Abstract
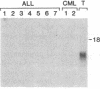
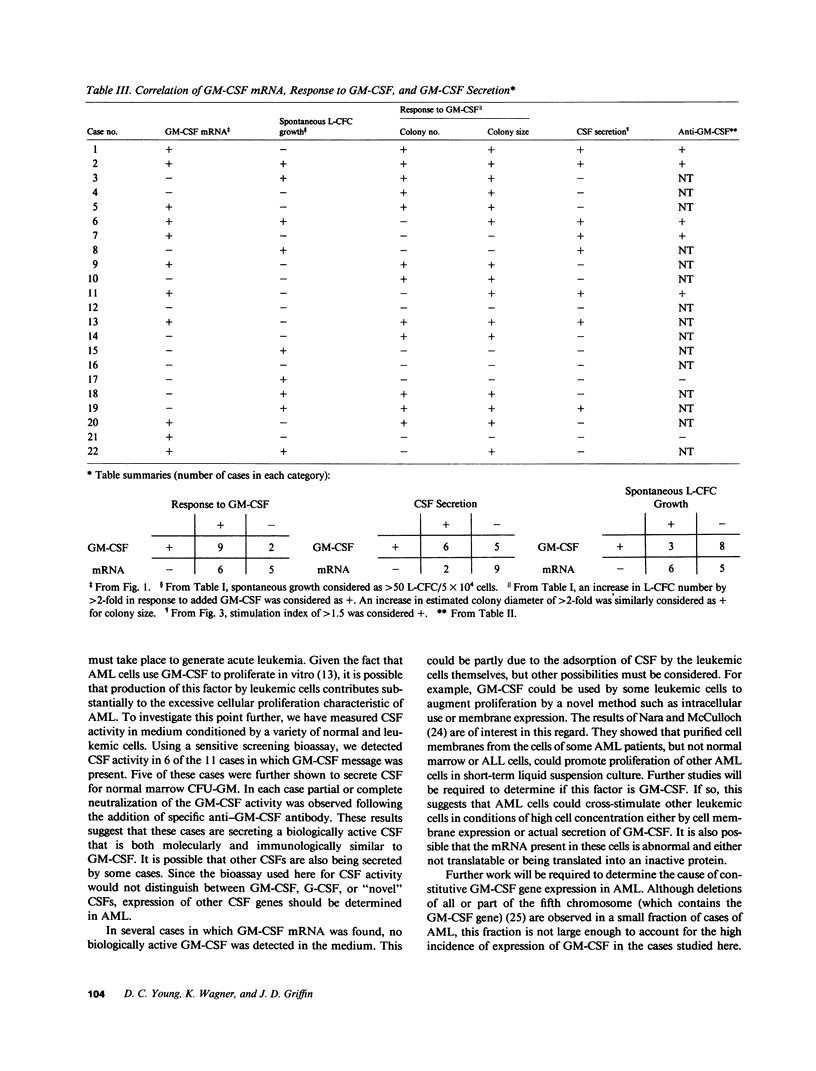
Expression of the granulocyte-macrophage colony-stimulating factor (GM-CSF) gene was studied by Northern blot analysis in normal human hematopoietic cells and a series of leukemias. GM-CSF messenger (m)RNA was detected in activated T cells, but not in normal bone marrow cells, monocytes, or nonactivated T cells. In contrast, leukemic cells from 11 of 22 cases of acute myeloblastic leukemia expressed GM-CSF transcripts. Biologically active CSF was detected in supernatant conditioned by 6 of these 11 leukemias. Expression of the GM-CSF gene was not detected in "common" (pre-B cell) acute lymphoblastic leukemia (11 cases tested) or chronic myeloid leukemia (4 cases tested). These results show that the GM-CSF gene is constitutively expressed in a subset of patients with AML, and further suggest that expression of this gene could contribute to the abnormal growth properties characteristic of AML.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985 Oct;103(4):620–625. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- Brennan J. K., Abboud C. N., DiPersio J. F., Barlow G. H., Lichtman M. A. Autostimulation of growth by human myelogenous leukemia cells (HL-60). Blood. 1981 Oct;58(4):803–812. [PubMed] [Google Scholar]
- Buick R. N., Minden M. D., McCulloch E. A. Self-renewal in culture of proliferative blast progenitor cells in acute myeloblastic leukemia. Blood. 1979 Jul;54(1):95–104. [PubMed] [Google Scholar]
- Buick R. N., Till J. E., McCulloch E. A. Colony assay for proliferative blast cells circulating in myeloblastic leukaemia. Lancet. 1977 Apr 16;1(8016):862–863. doi: 10.1016/s0140-6736(77)92818-5. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Di Persio J. F., Brennan J. K., Lichtman M. A., Speiser B. L. Human cell lines that elaborate colon-stimulating activity for the marrow cells of man and other species. Blood. 1978 Mar;51(3):507–519. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Graf T., von Weizsaecker F., Grieser S., Coll J., Stehelin D., Patschinsky T., Bister K., Bechade C., Calothy G., Leutz A. v-mil induces autocrine growth and enhanced tumorigenicity in v-myc-transformed avian macrophages. Cell. 1986 May 9;45(3):357–364. doi: 10.1016/0092-8674(86)90321-1. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Larcom P., Schlossman S. F. Use of surface markers to identify a subset of acute myelomonocytic leukemia cells with progenitor cell properties. Blood. 1983 Dec;62(6):1300–1303. [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Nadler L. M., Schlossman S. F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981 Oct;68(4):932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. D., Sullivan R., Beveridge R. P., Larcom P., Schlossman S. F. Induction of proliferation of purified human myeloid progenitor cells: a rapid assay for granulocyte colony-stimulating factors. Blood. 1984 Apr;63(4):904–911. [PubMed] [Google Scholar]
- Griffin J. D., Young D., Herrmann F., Wiper D., Wagner K., Sabbath K. D. Effects of recombinant human GM-CSF on proliferation of clonogenic cells in acute myeloblastic leukemia. Blood. 1986 May;67(5):1448–1453. [PubMed] [Google Scholar]
- Huebner K., Isobe M., Croce C. M., Golde D. W., Kaufman S. E., Gasson J. C. The human gene encoding GM-CSF is at 5q21-q32, the chromosome region deleted in the 5q- anomaly. Science. 1985 Dec 13;230(4731):1282–1285. doi: 10.1126/science.2999978. [DOI] [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Minden M. D., Till J. E., McCulloch E. A. Proliferative state of blast cell progenitors in acute myeloblastic leukemia (AML). Blood. 1978 Sep;52(3):592–600. [PubMed] [Google Scholar]
- Nadler L. M., Ritz J., Griffin J. D., Todd R. F., 3rd, Reinherz E. L., Schlossman S. F. Diagnosis and treatment of human leukemias and lymphomas utilizing monoclonal antibodies. Prog Hematol. 1981;12:187–225. [PubMed] [Google Scholar]
- Nagata S., Tsuchiya M., Asano S., Kaziro Y., Yamazaki T., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. 1986 Jan 30-Feb 5Nature. 319(6052):415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- Nara N., McCulloch E. A. Membranes replace irradiated blast cells as growth requirement for leukemic blast progenitors in suspension culture. J Exp Med. 1985 Nov 1;162(5):1435–1443. doi: 10.1084/jem.162.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Miura Y., Suda T., Motoyoshi K., Takaku F. In vitro differentiation of leukemic progenitor cells in various types of acute nonlymphocytic leukemia. Cancer Res. 1983 May;43(5):2334–2338. [PubMed] [Google Scholar]
- Royer H. D., Ramarli D., Acuto O., Campen T. J., Reinherz E. L. Genes encoding the T-cell receptor alpha and beta subunits are transcribed in an ordered manner during intrathymic ontogeny. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5510–5514. doi: 10.1073/pnas.82.16.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbath K. D., Ball E. D., Larcom P., Davis R. B., Griffin J. D. Heterogeneity of clonogenic cells in acute myeloblastic leukemia. J Clin Invest. 1985 Feb;75(2):746–753. doi: 10.1172/JCI111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Wouters R., Löwenberg B. On the maturation order of AML cells: a distinction on the basis of self-renewal properties and immunologic phenotypes. Blood. 1984 Mar;63(3):684–689. [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]