integrated with other interventions to reduce IMU. Employing this deprescribing process as a population-wide intervention to reduce IMU may not be the most efficient or effective method. Interventions where reimbursement for commonly overused medications have been restricted to only a few medically warranted indications has shown great reductions in overall prescribing [90,91]. While this type of intervention does not address how to cease the medication in patients already taking it, it could be paired with education on how to deprescribe the medication. For example, the Australian Department of Veterans Affairs funds a programme to improve quality use of medications, whereby targeted information sheets are sent to prescribers, pharmacists and patients. One of their interventions involved dissemination of an educational pamphlet, ‘PPIs in GORD: reduce the dose – keep the benefits’ [74], which provided practical guidance on which patients are suitable for dose reductions and how to perform this; a 14.5% increase in low-dose PPI prescribing was observed after the intervention [92]. Additionally, an educational campaign on appropriate indications for benzodiazepine use in a public hospital resulted in a significant increase in attempted dose reduction in patients who had been taking a benzodiazepine before admission (23 vs. 47% pre- vs. postintervention, P < 0.01) [93]. Alternatively, a deprescribing process could be amalgamated with previously existing medication optimization programmes, such as the home medication reviews in Australia [94]. More thought and discussion will be required to determine how wide-scale deprescribing can be best achieved.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: ER had support from the Australian Government (PhD candidacy funded by the Australian Postgraduate Award) for the submitted work; ER received an honorarium for a workshop presentation from the Australian Association of Consultant Pharmacists (AACP) and received payment for work conducted as part of a nonrelated study funded by the Society of Hospital Pharmacists Australia Celegene Information Technology in Hospital grant in the previous 3 years; SS, IH, MSR and MDW declare no support from any organization for the submitted work.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Overview of recommendations of elements required for deprescribing and how they correspond to the developed patient-centred deprescribing process
Summary of deprescribing process intervention studies and how the methods employed compare with the patient-centred deprescribing process
References
- 1.Steinman MA, Landefeld CS, Rosenthal GE, Berthenthal D, Sen S, Kaboli PJ. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516–1523. doi: 10.1111/j.1532-5415.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 2.Guaraldo L, Cano FG, Damasceno GS, Rozenfeld S. Inappropriate medication use among the elderly: a systematic review of administrative databases. BMC Geriatr. 2011;11:79. doi: 10.1186/1471-2318-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klarin I, Wimo A, Fastbom J. The association of inappropriate drug use with hospitalisation and mortality: a population-based study of the very old. Drugs Aging. 2005;22:69–82. doi: 10.2165/00002512-200522010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Lau DT, Kasper JD, Potter DE, Lyles A, Bennett RG. Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch Intern Med. 2005;165:68–74. doi: 10.1001/archinte.165.1.68. [DOI] [PubMed] [Google Scholar]
- 5.Lund BC, Carnahan RM, Egge JA, Chrischilles EA, Kaboli PJ. Inappropriate prescribing predicts adverse drug events in older adults. Ann Pharmacother. 2010;44:957–963. doi: 10.1345/aph.1m657. [DOI] [PubMed] [Google Scholar]
- 6.Forsetlund L, Eike MC, Gjerberg E, Vist GE. Effect of interventions to reduce potentially inappropriate use of drugs in nursing homes: a systematic review of randomised controlled trials. BMC Geriatr. 2011;11:16. doi: 10.1186/1471-2318-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnjidic D, Le Couteur DG, Kouladjian L, Hilmer SN. Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin Geriatr Med. 2012;28:237–253. doi: 10.1016/j.cger.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Kaur S, Mitchell G, Vitetta L, Roberts MS. Interventions that can reduce inappropriate prescribing in the elderly: a systematic review. Drugs Aging. 2009;26:1013–1028. doi: 10.2165/11318890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, Hanlon JT. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370:173–184. doi: 10.1016/S0140-6736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- 10.Ostini R, Hegney D, Jackson C, Tett SE. Knowing how to stop: ceasing prescribing when the medicine is no longer required. J Manag Care Pharm. 2012;18:68–72. doi: 10.18553/jmcp.2012.18.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brulhart M, Wermeille J. Multidisciplinary medication review: evaluation of a pharmaceutical care model for nursing homes. Int J Clin Pharm. 2011;33:549–557. doi: 10.1007/s11096-011-9506-1. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K, Pinholt EM. Reducing polypharmacy in the elderly. A controlled trial of physician feedback. J Am Geriatr Soc. 1990;38:31–36. doi: 10.1111/j.1532-5415.1990.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 13.Hamdy RC, Moore SW, Whalen K, Donnelly JP, Compton R, Testerman F, Haulsee P, Hughes J. Reducing polypharmacy in extended care. South Med J. 1995;88:534–538. doi: 10.1097/00007611-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Raghunath A, Hungin A, Cornford C, Featherstone V. Use of proton pump inhibitors: an exploration of the attitudes, knowledge and perceptions of general practitioners. Digestion. 2005;72:212–218. doi: 10.1159/000089727. [DOI] [PubMed] [Google Scholar]
- 15.Bain KT, Holmes HM, Beers MH, Maio V, Handler SM, Pauker SG. Discontinuing medications: a novel approach for revising the prescribing stage of the medication-use process. J Am Geriatr Soc. 2008;56:1946–1952. doi: 10.1111/j.1532-5415.2008.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coulson J, Routledge PA. Adverse reactions to drug withdrawal. Adverse Drug React Bull. 2008;252:967–970. [Google Scholar]
- 17.Wong I, Asherson P, Bilbow A, Clifford S, Coghill D, Desoysa R, Hollis C, McCarthy S, Murray M, Planner C, Potts L, Sayal K, Taylor E. Cessation of attention deficit hyperactivity disorder drugs in the young (CADDY) – a pharmacoepidemiological and qualitative study. Health Technol Assess. 2009;13:1–144. doi: 10.3310/hta13490. [DOI] [PubMed] [Google Scholar]
- 18.Viktil KK, Blix HS, Eek AK, Davies MN, Moger TA, Reikvam A. How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open. 2012;2:e001461. doi: 10.1136/bmjopen-2012-001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Linden CM, Kerskes MC, Bijl AM, Maas HA, Egberts AC, Jansen PA. Represcription after adverse drug reaction in the elderly: a descriptive study. Arch Intern Med. 2006;166:1666–1667. doi: 10.1001/archinte.166.15.1666. [DOI] [PubMed] [Google Scholar]
- 20.Lampela P, Hartikainen S, Lavikainen P, Sulkava R, Huupponen R. Effects of medication assessment as part of a comprehensive geriatric assessment on drug use over a 1-year period: a population-based intervention study. Drugs Aging. 2010;27:507–521. doi: 10.2165/11536650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Allen AS, Sequist TD. Pharmacy dispensing of electronically discontinued medications. Ann Intern Med. 2012;157:700–705. doi: 10.7326/0003-4819-157-10-201211200-00006. [DOI] [PubMed] [Google Scholar]
- 22.Woodward M. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:323–328. [Google Scholar]
- 23.Reeve E, Wiese MD, Hendrix I, Roberts M, Shakib S. People's attitudes, beliefs, and experiences regarding polypharmacy and willingness to deprescribe. J Am Geriatr Soc. 2013;61:1508–1514. doi: 10.1111/jgs.12418. [DOI] [PubMed] [Google Scholar]
- 24.Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging. 2013;30:793–807. doi: 10.1007/s40266-013-0106-8. [DOI] [PubMed] [Google Scholar]
- 25.Zikmund-Fisher BJ, Couper MP, Fagerlin A. Disparities in patient reports of communications to inform decision making in the DECISIONS survey. Patient Educ Couns. 2012;87:198–205. doi: 10.1016/j.pec.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Michie S, Miles J, Weinman J. Patient-centredness in chronic illness: what is it and does it matter? Patient Educ Couns. 2003;51:197–206. doi: 10.1016/s0738-3991(02)00194-5. [DOI] [PubMed] [Google Scholar]
- 27.Jahng KH, Martin LR, Golin CE, DiMatteo MR. Preferences for medical collaboration: patient-physician congruence and patient outcomes. Patient Educ Couns. 2005;57:308–314. doi: 10.1016/j.pec.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Barry MJ, Edgman-Levitan S. Shared decision making – the pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 29.Schuling J, Gebben H, Veehof LJG, Haaijer-Ruskamp FM. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56. doi: 10.1186/1471-2296-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levinson W, Kao A, Kuby A, Thisted R. Not all patients want to participate in decision making. J Gen Intern Med. 2005;20:531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostini R, Jackson C, Hegney D, Tett SE. How is medication prescribing ceased? A systematic review. Med Care. 2011;49:24–36. doi: 10.1097/MLR.0b013e3181ef9a7e. [DOI] [PubMed] [Google Scholar]
- 32.Hudon C, Fortin M, Haggerty JL, Lambert M, Poitras M-E. Measuring patients' perceptions of patient-centered care: a systematic review of tools for family medicine. Ann Fam Med. 2011;9:155–164. doi: 10.1370/afm.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitson A, Marshall A, Bassett K, Zeitz K. What are the core elements of patient-centred care? A narrative review and synthesis of the literature from health policy, medicine and nursing. J Adv Nurs. 2013;69:4–15. doi: 10.1111/j.1365-2648.2012.06064.x. [DOI] [PubMed] [Google Scholar]
- 34.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. 2000;51:1087–1110. doi: 10.1016/s0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 35.Stewart M. Towards a global definition of patient centred care: the patient should be the judge of patient centred care. BMJ. 2001;322:444–445. doi: 10.1136/bmj.322.7284.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson B. Benzodiazepines: guide to withdrawing in general practice. Prescriber. 2012;23:35–39. [Google Scholar]
- 37.Le Couteur DG, Banks E, Gnjidic D, McLachlan A. Deprescribing. Aust Prescriber. 2011;34:182–185. [Google Scholar]
- 38.Hardy JE, Hilmer SN. Deprescribing in the last year of life. J Pharm Pract Res. 2011;41:146–151. [Google Scholar]
- 39.Scott I, Gray L, Martin J, Mitchell C. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med. 2012;125:529–537. doi: 10.1016/j.amjmed.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Alexander GC, Sayla MA, Holmes HM, Sachs GA. Prioritizing and stopping prescription medicines. Can Med Assoc J. 2006;174:1083–1084. doi: 10.1503/cmaj.050837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandt NJ, Stefanacci RG. Discontinuation of unnecessary medications in older adults. Consult Pharm. 2011;26:845–854. doi: 10.4140/TCP.n.2011.845. [DOI] [PubMed] [Google Scholar]
- 42.Meeks TW, Culberson JW, Horton MS. Medications in long-term care: when less is more. Clin Geriatr Med. 2011;27:171–191. doi: 10.1016/j.cger.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Gordon SF, Dainty C, Smith T. Why and when to withdraw drugs in the elderly and frail. Prescriber. 2012;23:47–51. [Google Scholar]
- 44.Scott I, Gray L, Martin J, Mitchell C. Effects of a drug minimization guide on prescribing intentions in elderly persons with polypharmacy. Drugs Aging. 2012;29:659–667. doi: 10.1007/BF03262281. [DOI] [PubMed] [Google Scholar]
- 45.Bull SA, Hu XH, Hunkeler EM, Lee JY, Ming EE, Markson LE, Fireman B. Discontinuation of use and switching of antidepressants: influence of patient-physician communication. JAMA. 2002;288:1403–1409. doi: 10.1001/jama.288.11.1403. [DOI] [PubMed] [Google Scholar]
- 46.Ramaswamy R, Maio V, Diamond JJ, Talati AR, Hartmann CW, Arenson C, Roehl B. Potentially inappropriate prescribing in elderly: assessing doctor knowledge, confidence and barriers. J Eval Clin Pract. 2011;17:1153–1159. doi: 10.1111/j.1365-2753.2010.01494.x. [DOI] [PubMed] [Google Scholar]
- 47.SHPA committee of Specialty Practice in Clinical Pharmacy. SHPA standards of practice for clinical pharmacy. J Pharm Pract Res. 2005;35:122–146. [Google Scholar]
- 48.Institute for Safe Medication Practices. Making the Case for Medication Reconciliation. Washington, DC: American Pharmacists Association; 2012. Available at http://www.pharmacist.com/making-case-medication-reconciliation (last accessed 8 May 2013). Last updated 1 November 2012. [Google Scholar]
- 49.Frank C, Godwin M, Verma S, Kelly A, Birenbaum A, Seguin R, Anderson J. What drugs are our frail elderly patients taking? Do drugs they take or fail to take put them at increased risk of interactions and inappropriate medication use? Can Fam Physician. 2001;47:1198–1204. [PMC free article] [PubMed] [Google Scholar]
- 50.Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173:510–515. doi: 10.1503/cmaj.045311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27:481–493. doi: 10.1592/phco.27.4.481. [DOI] [PubMed] [Google Scholar]
- 52.Nester TM, Hale LS. Effectiveness of a pharmacist-acquired medication history in promoting patient safety. Am J Health Syst Pharm. 2002;59:2221–2225. doi: 10.1093/ajhp/59.22.2221. [DOI] [PubMed] [Google Scholar]
- 53.Gallagher P, Barry P, O'Mahony D. Inappropriate prescribing in the elderly. J Clin Pharm Ther. 2007;32:113–121. doi: 10.1111/j.1365-2710.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 54.Laroche ML, Charmes JP, Bouthier F, Merle L. Inappropriate medications in the elderly. Clin Pharmacol Ther. 2008;85:94–97. doi: 10.1038/clpt.2008.214. [DOI] [PubMed] [Google Scholar]
- 55.O'Connor M, Gallagher P, O'Mahony D. Inappropriate prescribing: criteria, detection and prevention. Drugs Aging. 2012;29:437–452. doi: 10.2165/11632610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 56.Hilmer SN, Gnjidic D. The effects of polypharmacy in older adults. Clin Pharmacol Ther. 2009;85:86–88. doi: 10.1038/clpt.2008.224. [DOI] [PubMed] [Google Scholar]
- 57.Hilmer SN, Gnjidic D, Le Couteur DG. Thinking through the medication list – appropriate prescribing and deprescribing in robust and frail older patients. Aust Fam Physician. 2012;41:924–928. [PubMed] [Google Scholar]
- 58.Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605–609. doi: 10.1001/archinte.166.6.605. [DOI] [PubMed] [Google Scholar]
- 59.O'Mahony D, Gallagher PF. Inappropriate prescribing in the older population: need for new criteria. Age Ageing. 2008;37:138–141. doi: 10.1093/ageing/afm189. [DOI] [PubMed] [Google Scholar]
- 60.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 61.Caslake R, Soiza RL, Mangoni AA. Practical advice for prescribing in old age. Medicine (Baltimore) 2013;41:9–12. [Google Scholar]
- 62.Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals. JAMA. 2007;297:1233–1240. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- 63.Elliott RA. Reducing medication regimen complexity for older patients prior to discharge from hospital: feasibility and barriers. J Clin Pharm Ther. 2012;37:637–642. doi: 10.1111/j.1365-2710.2012.01356.x. [DOI] [PubMed] [Google Scholar]
- 64.Williams ME, Pulliam CC, Hunter R, Johnson TM, Owens JE, Kincaid J, Porter C, Koch G. The short-term effect of interdisciplinary medication review on function and cost in ambulatory elderly people. J Am Geriatr Soc. 2004;52:93–98. doi: 10.1111/j.1532-5415.2004.52016.x. [DOI] [PubMed] [Google Scholar]
- 65.Nair K, Dolovich L, Cassels A, McCormack J, Levine M, Gray J, Mann K, Burns S. What patients want to know about their medications. Focus group study of patient and clinician perspectives. Can Fam Physician. 2002;48:104–110. [PMC free article] [PubMed] [Google Scholar]
- 66.Sellors J, Kaczorowski J, Sellors C, Dolovich L, Woodward C, Willan A, Goeree R, Cosby R, Trim K, Sebaldt R, Howard M, Hardcastle L, Poston J. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. CMAJ. 2003;169:17–22. [PMC free article] [PubMed] [Google Scholar]
- 67.Kilinç S, Campbell C. The experience of discontinuing antiepileptic drug treatment: an exploratory investigation. Seizure. 2008;17:505–513. doi: 10.1016/j.seizure.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Parr JM, Kavanagh DJ, Young RM, McCafferty K. Views of general practitioners and benzodiazepine users on benzodiazepines: a qualitative analysis. Soc Sci Med. 2006;62:1237–1249. doi: 10.1016/j.socscimed.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 69.Vine L, Philpott R, Fortun P. Proton pump inhibitors: how to withdraw treatment. Prescriber. 2012;23:12–16. [Google Scholar]
- 70.O'Mahony D, O'Connor MN. Pharmacotherapy at the end-of-life. Age Ageing. 2011;40:419–422. doi: 10.1093/ageing/afr059. [DOI] [PubMed] [Google Scholar]
- 71.Le Couteur DG, Hilmer SN, Glasgow N, Naganathan V, Cumming RG. Prescribing in older people. Aust Fam Physician. 2004;33:777–781. [PubMed] [Google Scholar]
- 72.Graves T, Hanlon JT, Schmader KE, Landsman PB, Samsa GP, Pieper CF, Weinberger M. Adverse events after discontinuing medications in elderly outpatients. Arch Intern Med. 1997;157:2205–2210. [PubMed] [Google Scholar]
- 73.Zermansky AG, Silcock J. Is medication review by primary-care pharmacists for older people cost effective?: a narrative review of the literature, focusing on costs and benefits. Pharmacoeconomics. 2009;27:11–24. doi: 10.2165/00019053-200927010-00003. [DOI] [PubMed] [Google Scholar]
- 74.Veterans' Medicines Advice and Therapeutics Education Services. Veterans' MATES Therapeutic Brief Edaffairs DoV. Adelaide: Australian Government Department of Veterans' Affairs; 2006. PPIs in GORD. Reduce the dose – keep the benefits. In:. Available at https://www.veteransmates.net.au/VeteransMATES/VeteransMATESServlet?page=site&m=10020 (last accessed 14 April 2014) [Google Scholar]
- 75.Tinetti ME. Preventing falls in elderly persons. N Engl J Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 76.Verbeek-Heida P, Mathot E. Better safe than sorry – why patients prefer to stop using selective serotonin reuptake inhibitor (SSRI) antidepressants but are afraid to do so: results of a qualitative study. Chronic Illn. 2006;2:133–142. doi: 10.1177/17423953060020020801. [DOI] [PubMed] [Google Scholar]
- 77.Iyer S, Naganathan V, McLachlan AJ, Le Couteur DG. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25:1021–1031. doi: 10.2165/0002512-200825120-00004. [DOI] [PubMed] [Google Scholar]
- 78.Uijtendaal EV, Zwart-van Rijkom JE, van Solinge WW, Egberts TC. Serum potassium influencing interacting drugs: risk-modifying strategies also needed at discontinuation. Ann Pharmacother. 2012;46:176–182. doi: 10.1345/aph.1Q542. [DOI] [PubMed] [Google Scholar]
- 79.Smeets HM, De Wit NJ, Delnoij DMJ, Hoes AW. Patient attitudes towards and experiences with an intervention programme to reduce chronic acid-suppressing drug intake in primary care. Eur J Gen Pract. 2009;15:219–225. doi: 10.3109/13814780903452168. [DOI] [PubMed] [Google Scholar]
- 80.Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177. doi: 10.1186/1472-6963-7-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vira T, Colquhoun M, Etchells E. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care. 2006;15:122–126. doi: 10.1136/qshc.2005.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barnsteiner JH. Medication reconciliation: transfer of medication information across settings – keeping it free from error. Am J Nurs. 2005;105:31–36. doi: 10.1097/00000446-200503001-00007. [DOI] [PubMed] [Google Scholar]
- 83.Beer C, Loh PK, Peng YG, Potter K, Millar A. A pilot randomized controlled trial of deprescribing. Ther Adv Drug Saf. 2011;2:37–43. doi: 10.1177/2042098611400332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648–1654. doi: 10.1001/archinternmed.2010.355. [DOI] [PubMed] [Google Scholar]
- 85.van Duijn HJ, Belo JN, Blom JW, Velberg ID, Assendelft WJ. Revised guidelines for cardiovascular risk management time to stop medication? A practice-based intervention study. Br J Gen Pract. 2011;61:e347–352. doi: 10.3399/bjgp11X578025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horne R, Graupner L, Frost S, Weinman J, Wright SM, Hankins M. Medicine in a multi-cultural society: the effect of cultural background on beliefs about medications. Soc Sci Med. 2004;59:1307–1313. doi: 10.1016/j.socscimed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 87.Chia LR, Schlenk EA, Dunbar-Jacob J. Effect of personal and cultural beliefs on medication adherence in the elderly. Drugs Aging. 2006;23:191–202. doi: 10.2165/00002512-200623030-00002. [DOI] [PubMed] [Google Scholar]
- 88.Bain KT. Barriers and strategies to influencing physician behavior. Am J Med Qual. 2007;22:5–7. doi: 10.1177/1062860606296147. [DOI] [PubMed] [Google Scholar]
- 89.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362:1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 90.Ramser KL, Sprabery LR, Hamann GL, George CM, Will A. Results of an intervention in an academic Internal Medicine Clinic to continue, step-down, or discontinue proton pump inhibitor therapy related to a tennessee medicaid formulary change. J Manag Care Pharm. 2009;15:344–350. doi: 10.18553/jmcp.2009.15.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kollen BJ, van der Veen WJ, Groenhof F, Donker GA, van der Meer K. Discontinuation of reimbursement of benzodiazepines in the Netherlands: does it make a difference? BMC Fam Pract. 2012;13:111. doi: 10.1186/1471-2296-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roughead EE, Ellett LMK, Ramsay EN, Pratt NL, Barratt JD, LeBlanc VT, Ryan P, Peck R, Killer G, Gilbert AL. Bridging evidence-practice gaps: improving use of medicines in elderly Australian veterans. BMC Health Serv Res. 2013;13:514. doi: 10.1186/1472-6963-13-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elliott R, Woodward M, Oborne C. Improving benzodiazepine prescribing for elderly hospital inpatients using audit and multidisciplinary feedback. Intern Med J. 2001;31:529–535. doi: 10.1046/j.1445-5994.2001.00139.x. [DOI] [PubMed] [Google Scholar]
- 94.Stafford AC, Tenni PC, Peterson GM, Jackson SL, Hejlesen A, Villesen C, Rasmussen M. Drug-related problems identified in medication reviews by Australian pharmacists. Pharm World Sci. 2009;31:216–223. doi: 10.1007/s11096-009-9287-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of recommendations of elements required for deprescribing and how they correspond to the developed patient-centred deprescribing process
Summary of deprescribing process intervention studies and how the methods employed compare with the patient-centred deprescribing process


