Abstract
Aims
Congestive heart failure (CHF) associated with vascular endothelial growth factor tyrosine-kinase inhibitors (VEGFR-TKIs) has emerged as a relevant problem in clinical and scientific communities. We performed an up-to-date, comprehensive meta-analysis to determine the overall incidence and risk of CHF in cancer patients receiving VEGFR-TKIs.
Methods
The databases of PubMed, Web of Science and abstracts presented at the American Society of Clinical Oncology up to August 31 2013 were searched for relevant articles. Statistical analyses were conducted to calculate the summary incidence, odds ratio (OR) and 95% confidence intervals (CIs) by using either random effects or fixed effect models according to the heterogeneity of included studies.
Results
A total of 10 553 patients from 36 clinical trials were included. The overall incidence of all grade and high grade CHF associated with VEGFR-TKIs was 3.2% (95% CI 1.8%, 5.8%) and 1.4% (95% CI 0.9%, 2.3%), respectively. The use of VEGFR-TKIs significantly increased the risk of developing all grade (OR 2.37, 95% CI 1.76, 3.20, P < 0.001) and high grade (OR 3.51, 95% CI 1.74, 7.05, P < 0.001) CHF. In subgroup analyses, the risk of CHF did not significantly vary with tumour types (P = 0.071 for all grade; P = 0.72 for high grade) and VEGFR-TKIs (P = 0.55 for all grade; P = 0.99 for high grade). Meta-regression indicated that CHF might possibly occur early in the treatment of VEGFR-TKIs. No evidence of publication bias was observed.
Conclusion
The use of VEGFR-TKIs is associated with a significantly increased risk of developing congestive heart failure in cancer patients. Clinicians should be aware of this risk and provide close monitoring in patients receiving these therapies.
Keywords: congestive heart failure, meta-analysis, VEGFR-TKIs
Introduction
In recent years, anti-angiogenesis targeted therapies have proven to be a promising therapeutic strategy in patients with cancer [1,2]. Several newly-developed agents that target the vascular endothelial growth factor (VEGF) signalling pathway, such as the small molecular VEGF receptor inhibitors (e.g. sunitinib, sorafenib, vandetanib, pazopanib, axitinib, cediranib, tivozanib, regorafenib, cabozantinib, brivanib and ramucirumab) and the anti-VEGF monoclonal antibody bevacizumab, have shown encouraging treatment benefits in patients with various types of solid tumours [3–16]. However, as the VEGF pathway is not only essential for normal growth and development, but also critical to physiological response and homeostasis in many organs and functions in adulthood [17], a variety of adverse effects are anticipated with pharmacological blockage of this pathway. Indeed, the clinical adverse event profiles are extensive [18–20]. The adverse effects attributed to VEGF inhibition include hypertension, arterial thromboembolic events (ATEs), proteinuria or renal dysfunction, wound complications, haemorrhage and gastrointestinal perforation, which have been systematically defined in previous studies [21–36].
Congestive heart failure (CHF) is a rare but serious adverse event associated with VEGF-targeted agents. A previous meta-analysis demonstrated that the use of bevacizumab significantly increased the risk of CHF when compared with controls (relative risk (RR) 4.74, 95% CI 1.66, 11.18, P = 0.001) [37]. The VEGFR-TKI agent sunitinib has been also associated with an increased risk of CHF in one meta-analysis [38]. However, that report has several limitations. Although the meta-analysis included 16 clinical trials, most of these were single arm trials, and only four randomized controlled trials (RCTs) were included in the meta-analysis and thus the power to investigate the risk of CHF with sunitinib was small and the combined results might have been affected by a single large RCT. In addition, several newly developed VEGFR-TKIs which share a similar spectrum of target receptors with sunitinib might be also associated with increased risk of developing CHF. Indeed, CHF related to these drugs has been sporadically reported in recent clinical trials [7,39–43]. However the contributions of these newly developed VEGFR-TKIs to CHF are still unknown. As a result, we conducted this meta-analysis of all available clinical trials to determine the overall incidence and risk of CHF associated with VEGFR-TKIs.
Methods
Data sources
We conducted an independent review of citations from PubMed between January 1 1966 and August 31 2013. Keywords were sorafenib, nexavar, BAY43-9006, sunitinib, sutent, SU11248, pazopanib, votrient, GW786034, vandetanib, caprelsa, ZD6474, axitinib, cediranib, tivozanib, regorafenib, cabozantinib, brivanib, ramucirumab, clinical trials and cancer. The search was limited to prospective clinical trials published in English. The search strategy also used text terms such as angiogenesis inhibitors and vascular endothelial growth factor receptor-tyrosine kinase inhibitors to identify relevant information. We also performed independent searches using Web of Science databases between January 1 1966 and August 31 2013, to ensure that no clinical trials were overlooked. Additionally, we searched the clinical trial registration website (http://www.ClinicalTrials.gov) to obtain information on the registered prospective trials. We also searched abstracts and virtual meeting presentations from the American Society of Clinical Oncology (http://www.asco.org/ASCO) conferences that took place between January 2004 and January 2013. Reference lists from relevant primary studies and review articles were also examined to find additional publications. Each publication was reviewed and in cases of duplicate publication only the most complete, recent and updated report of the clinical trial was included in the meta-analysis.
Study selection was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [44]. Clinical trials that met the following criteria were included: (1) prospective phase II and III trials, expanded access protocols (EAPs), (2) participants assigned to treatment with VEGFR-TKIs (alone or in combination at any dosage or frequency) and (3) available data regarding events or incidence of CHF and sample size. Phase I trials were excluded because of inter-study variability in drug dosing as well as the small number of patients in these trials.
Data extraction
Data abstraction was conducted independently by two investigators (WXQ and ZS), and any discrepancy between the reviewers was resolved by consensus. For each study, the following information was extracted: first author's name, year of publication, trial phase, number of enrolled subjects, treatment arms, number of patients in treatment and controlled groups, underlying malignancy, median age, median treatment duration, median progression-free survival, number of CHF events, name and dosage of the VEGFR-TKIs agents. We considered the reporting of left ventricular ejection fraction (LVEF) decline or dysfunction and CHF not otherwise specified as CHF-related adverse events. Adverse events of all and high grade (≥ grade 3), as recorded according to the National Cancer Institute's common terminology criteria for adverse events (version 2 or 3), were extracted for analysis [45]. The quantitative five point Jadad scale was used to assess the quality of the included RCTs based on the reporting of the studies' methods and results [46].
Statistical analysis
The principal summary measures were incidence, odds ratio (OR) and corresponding 95% confidence intervals (CIs). For the calculation of incidence, the number of patients experiencing CHF and total number of patients treated with VEGFR-TKIs were extracted from the safety profiles of all selected clinical trials; the proportion of patients with CHF and 95% CI were derived for each study. We used the Peto method to calculate the ORs and 95% CIs because this method provided the best CI coverage and was more powerful and relatively less biased than the fixed or random effects analysis when dealing with low event rates [47]. Between study heterogeneity was estimated using the χ2-based Q statistic [48]. Heterogeneity was considered statistically significant when Pheterogeneity < 0.1. If heterogeneity existed, data were analyzed using a random effects model. In the absence of heterogeneity, a fixed effects model was used. A statistical test with a P value less than 0.05 was considered significant. To assess the stability of the results, sensitivity analysis was carried out by sequential omission of individual studies. Additionally, to test whether effect sizes were moderated by differences in the length of treatment, we had carried out meta-regressions with differences in the median length of experimental treatments (expressed in months) as a predictor and the odds ratio as a dependent variable. The presence of publication bias was evaluated by using the Egger tests. All statistical analyses were performed by using Stata version 12.0 software (Stata Corporation, College Station, Texas, USA) and Open Meta-Analyst software version 4.16.12 (Tufts University).
Results
Search results
Our search yielded 927 clinical studies relevant to VEGFR-TKIs (sunitinib, sorafenib, pazopanib, vandetanib, axitinib, cediranib, tivozanib, regorafenib, cabozantinib, brivanib and ramucirumab). After excluding review articles, phase I studies, case reports, meta-analyses and observation studies (Figure 1), we selected 36 clinical trials, including eight phase III, 27 phase II trials and one extended access programme (EAP) trial, for the purposes of analysis (Table 2). A total of 10 553 patients from 36 clinical trials were included for analysis. The characteristics of patients and studies are listed in Table 1. According to the inclusion criteria of each trial, patients were required to have adequate hepatic, renal and haematological function. Underlying malignancies included renal cell cancer [14,15,40,49–57] (12 trials), breast cancer [58–63] (six trials), sarcoma [7,39,64,65] (four trials), thyroid cancer [42,66,67] (three trials), gastro-intestinal stromal tumour (GIST) [13,68] (two trials), primitive neuroectodermal tumour (PNET) [69] (one trial), hepatocellular carcinoma [70] (one trial), pancreatic cancer [43] (one trial), cervical cancer [71] (one trial), colorectal cancer (one trial) [16], gastric cancer [41] (one trial), prostate cancer [72] (one trial), non-small cell lung cancer (NSCLC) [73] (one trial) and advanced neuroendocrine tumours [74] (one trial). The most commonly reported adverse event meeting our criteria was LVEF decline (16 studies), with congestive failure in 12 studies and LV dysfunction in eight studies. The quality of 15 included RCTs was high. Ten trials had Jadad scores of 5 and four trials did not mention the blinding of allocation clearly in the randomization process and thus had Jadad scores of 3. Another one trial had a Jadad score of 2.
Figure 1.
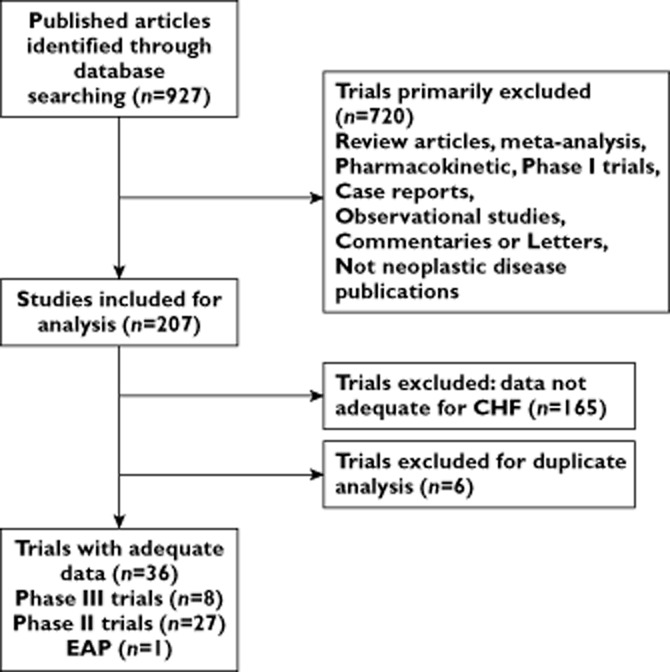
Selection process for prospective clinical trials included in the meta-analysis
Table 2.
Incidence of CHF based on prespecified subgroups
| Grades | Subgroup | Number of trials | CHF events | Total number of patients | I2 (%) | Incidence (95% CI) | P for group difference |
|---|---|---|---|---|---|---|---|
| All grade | Overall | 27 | 174 | 6903 | 90 | 3.2 (1.8, 5.8) | NA |
| Tumour types | |||||||
| RCC | 10 | 119 | 5228 | 96 | 4.5 (1.4, 13.6) | 0.52 | |
| Non-RCC | 17 | 55 | 1675 | 63 | 3.0 (1.7, 5.1) | ||
| VEGFR-TKIs | |||||||
| Sunitinib | 14 | 136 | 5638 | 94.3 | 3.6 (1.4, 8.8) | 0.025 | |
| Sorafenib | 4 | 9 | 269 | 66.2 | 3.4 (0.9, 12.5) | ||
| Axitinib | 2 | 3 | 114 | 0 | 2.7 (0.9, 8.1) | ||
| Cediranib | 2 | 6 | 99 | 48.0 | 5.9 (1.5, 20.6) | ||
| Vandetanib | 1 | 1 | 231 | 0 | 0.4 (0.1, 3.0) | ||
| Pazopanib | 3 | 18 | 314 | 0 | 6.1 (3.9, 9.5) | ||
| Ramucirumab | 1 | 1 | 238 | 0 | 0.4 (0.1, 2.9) | ||
| Phase of trials | |||||||
| Phase II | 21 | 45 | 1240 | 32.8 | 4.5 (3.1, 6.6) | 0.42 | |
| Phase III | 6 | 129 | 5663 | 97.8 | 2.4 (0.5, 10.2) | ||
| High grade | Overall | 26 | 48 | 6896 | 58 | 1.4 (0.9, 2.3) | NA |
| Tumour types | |||||||
| RCC | 10 | 35 | 5219 | 0 | 1.8 (0.7, 4.4) | 0.55 | |
| Non-RCC | 16 | 13 | 1677 | 96 | 1.3 (0.8, 2.0) | ||
| VEGFR-TKIs | |||||||
| Sunitinib | 15 | 37 | 5724 | 74.2 | 1.5 (0.7, 3.1) | 0.72 | |
| Sorafenib | 3 | 3 | 238 | 0 | 1.5 (0.5, 4.5) | ||
| Axitinib | 1 | 1 | 52 | 0 | 1.9 (0.3, 12.4) | ||
| Cediranib | 2 | 2 | 99 | 0 | 2.0 (0.5, 7.7) | ||
| Vandetanib | 1 | 1 | 231 | 0 | 0.4 (0.1, 3.0) | ||
| Pazopanib | 3 | 4 | 314 | 0 | 1.5 (0.6, 3.7) | ||
| Ramucirumab | 1 | 0 | 238 | 0 | 0 | ||
| Phase of trials | |||||||
| Phase II | 19 | 16 | 1147 | 97.4 | 2.1 (1.4, 3.3) | 0.18 | |
| Phase III | 7 | 32 | 5749 | 0 | 0.9 (0.3, 2.8) | ||
RCC, renal cell carcinoma; NA, not available.
Table 1.
Baseline characteristics of the 36 trials included in the meta-analysis (n = 10 553)
| Authors/phase | Histology | Patients enrolled | Treatment arm | Number for analysis | Median age (years) | Median treatment duration (months) | Median PFS/TTP (months) | Median OS (months) | Number of high grade CHF | Reported events | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demetri et al. [13]/III* | GIST | 312 | Sunitinib 50 mg d1−1-28, q6w | 202 | 58 | 1.9 | 6 | NR | 3 | LVEF decline | 5 |
| Placebo | 102 | 55 | 1 | 1.5 | NR | 0 | |||||
| Motzer et al. [15]/III* | RCC | 750 | Sunitinib 50 mg d1−1-28, q6w | 375 | 62 | 6 | 11 | NR | 13 | LVEF decline | 3 |
| INF 9 MUI 3 times weekly | 360 | 59 | 4 | 2 | NR | 4† | |||||
| Barrios et al. [58]/III* | HER-2 negative BC | 482 | Sunitinib 37.5 mg qd + capecitabine 1 250 mg m−2 d1–14 bid po, q3w.§ | 238 | 53 | 2 | 2.8 | 15.3 | 1 | Congestive failure | 3 |
| Capecitabine 1 250 mg m−2 d1–14 bid po, q3w | 244 | 53 | 2 | 4.2 | 24.6 | 0 | |||||
| Abou-Alfa et al. [70]/II* | HCC | 96 | Doxorubicin + sorafenib 400 mg bid po qd | 47 | 66 | 4 | 6 | 13.7 | 1 | LV dysfunction | 5 |
| Doxorubicin + placebo | 49 | 65 | 1.9 | 2.7 | 6.5 | 0 | |||||
| Raymond et al. [69]/III* | PNET | 171 | Sunitinib 37.5 mg qd po | 86 | 56 | 4.6 | 11.4 | NR | 2 | Cardiac failure | 5 |
| placebo | 85 | 47 | 3.7 | 5.5 | NR | 0 | |||||
| Kindler et al. [43]/II* | Pancreatic carcinoma | 632 | Axitinib 5 mg bid po + gemcitabine 1000 mg m−2 d 1, 8, 15 q4w. | 314 | 61 | 2.8 | 4.4 | 8.5 | 1 | Cardiac failure | 5 |
| Placebo + gemcitabine 1000 mg m−2 d1, 8, 15. q4w. | 316 | 62 | 2.3 | 4.4 | 8.3 | 0 | |||||
| Bergh et al. [59]/III* | MBC | 593 | Sunitinib 37.5 mg qd po q3w. + docetaxel | 296 | 54 | 6.1 | 8.6 | 24.8 | 1 | Cardiac failure | 5 |
| Placebo + ocetaxel | 297 | 56 | 4.2 | 8.3 | 25.5 | 0 | |||||
| Mulders et al. [57]/II* | RCC | 71 | Cediranib 45 mg qd po | 53 | 60 | 12 | 12.1 | NR | 1 | LVEF decline | 5 |
| placebo | 18 | 61 | NR | 2.8 | NR | 0 | |||||
| Wells et al. [42]/III* | Thyroid cancer | 331 | Vandetanib 300 mg qd po | 231 | 50.7 | 21 | 30.5 | NR | 1 | Cardiac failure | 5 |
| placebo | 100 | 53.4 | 9.3 | 19.3 | NR | 0 | |||||
| van der Graaf et al. [7]/III* | STS | 372 | Pazopanib 800 mg qd po | 246 | 51.9 | 3.83 | 4.6 | 12.5 | 3 | LVEF decline | 5 |
| placebo | 123 | 56.7 | 1.89 | 1.6 | 10.7 | 0 | |||||
| Cristofanilli et al. [60]/II* | Inflammatory BC | 163 | Pazopanib 800 mg qd po + lapatinib 1500 mg | 38 | 52 | 2.8 | NR | 16.2 | 0 | LVEF decline | 3 |
| Placebo + apatinib 1500 mg | 38 | 52 | 3.8 | NR | 14.7 | 0 | |||||
| Placebo + apatinib 1500 mg | 36 | 53 | 3.76 | NR | 15.9 | 0 | |||||
| pazopanib 400 mg qd po + lapatinib 1000 mg | 38 | 54 | 2.96 | NR | NR | 0 | |||||
| pazopanib 800 mg qd po | 13 | 55 | 1.73 | NR | NR | 0 | |||||
| Curigliano et al. [61]/II* | Triple-negative BC | 217 | Sunitinib 37.5 mg qd po q3w. | 113 | 52 | NR | 2 | 9.4 | 0 | Cardiac failure | 2 |
| standard of care | 104 | 52 | NR | 2.7 | 10.5 | 1 | |||||
| Fuchs et al. [41]/III* | Gastric or gastro-oesophageal junction cancer | 355 | Ramucirumab 8 mg kg−1 q2w. | 238 | 60 | 1.87 | 2.1 | 5.2 | 0 | Cardiac failure | 5 |
| placebo | 117 | 60 | 1.4 | 1.3 | 3.8 | 0 | |||||
| Hyams et al. [62]/II* | MBC | 62 | Cediranib 45 mg qd po + fulvestrant 500 mg q4w. | 31 | NR | NR | 7.4 | NR | 0 | LVEF decline | 5 |
| Placebo + ulvestrant 500 mg q4w. | 31 | NR | NR | 3.7 | NR | 0 | |||||
| Johnston et al. [63]/II* | HER-2 positive BC | 177 | Pazopanib 400 mg qd po + lapatinib 1000 mg | 69 | 50 | NR | NR | NR | 0 | LV dysfunction | 3 |
| Placebo + lapatinib 1500 mg | 72 | 54 | NR | NR | NR | 0 | |||||
| Lapatinib + pazopanib 800 mg qd po | 36 | 54 | NR | NR | NR | 0 | |||||
| Motzer et al. [14]/II | RCC | 63 | Sunitinib 50 mg d1−1-28 q6w | 63 | 60 | 9 | 8.7 | NR | 1 | LVEF decline | NA |
| Motzer et al. [49]/II | RCC | 106 | Sunitinib 50 mg d1−1-28 q6w | 105 | 56 | 7.5 | 8.3 | NR | 5 | LVEF decline | NA |
| Saltz et al. [16]/II | CRC | 84 | Sunitinib 50 mg d1−1-28 q6w, bevacizumab native | 40 | 56.5 | NR | 2.3 | NR | 0 | LVEF decline | NA |
| Sunitinib 50 mg d1−1-28 q6w, pretreated with bevacizumab | 42 | 57.5 | NR | 2.8 | NR | 0 | |||||
| Rixe et al. [50]/II | RCC | 52 | Axitinib 5 mg bid po qd | 52 | 59 | 9.4 | 15.7 | 29.9 | 1 | LVEF decline | NA |
| Kulke et al. [74]/II | pancreatic carcinoid | 107 | Sunitinib 50 mg d1−1-28 q6w | 107 | 56 | 12.5 | 7.7 | 16.4 | 1 | CHF | NA |
| Dror Michaelson et al. [72]/II | prostate cancer | 34 | Sunitinib 50 mg d1−1-28 q6w | 34 | 71 | 1.5 | NR | NR | 1 | LV dysfunction | NA |
| Escudier et al. [9]/II | RCC | 107 | Sunitinib 37.5 qd po | 105 | 59 | 8.4 | 8.2 | 19.8 | 1 | LVEF decline | NA |
| Gore et al. [53]/II | RCC | 4564 | Sunitinib 50 mg d1−1-28 q6w | 4371 | 59 | 7.5 | 10.9 | 18.4 | 10 | CHF | NA |
| Hensley et al. [64]/II | Uterine leiomyosarcoma | 25 | Sunitinib 50 mg d1−1-28 q6w | 23 | 56 | NR | 1.54 | 15.1 | 0 | LVEF decline | NA |
| Kontovinis et al. [54]/II | RCC | 42 | Sunitinib 50 mg d1−1-28 q6w | 42 | 64 | NR | 8.9 | 16.23 | 1 | LVEF decline | NA |
| Di Lorenzo et al. [51]/II | RCC | 52 | Sorafenib 400 mg qd po q8w. | 52 | 60 | 4.1 | 3.73 | 7.5 | 1 | LV dysfunction | NA |
| Hoftijzer et al. [66]/II | Thyroid carcinoma | 31 | Sorafenib 400 mg qd po q8w. | 31 | 65 | NR | 13.5 | NR | NR | CHF | NA |
| Kloos et al. [67]/II | Thyroid carcinoma | 41 | Sorafenib 400 mg qd po q8w. Previously treated | 19 | 67 | 8.73 | 16 | 23 | 1 | LV dysfunction | NA |
| Sorafenib 400 mg qd po q8w. Native | 22 | 56 | 9.87 | 10 | 37.5 | 0 | |||||
| Maki et al. [65]/II | Sarcoma | 147 | Sorafenib 400 mg qd po q4w. | 145 | 55 | NR | 3.2 | 14.3 | 1 | Cardiac ejection fraction | NA |
| Rini et al. [6]/II | RCC | 62 | Axitinib 5 mg bid po qd | 62 | 60 | 6.2 | 7.4 | 13.6 | NR | CHF | NA |
| MacKay et al. [71]/II | Cervical cancer | 19 | Sunitinib 50 mg d1−1-28 q6w | 19 | 44 | NR | 3.5 | NR | 0 | LV dysfunction | NA |
| Tomita et al. [56]/II | RCC | 51 | Sunitinib 50 mg d1−1-28 q6w | 51 | 56.6 | 4.9 | 12.2 | 33.1 | 1 | LVEF decline | NA |
| 61.1 | 6.5 | 10.6 | 32.5 | ||||||||
| Matsumoto et al. [68]/II | GIST | 18 | Sunitinib 50 mg d1−1-28 q6w | 18 | 58.7 | NR | 5.3 | NR | 0 | LVEF decline | NA |
| Hainsworth et al. [40]/II | RCC | 55 | Pazopanib 800 mg qd po q8w. | 55 | 60 | 6 | 7.5 | NR | 1 | LV dysfunction | NA |
| Kummar et al. [39]/II | STS | 46 | Cediranib 30 mg qd po q4w. | 46 | 27 | 1.87 | NR | NR | 1 | LV dysfunction | NA |
| Reynolds et al. [73]/II | NSCLC | 63 | Sunitinib 37.5 mg qd po q6w. | 63 | 78.4 | 2.8 | 3 | NR | 0 | CHF | NA |
Randomized controlled trial.
Data retrieved from drug package insert.
1200 mg m−2 in patients age ≥65 years. PFS, progression-free survival; TTP, time to progression; OS, overall survival; LVEF, left ventricular ejection fraction; CHF, congestive heart failure; RCC, renal cell cancer; STS, soft tissue sarcoma; MBC, metastatic breast cancer; NSCLC, non-small-cell lung carcinoma; GIST, gastrointestinal stromal tumours; CRC, colorectal cancer; HCC, hepatocellular carcinoma; PNET, pancreatic neuroendocrine tumours; NR, not reported; NA, not available. Qd daily; bid twice daily; q2w every 2 weeks; q3w every 3 weeks; q4w every 4 weeks; q6w every 6 weeks; q8w every 8 weeks.
Incidence of CHF
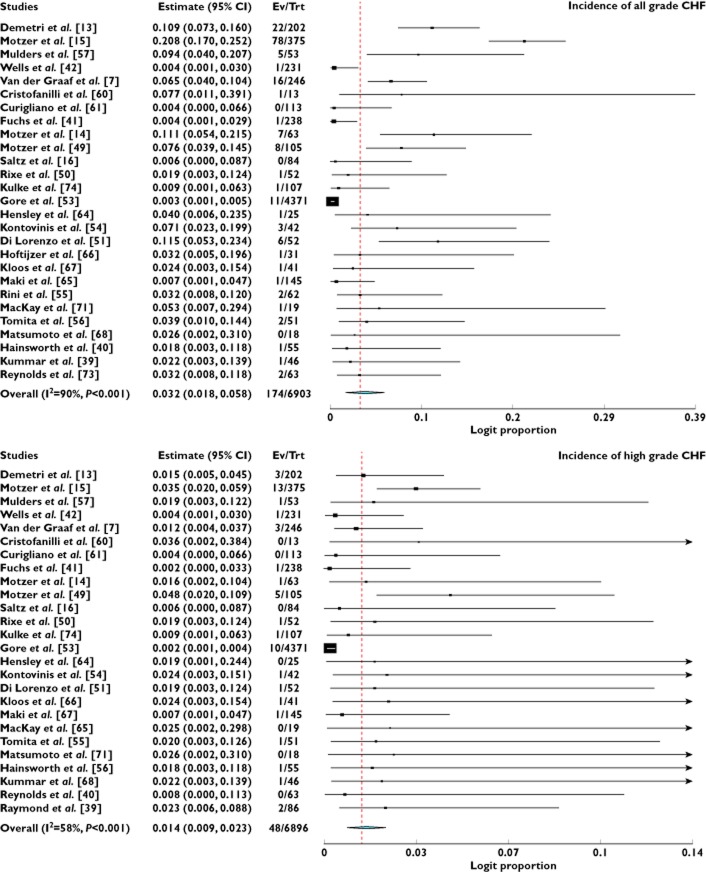
A total of 6903 patients from 27 treatment arms who received VEGFR-TKIs as a single agent were available for all grade CHF analysis. There were 174 total CHF events among these patients. The incidence of all grade CHF ranged between 0% and 11.5%. Using a random effect model (χ2-based Q statistic test: Q = 269.35, P < 0.001, I2 = 90%), the overall incidence of all grade CHF was 3.2% (95% CI 1.8, 5.8%, Figure 2). High grade CHF was associated with increased morbidity and could result in dose modification or treatment interruption. A total of 6896 patients prescribed VEGFR-TKIs as a single agent from 26 treatment arms were included for analysis. The incidence of high grade CHF ranged from 0% to 4.8%. The summary incidence of high grade CHF was 1.4% (95% CI 0.9, 2.3%, Figure 2) according to the random effects model. We also performed a sub-group analysis to investigate the incidence difference according to tumour types, VEGFR-TKIs and phase of trials. Our results demonstrated that the incidence of CHF did not significantly vary with tumour types and phase of trials (Table 2). The incidence of all grade CHF associated pazopanib (6.1%) and cediranib (5.9%) was higher than that of vandetanib (0.4%) and ramucirumab (0.4%), and there were significant variations in the incidence of all grade CHF among different VEGFR-TKIs (P = 0.025), but not for high grade CHF (P = 0.72).
Figure 2.
Incidence of all and high grade CHF associated with VEGFR-TKIs
Odds ratio of CHF
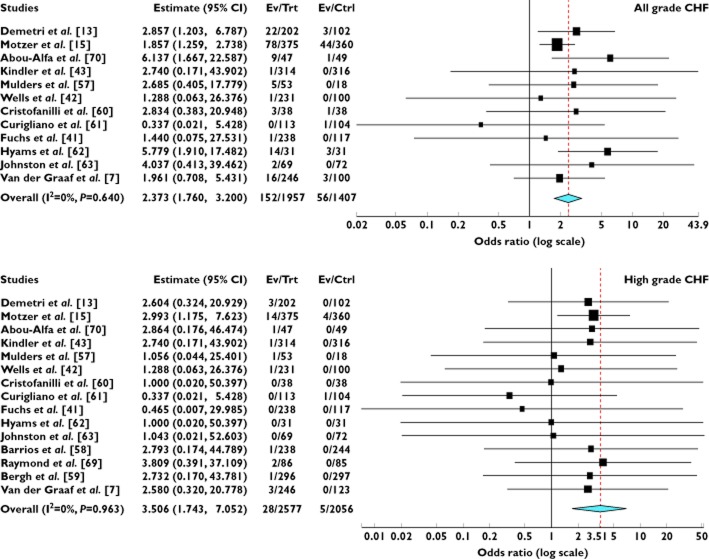
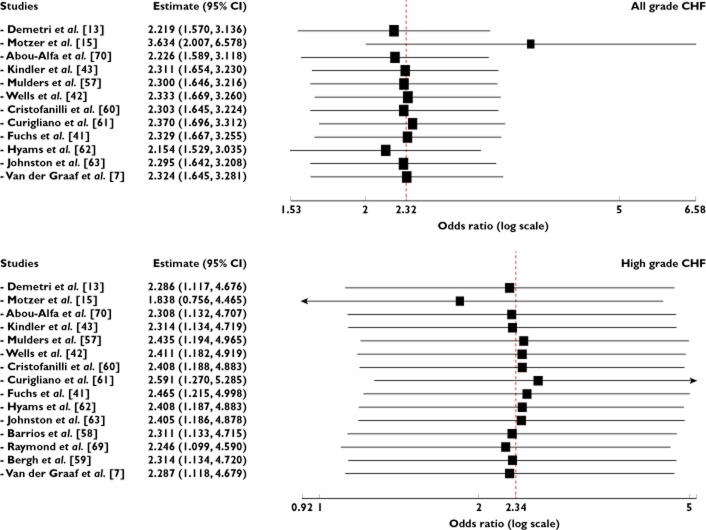
A meta-analysis of the OR for all grade CHF attributable to VEGFR-TKIs compared with controls was performed on 12 randomized controlled trials. The overall OR for all grade CHF was 2.37 (95% CI 1.76, 3.20, P < 0.001, Figure 3), according to the fixed effects model. As for high grade CHF, 15 RCTs were available for analysis. The combined OR also demonstrated that VEGFR-TKIs significantly increased the risk of developing CHF (OR = 3.51, 95% CI 1.74, 7.05, P < 0.001, Figure 3) using a fixed effects model. We also did a sensitivity analysis to examine the stability and reliability of pooled ORs by sequential omission of individual studies. The results indicated that the significance estimate of pooled all grade ORs was not significantly influenced by omitting any single study. As for high grade ORs, there was a non-significantly increased risk of developing high grade CHF after excluding the trial conducted by Motzer et al. [15] (Figure 4). Since in three studies, data on the length of treatment were not reported, nine of 12 studies were included in the analysis. The result indicated that the OR tended to be lower in the studies in which the experimental treatment was longer, and this effect was statistically significant (β = 1.15, P = 0.13, Figure 5). As for high grade CHF, a similar result was also observed (β = 1.26, P = 0.52, Figure 5). Based on these results, we believe that CHF might possibly occur early in treatment regimens.
Figure 3.
Odds ratio of all and high grade CHF associated with VEGFR-TKIs vs. control
Figure 4.
Meta-analysis of all and high grade CHF associated with VEGFR-TKIs vs. control: ‘leave-one-out’ sensitivity analysis
Figure 5.
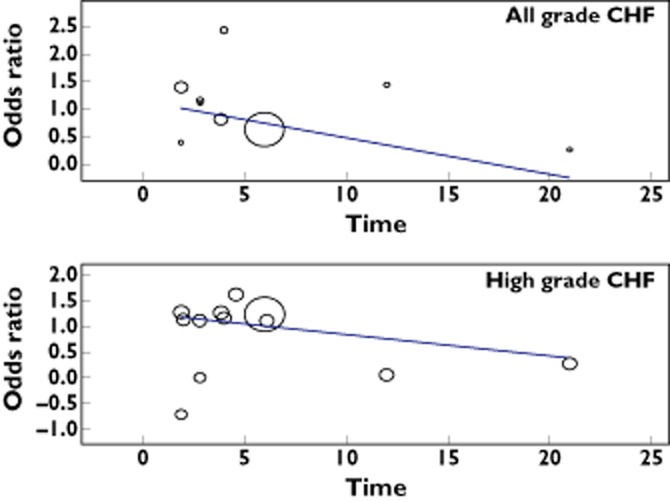
Meta-regression analysis of trends between treatment duration (months) and all and high grade odds ratio. Symbols: each study is represented by a circle the diameter of which is proportional to its statistical weight
Risk of CHF according to different tumour types, VEGFR-TKIs and phase of trials
To determine whether the observed increase in ORs of developing all and high grade CHF was the result of confounding bias, we preformed subgroup analyses of renal cell carcinoma (RCC) vs. other malignancies, phase II vs. phase III trials and trials with different VEGFR-TKIs. No significant differences were observed for ORs of all (1.91 vs. 3.36, P = 0.071) or high grade (3.02 vs. 4.31, P = 0.72) between patients with RCC and non-RCC. Similarly, no significant differences in ORs were found among different VEGFR-TKIs (all grade: P = 0.55, high grade: P = 0.99, Table 3). Interestingly, the ORs of all grade CHF were significantly higher in phase II trials than in phase III trials (4.77 vs. 2.01, P = 0.026), but not for high grade CHF (2.21 vs. 3.73, P = 0.67).
Table 3.
Odds ratio of CHF based on pre-specified subgroups
| Grades | Subgroup | Number of trials | VEGFR-TKIs | Control | I2 (%) | OR (95% CI) | P value | P for group difference |
|---|---|---|---|---|---|---|---|---|
| Events/total, n | Events/total, n | |||||||
| All grade | Overall | 12 | 152/1957 | 56/1407 | 0 | 2.37 (1.76, 3.20) | <0.001 | NA |
| Tumour types | ||||||||
| RCC | 2 | 83/428 | 44/378 | 0 | 1.91 (1.30, 2.80) | 0.001 | 0.071 | |
| Non-RCC | 10 | 69/1529 | 12/1029 | 0 | 3.36 (2.08, 5.43) | <0.001 | ||
| VEGFR-TKIs | ||||||||
| Sunitinib | 3 | 100/690 | 48/566 | 26.0 | 1.95 (1.37, 2.78) | <0.001 | 0.55 | |
| Sorafenib | 1 | 9/47 | 1/49 | 0 | 6.14 (1.67, 22.59) | 0.006 | ||
| Axitinib | 1 | 1/314 | 0/316 | 0 | 7.44 (0.15, 374.8) | 0.32 | ||
| Cediranib | 2 | 19/84 | 3/49 | 0 | 5.37 (2.02, 14.3) | 0.001 | ||
| Vandetanib | 1 | 1/231 | 0/100 | 0 | 4.19 (0.06, 299.27) | 0.51 | ||
| Pazopanib | 3 | 21/353 | 4/210 | 0 | 2.40 (1.01, 5.69) | 0.047 | ||
| Ramucirumab | 1 | 1/238 | 0/117 | 0 | 4.44 (0.07, 287.50) | 0.48 | ||
| Phase of trials | ||||||||
| Phase II | 7 | 34/665 | 6/628 | 0 | 4.77 (2.41, 9.43) | <0.001 | 0.026 | |
| Phase III | 5 | 118/1292 | 50/779 | 0 | 2.01 (1.44, 2.81) | <0.001 | ||
| High grade | Overall | 15 | 28/2577 | 5/2056 | 0 | 3.51 (1.74, 7.05) | <0.001 | NA |
| Tumour types | ||||||||
| RCC | 2 | 15/428 | 4/378 | 0 | 3.02 (1.21, 7.55) | 0.008 | 0.72 | |
| Non-RCC | 13 | 13/2149 | 1/1678 | 0 | 4.31 (1.46, 12.72) | 0.018 | ||
| VEGFR-TKIs | ||||||||
| Sunitinib | 6 | 21/1310 | 5/1192 | 0 | 3.19 (1.46, 6.95) | 0.004 | 0.99 | |
| Sorafenib | 1 | 1/47 | 0/49 | 0 | 7.71 (0.15, 388.9) | 0.31 | ||
| Axitinib | 1 | 1/314 | 0/316 | 0 | 7.44 (0.15, 374.8) | 0.32 | ||
| Cediranib | 2 | 1/53 | 0/18 | 0 | 3.82 (0.04, 345.5) | 0.56 | ||
| Vandetanib | 1 | 1/231 | 0/100 | 0 | 4.19 (0.06, 299.3) | 0.51 | ||
| Pazopanib | 3 | 3/353 | 0/233 | 0 | 4.52 (0.41, 50.16) | 0.22 | ||
| Ramucirumab | 1 | 0/238 | 0/117 | 0 | NA | NA | ||
| Phase of trials | ||||||||
| Phase II | 7 | 3/665 | 1/628 | 0 | 2.21 (0.29, 16.72) | 0.44 | 0.67 | |
| Phase III | 8 | 25/1912 | 4/1428 | 0 | 3.73 (1.77, 7.86) | <0.001 | ||
RCC, renal cell carcinoma; NA, not available.
Publication bias
No evidence of publication bias was detected for the OR of all grade and high grade ILD in this study by Egger's test (OR of all grade: P = 0.18, OR of high grade: P = 0.66).
Discussion
CHF is a rare but potentially life-threatening complication during anti-VEGF therapy [75,76]. Concerns have arisen regarding the risk of CHF with the use of these drugs. A previous meta-analysis demonstrated that the incidence of high grade CHF was 1.6% (95% CI 1.0, 2.6%) among patients receiving the VEGF antibody bevacizumab, and patients treated with bevacizumab had a significantly increased risk of developing CHF (RR 4.74, 95% CI 1.66, 11.18, P = 0.001) [37]. However, the association between CHF and VEGFR-TKIs, which also target VEGF signalling pathways, has not been systematically defined. As a result, we conducted this study to investigate the overall incidence and risk of CHF in cancer patients treated with VEGFR-TKIs.
Our study included 10 553 patients from 36 clinical trials and demonstrated that the pooled incidence of VEGFR-TKIs associated all and high grade CHF was 3.2% (95% CI 1.8, 5.8%) and 1.4% (95% CI 0.9, 2.3%), respectively. Additionally, we also found that the use of VEGFR-TKIs was associated with a significantly increased risk of all and high grade CHF when compared with controls. Sensitivity analysis indicated that the significance estimate of pooled all grade ORs was not significantly influenced by omitting any single study. As for high grade ORs, there is a non-significantly increased risk of developing high grade CHF after excluding the trial conducted by Motzer et al. [15]. In addition, the meta-regression indicated that the OR of CHF tended to be lower in studies in which the experimental treatment was longer, and the effect was statistically significant. Based on our findings, we could conclude that while VEGFR-TKIs are associated with an increased risk of developing CHF in cancer patients, the absolute incidence and risk of CHF appears low and the use of VEGFR-TKIs should be considered in the context of overall survival benefits. Moreover, as CHF commonly occurs early in the treatment with VEGFR-TKIs, close cardiac monitoring for patients receiving VEGFR-TKIs is recommended, especially during the initial of the regimens.
We also carried out a subgroup risk analysis stratified according to tumour type, VEGFR-TKIs agents, and phase of trials. Our results show that the incidence and risk of CHF associated with VEGFR-TKIs does not significantly vary with tumour types (all grade: P = 0.071, high grade P = 0.72). Then, we explored the incidence and risk of CHF among different VEGFR-TKIs. The incidence of CHF varied significantly with different VEGFR-TKIs (P = 0.025), reflecting the nature of the underlying tumour biology or the different spectrum of target receptors of VEGFR-TKIs. However, our study shows that the OR of CHF did not vary significantly with VEGFR-TKIs (all grade P = 0.55; high grade P = 0.99), but this is unclear with the sample size in this analysis. Additionally, we found that the risk of CHF was substantially higher in phase II trials than that in phase III trials (P = 0.026), but not for high grade CHF (P = 0.67).
The pathogenesis of angiogenesis inhibitor related CHF is currently unknown, and multiple mechanisms might be involved in the pathogenesis of CHF. VEGFR-TKIs, such as sorafenib, sunitinib, vandetanib, pazopanib, axitinib and regorafenib, have been shown to increase the risk of hypertension. The RR for hypertension with these VEGF-TKIs has been shown to range between 1.71 and 8.06 [23,25,26,30,32,33]. Hypertension is a well-known risk factor for development of CHF, and it is possible that VEGFR-TKIs use increases CHF through this mechanism [77]. Another potential mechanism of cardiotoxicity is through inhibition of the VEGF signal pathway. Inactivation of endogenous VEGF with an adenoviral vector encoding a decoy VEGFR could lead to a net reduction in capillary density, impaired cardiac hypertrophy and loss of contractile function after pressure overload in mice subjected to transverse aortic constriction [78], while micro-vascular plasticity allows adaptation of the vascular network, and thus oxygen supply to enhanced metabolic demand due to pressure overload [79]. Inhibiting the VEGF pathway blocks such plasticity, contributing to maladaptive hypertrophy of cardiomyocytes. Additionally, the platelet-derived growth factor (PDGF) signalling pathway also plays a crucial role in the heart. Inhibition of PDGFR-β in cardiomyocytes has been shown to induce heart failure in mice exposed to high vascular pressures [80].
Meta-analysis is considered as a useful tool for analyzing rare and unintended effects of a treatment because it could allow synthesis of data and achieve more stable estimates of effects. However, there are several limitations needed to be considered in our meta-analysis. First, these studies were conducted at various international institutions by different investigators and may have potential bias in reporting the types of adverse events. In particular, the frequency of CHF is under-reported in clinical trials. Second, although CHF events were prospectively collected for each individual study, this analysis was retrospective, and there are potentially important differences among the studies, including differing tumour types, dosage and administration schedule of VEGFR-TKIs, periods of study conduct and study investigators. All of these would increase the clinical heterogeneity among included trials, which also made the interpretation of a meta-analysis more problematic. Thirdly, VEGFR-TKI treatment has also been associated with a significant increase in the risk of hypertension and ATEs. Therefore, an increase in the risk of CHF may have been secondary to an increased incidence of hypertension and/or ATE [35]. However, we could not correlate the incidence of CHF with secondary hypertension or ATE, as neither the causality nor association was reported in any trial. In addition, we were not able to correlate our data with dose delays/interruptions or discontinuations secondary to CHF in the analysis. Fourthly, all these studies excluded patients with poor renal, haematological and hepatic function, and are performed mostly at major academic centres and research institutions. The analysis of these studies may not apply to patients with organ dysfunction and in the community, and the overall incidence and risk of CHF may be higher in medical practice. Finally, our study was a study-level meta-analysis and individual patient information was not available. Therefore, establishment of risk factors for the development of CHF, including prior exposure to cardiotoxic agents, or of potentially contributing comorbid conditions, including prior cardiovascular disease, was not possible in this analysis. Also, we could not determine the potential association between patients who developed CHF during VEGFR-TKIs treatment and efficacy of these drugs. Thus further studies are recommended to investigate this association.
In conclusion, our study suggests that the use of VEGFR-TKIs is associated with an increased risk of developing CHF. As these drugs are increasingly used in the routine treatment of cancer patients and in the setting of clinical trials in combination with other agents, physicians and investigators should be aware of this adverse effect and should monitor patients receiving VEGFR-TKIs closely to offer early intervention and to optimize the balance between oncologic clinical benefit and life-threatening adverse events.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
We are indebted to the authors of the primary studies, for without their contributions, this work would have been impossible.
Funding
None.
References
- 1.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Iacovelli R, Alesini D, Palazzo A, Trenta P, Santoni M, De Marchis L, Cascinu S, Naso G, Cortesi E. Targeted therapies and complete responses in first line treatment of metastatic renal cell carcinoma. A meta-analysis of published trials. Cancer Treat Rev. 2014;40:271–275. doi: 10.1016/j.ctrv.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 7.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, Schoffski P, Aglietta M, Staddon AP, Beppu Y, Le Cesne A, Gelderblom H, Judson IR, Araki N, Ouali M, Marreaud S, Hodge R, Dewji MR, Coens C, Demetri GD, Fletcher CD, Dei Tos AP, Hohenberger P. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 8.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D, Group CS. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 10.Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT, Loevner LA, O'Dwyer PJ, Brose MS. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 12.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 13.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, Baum CM, Michaelson MD. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 16.Saltz LB, Rosen LS, Marshall JL, Belt RJ, Hurwitz HI, Eckhardt SG, Bergsland EK, Haller DG, Lockhart AC, Rocha Lima CM, Huang X, DePrimo SE, Chow-Maneval E, Chao RC, Lenz HJ. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol. 2007;25:4793–4799. doi: 10.1200/JCO.2007.12.8637. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 18.Iacovelli R, Palazzo A, Procopio G, Santoni M, Trenta P, de Benedetto A, Mezi S, Cortesi E. Incidence and relative risk of hepatic toxicity in patients treated with anti-angiogenic tyrosine kinase inhibitors for malignancy. Br J Clin Pharmacol. 77:929–938. doi: 10.1111/bcp.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santoni M, Conti A, De Giorgi U, Iacovelli R, Pantano F, Burattini L, Muzzonigro G, Berardi R, Santini D, Cascinu S. Risk of gastrointestinal events with sorafenib, sunitinib and pazopanib in patients with solid tumors: a systematic review and meta-analysis of clinical trials. Int J Cancer. 2014;135:763–773. doi: 10.1002/ijc.28544. [DOI] [PubMed] [Google Scholar]
- 20.Santoni M, Conti A, Massari F, Arnaldi G, Iacovelli R, Rizzo M, De Giorgi U, Trementino L, Procopio G, Tortora G, Cascinu S. Treatment-related fatigue with sorafenib, sunitinib and pazopanib in patients with advanced solid tumors: an up-to-date review and meta-analysis of clinical trials. Int J Cancer. 2014 doi: 10.1002/ijc.28715. doi: 10.1002/ijc.28715 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Qi WX, Tang LN, Sun YJ, He AN, Lin F, Shen Z, Yao Y. Incidence and risk of hemorrhagic events with vascular endothelial growth factor receptor tyrosine-kinase inhibitors: an up-to-date meta-analysis of 27 randomized controlled trials. Ann Oncol. 2013;24:2943–2952. doi: 10.1093/annonc/mdt292. [DOI] [PubMed] [Google Scholar]
- 22.Qi WX, Sun YJ, Tang LN, Shen Z, Yao Y. Risk of gastrointestinal perforation in cancer patients treated with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2014;89:394–403. doi: 10.1016/j.critrevonc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Qi WX, Shen Z, Lin F, Sun YJ, Min DL, Tang LN, He AN, Yao Y. Incidence and risk of hypertension with vandetanib in cancer patients: a systematic review and meta-analysis of clinical trials. Br J Clin Pharmacol. 2013;75:919–930. doi: 10.1111/j.1365-2125.2012.04417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi WX, Min DL, Shen Z, Sun YJ, Lin F, Tang LN, He AN, Yao Y. Risk of venous thromboembolic events associated with VEGFR-TKIs: a systematic review and meta-analysis. Int J Cancer. 2013;132:2967–2974. doi: 10.1002/ijc.27979. [DOI] [PubMed] [Google Scholar]
- 25.Qi WX, Lin F, Sun YJ, Tang LN, He AN, Yao Y, Shen Z. Incidence and risk of hypertension with pazopanib in patients with cancer: a meta-analysis. Cancer Chemother Pharmacol. 2013;71:431–439. doi: 10.1007/s00280-012-2025-5. [DOI] [PubMed] [Google Scholar]
- 26.Qi WX, He AN, Shen Z, Yao Y. Incidence and risk of hypertension with a novel multi-targeted kinase inhibitor axitinib in cancer patients: a systematic review and meta-analysis. Br J Clin Pharmacol. 2013;76:348–357. doi: 10.1111/bcp.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, Bergsland E, Ngai J, Holmgren E, Wang J, Hurwitz H. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 29.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 30.Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9:117–123. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 31.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559–568. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 32.Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10:967–974. doi: 10.1016/S1470-2045(09)70222-0. [DOI] [PubMed] [Google Scholar]
- 33.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol. 2009;48:9–17. doi: 10.1080/02841860802314720. [DOI] [PubMed] [Google Scholar]
- 34.Hapani S, Sher A, Chu D, Wu S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology. 2010;79:27–38. doi: 10.1159/000314980. [DOI] [PubMed] [Google Scholar]
- 35.Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–2285. doi: 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- 36.Qi WX, Shen Z, Tang LN, Yao Y. Risk of hypertension in cancer patients treated with aflibercept: a systematic review and meta-analysis. Clin Drug Investig. 2014;34:231–240. doi: 10.1007/s40261-014-0174-5. [DOI] [PubMed] [Google Scholar]
- 37.Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, Bellmunt J, Burstein HJ, Schutz FA. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol. 2011;29:632–638. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- 38.Richards CJ, Je Y, Schutz FA, Heng DY, Dallabrida SM, Moslehi JJ, Choueiri TK. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29:3450–3456. doi: 10.1200/JCO.2010.34.4309. [DOI] [PubMed] [Google Scholar]
- 39.Kummar S, Allen D, Monks A, Polley EC, Hose CD, Ivy SP, Turkbey IB, Lawrence S, Kinders RJ, Choyke P, Simon R, Steinberg SM, Doroshow JH, Helman L. Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol. 2013;31:2296–2302. doi: 10.1200/JCO.2012.47.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hainsworth JD, Rubin MS, Arrowsmith ER, Khatcheressian J, Crane EJ, Franco LA. Pazopanib as second-line treatment after sunitinib or bevacizumab in patients with advanced renal cell carcinoma: a Sarah Cannon oncology research consortium phase II trial. Clin Genitourin Cancer. 2013;11:270–275. doi: 10.1016/j.clgc.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J for the RTI. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2013;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 42.Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kindler HL, Ioka T, Richel DJ, Bennouna J, Letourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, Springett GM, Wasan HS, Trask PC, Bycott P, Ricart AD, Kim S, Van Cutsem E. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 45.NCI, Cancer Therapy Evaluation Program. CTC v 2.0 and common terminology criteria for adverse events criteria V3.0 (CTCAE). Available at http://ctepcancergov/protocolDevelopment/electronic_applications/ctchtm (last assessed 27 January 2013)
- 46.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 47.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 48.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28:123–137. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 49.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 50.Rixe O, Bukowski RM, Michaelson MD, Wilding G, Hudes GR, Bolte O, Motzer RJ, Bycott P, Liau KF, Freddo J, Trask PC, Kim S, Rini BI. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8:975–984. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 51.Di Lorenzo G, Carteni G, Autorino R, Bruni G, Tudini M, Rizzo M, Aieta M, Gonnella A, Rescigno P, Perdona S, Giannarini G, Pignata S, Longo N, Palmieri G, Imbimbo C, De Laurentiis M, Mirone V, Ficorella C, De Placido S. Phase II study of sorafenib in patients with sunitinib-refractory metastatic renal cell cancer. J Clin Oncol. 2009;27:4469–4474. doi: 10.1200/JCO.2009.22.6480. [DOI] [PubMed] [Google Scholar]
- 52.Escudier B, Roigas J, Gillessen S, Harmenberg U, Srinivas S, Mulder SF, Fountzilas G, Peschel C, Flodgren P, Maneval EC, Chen I, Vogelzang NJ. Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4068–4075. doi: 10.1200/JCO.2008.20.5476. [DOI] [PubMed] [Google Scholar]
- 53.Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, Hariharan S, Lee SH, Haanen J, Castellano D, Vrdoljak E, Schoffski P, Mainwaring P, Nieto A, Yuan J, Bukowski R. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10:757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 54.Kontovinis LF, Papazisis KT, Touplikioti P, Andreadis C, Mouratidou D, Kortsaris AH. Sunitinib treatment for patients with clear-cell metastatic renal cell carcinoma: clinical outcomes and plasma angiogenesis markers. BMC Cancer. 2009;9:82. doi: 10.1186/1471-2407-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rini BI, Wilding G, Hudes G, Stadler WM, Kim S, Tarazi J, Rosbrook B, Trask PC, Wood L, Dutcher JP. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4462–4468. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 56.Tomita Y, Shinohara N, Yuasa T, Fujimoto H, Niwakawa M, Mugiya S, Miki T, Uemura H, Nonomura N, Takahashi M, Hasegawa Y, Agata N, Houk B, Naito S, Akaza H. Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol. 2010;40:1166–1172. doi: 10.1093/jjco/hyq146. [DOI] [PubMed] [Google Scholar]
- 57.Mulders P, Hawkins R, Nathan P, de Jong I, Osanto S, Porfiri E, Protheroe A, van Herpen CM, Mookerjee B, Pike L, Jurgensmeier JM, Gore ME. Cediranib monotherapy in patients with advanced renal cell carcinoma: results of a randomised phase II study. Eur J Cancer. 2012;48:527–537. doi: 10.1016/j.ejca.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 58.Barrios CH, Liu MC, Lee SC, Vanlemmens L, Ferrero JM, Tabei T, Pivot X, Iwata H, Aogi K, Lugo-Quintana R, Harbeck N, Brickman MJ, Zhang K, Kern KA, Martin M. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat. 2010;121:121–131. doi: 10.1007/s10549-010-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bergh J, Bondarenko IM, Lichinitser MR, Liljegren A, Greil R, Voytko NL, Makhson AN, Cortes J, Lortholary A, Bischoff J, Chan A, Delaloge S, Huang X, Kern KA, Giorgetti C. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J Clin Oncol. 2012;30:921–929. doi: 10.1200/JCO.2011.35.7376. [DOI] [PubMed] [Google Scholar]
- 60.Cristofanilli M, Johnston SR, Manikhas A, Gomez HL, Gladkov O, Shao Z, Safina S, Blackwell KL, Alvarez RH, Rubin SD, Ranganathan S, Redhu S, Trudeau ME. A randomized phase II study of lapatinib + pazopanib versus lapatinib in patients with HER2+ inflammatory breast cancer. Breast Cancer Res Treat. 2013;137:471–482. doi: 10.1007/s10549-012-2369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curigliano G, Pivot X, Cortes J, Elias A, Cesari R, Khosravan R, Collier M, Huang X, Cataruozolo PE, Kern KA, Goldhirsch A. Randomized phase II study of sunitinib versus standard of care for patients with previously treated advanced triple-negative breast cancer. Breast. 2013;22:650–656. doi: 10.1016/j.breast.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 62.Hyams DM, Chan A, de Oliveira C, Snyder R, Vinholes J, Audeh MW, Alencar VM, Lombard J, Mookerjee B, Xu J, Brown K, Klein P. Cediranib in combination with fulvestrant in hormone-sensitive metastatic breast cancer: a randomized Phase II study. Invest New Drugs. 2013;31:1345–1354. doi: 10.1007/s10637-013-9991-2. [DOI] [PubMed] [Google Scholar]
- 63.Johnston SR, Gomez H, Stemmer SM, Richie M, Durante M, Pandite L, Goodman V, Slamon D. A randomized and open-label trial evaluating the addition of pazopanib to lapatinib as first-line therapy in patients with HER2-positive advanced breast cancer. Breast Cancer Res Treat. 2013;137:755–766. doi: 10.1007/s10549-012-2399-4. [DOI] [PubMed] [Google Scholar]
- 64.Hensley ML, Sill MW, Scribner DR, Jr, Brown J, Debernardo RL, Hartenbach EM, McCourt CK, Bosscher JR, Gehrig PA. Sunitinib malate in the treatment of recurrent or persistent uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol. 2009;115:460–465. doi: 10.1016/j.ygyno.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maki RG, D'Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, Livingston MB, Cooney MM, Hensley ML, Mita MM, Takimoto CH, Kraft AS, Elias AD, Brockstein B, Blachere NE, Edgar MA, Schwartz LH, Qin LX, Antonescu CR, Schwartz GK. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–3140. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoftijzer H, Heemstra KA, Morreau H, Stokkel MP, Corssmit EP, Gelderblom H, Weijers K, Pereira AM, Huijberts M, Kapiteijn E, Romijn JA, Smit JW. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161:923–931. doi: 10.1530/EJE-09-0702. [DOI] [PubMed] [Google Scholar]
- 67.Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE, Jr, Vasko VV, Saji M, Rittenberry J, Wei L, Arbogast D, Collamore M, Wright JJ, Grever M, Shah MH. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsumoto K, Sawaki A, Mizuno N, Hara K, Hijioka S, Niwa Y, Tajika M, Kawai H, Kondo S, Yamao K. Clinical efficacy and safety of sunitinib after imatinib failure in Japanese patients with gastrointestinal stromal tumor. Jpn J Clin Oncol. 2011;41:57–62. doi: 10.1093/jjco/hyq164. [DOI] [PubMed] [Google Scholar]
- 69.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Horsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 70.Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, Leung T, Gansukh B, Saltz LB. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 71.Mackay HJ, Tinker A, Winquist E, Thomas G, Swenerton K, Oza A, Sederias J, Ivy P, Eisenhauer EA. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG Trial IND.184. Gynecol Oncol. 2010;116:163–167. doi: 10.1016/j.ygyno.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 72.Dror Michaelson M, Regan MM, Oh WK, Kaufman DS, Olivier K, Michaelson SZ, Spicer B, Gurski C, Kantoff PW, Smith MR. Phase II study of sunitinib in men with advanced prostate cancer. Ann Oncol. 2009;20:913–920. doi: 10.1093/annonc/mdp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reynolds C, Spira AI, Gluck L, Mueller SE, Zhan F, Boehm KA, Asmar L. Sunitinib malate in previously untreated, nonsquamous, non-small cell lung cancer patients over the age of 70 years: results of a Phase II trial. Invest New Drugs. 2013;31:1330–1338. doi: 10.1007/s10637-013-9985-0. [DOI] [PubMed] [Google Scholar]
- 74.Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, Bergsland E, Stuart K, Tye L, Huang X, Li JZ, Baum CM, Fuchs CS. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–3410. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- 75.Schutz FA, Je Y, Richards CJ, Choueiri TK. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J Clin Oncol. 2012;30:871–877. doi: 10.1200/JCO.2011.37.1195. [DOI] [PubMed] [Google Scholar]
- 76.Sivendran S, Liu Z, Portas LJ, Jr, Yu M, Hahn N, Sonpavde G, Oh WK, Galsky MD. Treatment-related mortality with vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy in patients with advanced solid tumors: a meta-analysis. Cancer Treat Rev. 2012;38:919–925. doi: 10.1016/j.ctrv.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Moser M, Hebert PR. Prevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials. J Am Coll Cardiol. 1996;27:1214–1218. doi: 10.1016/0735-1097(95)00606-0. [DOI] [PubMed] [Google Scholar]
- 78.Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–893. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levy BI. Microvascular plasticity and experimental heart failure. Hypertension. 2006;47:827–829. doi: 10.1161/01.HYP.0000215283.53943.39. [DOI] [PubMed] [Google Scholar]
- 80.Chintalgattu V, Ai D, Langley RR, Zhang J, Bankson JA, Shih TL, Reddy AK, Coombes KR, Daher IN, Pati S, Patel SS, Pocius JS, Taffet GE, Buja LM, Entman ML, Khakoo AY. Cardiomyocyte PDGFR-beta signaling is an essential component of the mouse cardiac response to load-induced stress. J Clin Invest. 2010;120:472–484. doi: 10.1172/JCI39434. [DOI] [PMC free article] [PubMed] [Google Scholar]