Abstract
Aim
The aim of the study was to investigate the pharmacokinetics and pharmacodynamics of norepinephrine in hypotensive critically ill children, including associated variability factors.
Methods
This was a prospective study in an 18-bed neonatal and paediatric intensive care unit. All children were aged less than 18 years, weighed more than 1500 g and required norepinephrine for systemic arterial hypotension. The pharmacokinetics and haemodynamic effects were described using the non-linear mixed effect modelling software MONOLIX.
Results
Norepinephrine dosing infusions ranging from 0.05 to 2 μg kg−1 min−1 were administered to 38 children whose weight ranged from 2 to 85 kg. A one compartment open model with linear elimination adequately described the norepinephrine concentration–time courses. Bodyweight (BW) was the main covariate influencing norepinephrine clearance (CL) and endogenous norepinephrine production rate (q0) via an allometric relationship: CL(BWi) = θCL × (BWi)3/4 and q0(BWi) = θq0 × (BWi)3/4. The increase in mean arterial pressure (MAP) as a function of norepinephrine concentration was well described using an Emax model. The effects of post-conceptional age (PCA) and number of organ dysfunctions were significant on basal MAP level (MAP0i = MAP0 × PCA/9i0.166) and on the maximal increase in MAP (32 mmHg and 12 mmHg for a number of organ dysfunctions ≤3 and ≥4, respectively).
Conclusion
The pharmacokinetics and haemodynamic effects of norepinephrine in hypotensive critically ill children highlight the between-subject variability which is related to the substantial role of age, BW and severity of illness. Taking into account these individual characteristics may help clinicians in determining an appropriate initial a priori dosing regimen.
Keywords: children, hypotension, norepinephrine, pharmacokinetic/pharmacodynamic model
What is already known about this subject
Haemodynamic response to norepinephrine is variable and unpredictable.
The pharmacokinetics and pharmacodynamics of norepinephrine in adults are well described by a one compartment model with a first order elimination and Emax model, respectively. Clearance is influenced by the severity of illness.
Norepinephrine kinetics and dynamics in children are unknown.
What this study adds
In children, the pharmacokinetics and pharmacodynamics of norepinephrine on between-subject variability are related to age, bodyweight and severity of illness.
The lower the age, the higher the number of organ dysfunctions and the lower the amplitude of the increase in mean arterial pressure.
Introduction
Norepinephrine is currently administered to patients with hypotensive distributive shock 1,2. In children, it has supplanted dopamine over the past few years as the preferred drug for sustaining and increasing systemic arterial pressure, although evidenced-based data are poor 3,4. The amplitude of the haemodynamic response, which is primarily dependent on norepinephrine concentrations, is difficult to predict given the multitude of factors involved and clinical experience suggests broad between-subject variability 5,6. Such variability is supported by previous studies with other catecholamines such as dopamine, dobutamine and epinephrine, both in volunteers and in critically ill children 7–10. While adult norepinephrine kinetic studies using mixed effect modelling have shown a one compartment linear model with clearance negatively related to the Simplified Acute Physiology Score II (SAPS II), these studies failed to find any clear factor explaining the variability in pharmacodynamics 6,11. Paediatric dosages of norepinephrine are usually extrapolated from adult studies albeit without any tangible evidence, while recent small descriptive studies suggest higher dosing regimens 3. However, in children, current knowledge regarding norepinephrine pharmacokinetics and pharmacodynamics are lacking and the developmental effect of age could impact on the kinetics and dynamic modelling of norepinephrine.
The purpose of the present study was to investigate, using a population approach, the pharmacokinetics and pharmacodynamics of norepinephrine in hypotensive critically ill children, including associated variability factors. The effects of developmental and other factors on norepinephrine pharmacokinetics and pharmacodynamics were investigated in order to explain better between-subject variabilities and to ultimately suggest initial individualized dosage regimens.
Methods
Setting
This prospective study was conducted in an 18-bed neonatal and paediatric intensive care unit of a teaching hospital in France from January 2011 to December 2012. The Ethics committee of the Necker Enfants Malades Hospital (Comité de protection des personnes, number S.C 2776) approved the study provided that appropriate written consent was obtained from the child's parent(s) after they were informed of the objectives.
All consecutive children aged less than 18 years and weighing more than 1200 g requiring norepinephrine infusion were included. Exclusion criteria were: unknown initial time infusion of norepinephrine, unknown time of norepinephrine flow rate changes or unknown time of blood sampling. Children were enrolled prior to the onset of infusion and for a period lasting 6 to 24 h h after the initiation of norepinephrine administration. Patients receiving dopamine as well as patients in whom other catecholamines were started and/or if their dosing regimen was changed during the course of the study period were excluded.
Intervention
Systemic arterial hypotension pressure was defined when mean arterial pressure was below normal values adjusted for age according to the International Paediatric Consensus 12,13. Blood pressure was measured with invasive (femoral or radial arterial catheter) or non-invasive techniques.
All causes of systemic arterial hypotension were investigated: septic shock, heavy sedation, non-traumatic cerebral injury and non-hypovolaemic hypotension in the neonate. Details of the respective causes and definitions are provided in the Appendix S1. Norepinephrine infusion was initiated after or during fluid therapy aimed at normalizing preload status (based on echocardiographic data) in hypotensive children, alone or in association with dobutamine or epinephrine in cases of myocardial dysfunction (defined by a decrease in shortening fraction less than 25%).
Norepinephrine (noradrénaline 2 mg ml−1, Renaudin™ diluted to 200 μg ml−1, 100 μg ml−1, 50 μg ml−1, 40 μg ml−1 or 20 μg ml−1 in glucose 5% Baxter™, UK) was infused using a programmable electric syringe pump (DPS, Fresenius Vial™, Brezins, France) through a double lumen central venous catheter (CVC) (Arrow, Teleflex™, PA 19605, USA) with a flow rate varying from 1 ml h−1 to 2 ml h−1 according to local implemented protocol. Titration, frequency and amplitude of the varying flow rate were adjusted according to severity of hypotension and the normal targeted mean arterial pressure (MAP).
In addition to intravenous antibiotic therapy in septic shock cases, adequate analgesia and sedation were ensured by continuous intravenous morphine sulfate and midazolam, respectively. Mechanical ventilation was performed with appropriate pressure levels, oxygen inspired fraction and inhaled nitric oxide in cases of pulmonary arterial hypertension. The daily amount of intravenous glucose was recorded. Hydrocortisone was administered in all hypotensive newborns and at the physician's discretion for the remaining patients. Continuous veno-venous renal replacement therapy was usually performed in cases of persistent oliguria and/or metabolic acidosis and/or severe electrolytic disorders and/or excessive oedema.
Blood sampling
An initial blood sample (C0) was collected prior to initiation of norepinephrine infusion. A second blood sample (C1) was drawn at least 60 min after initiating norepinephrine infusion or at least 40 min after a change in flow rate. A last blood sample (C2) was drawn at least 40 min after a change in flow rate or more than 6 h and prior to 24 h after initiating norepinephrine infusion in the case of a constant flow rate.
The 60 min steady-state interval was chosen according to at least five times the norepinephrine plasma half-life in healthy subjects (approximately 20 min) and the dead volume of the CVC used to infuse norepinephrine at a flow rate of 1 to 2 ml h−1 (time to reach steady state drug infusion which approximates 20 to 40 min).
C0 was used to assess plasma concentrations of endogenous norepinephrine. Only C0 and C1 were drawn in patients who weighed less than 2500 g, according to the percentage of blood volume allowed by the Ethics Committee of our institution.
Sample handling
Blood assigned to catecholamine assays was sampled in EDTA-tubes plunged in an ice bucket and immediately centrifuged at 3000 g for 5 min. The plasma samples were then separated and immediately stored at −20°C and thereafter at −80°C before 24 h running.
Assay
Norepinephrine concentrations were blindly determined by means of high performance liquid chromatography (HPLC) with colorimetric detection 14 using a chromatographic system comprised of a column (25 cm × 4.6 mm inner diameter, 5 μm Supelcosil LC-18 Supelco™), an electrochemical ESA colorimetric detector (Model Coulochem III, Eurosep™) and dual analytical cells (ESA cell Model 5011). The limit of quantification (defined by a variability between measurements of <10%) for HPLC was 0.10 nmol l−1. The norepinephrine concentration measured at C1 or at C2 represented the sum of endogenous and exogenous norepinephrine as the two compounds are strictly identical with regard to chromatographic detection, whereas C0 solely reflected endogenous norepinephrine.
Patient data
Baseline patient characteristics were recorded, including medical histories, gender, age, bodyweight (BW), Score for Neonatal Acute Physiology (SNAP-II) 15, Paediatric Logistic Organ Dysfunction (PELOD) score 16 for all non-premature children, type of shock, duration of intensive care unit (ICU) stay, duration of mechanical endotracheal ventilation, continuous renal replacement therapy and death during ICU stay. Clinical and biological parameters were recorded in order to estimate type and calculate the number of organ dysfunctions according to the International Consensus Conference on Paediatric Sepsis definitions 13.
Duration of norepinephrine and shock were also recorded while variation of infused doses was recorded at the collection of blood samples (more than 6 h and less than 24 h after norepinephrine initiation.
Heart rate (HR) (beats min−1), systolic, diastolic and MAP (mmHg) data were recorded at initiation of norepinephrine, and thereafter every hour or less if needed. Quantity of fluid therapy was noted. Left ventricular shortening fraction (%) was measured at least once during the 6 to 24 h period. Temperature (°C) and urine outputs (ml kg−1 h−1) were recorded.
Plasma lactate and glucose concentrations (mmol l−1) were recorded before norepinephrine infusion and at least once thereafter during the following 6 to 24 h. pH, ionized plasma calcium concentrations (mmol l−1) and plasma HCO3- concentrations (mmol l−1) were recorded during the first 6 to 24 h. Hepatic and renal functions were also recorded using factor V activity (%) and creatinine clearance (ml min−1 1.73 m−2, by original Schwartz estimate), respectively.
Results are expressed as raw numbers (%) or medians (ranges). A non-parametric Wilcoxon test was performed to compare pharmacokinetic and pharmacodynamic values before and under norepinephrine infusion. P < 0.05 was considered statistically significant.
Pharmacokinetic–pharmacodynamic modelling
Norepinephrine concentration−time courses were described by a one-compartment open model with first order elimination whose parameters were elimination clearance (CL) and volume of distribution (V).The differential equation connected to this model is thus
| (1) |
| (2) |
where A(t) and C(t) denote the amount of drug and concentration of drug in the body at time t. Rate and q0 denote the exogenous infusion and endogenous production rates.
The effect of bodyweight (BW) was investigated in the pharmacokinetic model via an allometric relationship 17:
| (3) |
where P, PTYP and PWR are the individual parameter, typical parameter and power exponent, respectively. The PWR exponent was estimated in a first attempt and then eventually fixed to ¾ for CL and q0 terms according to the typical weight-based allometric rule.
The circulating volume, VCirc (l), was related to BW as follows 18:
| (4) |
Since kinetics were ascribed using a one compartment model with first order elimination, the half-life (t1/2) is related to V and CL as t1/2 = ln2 V/CL.
The MAP response, MAP(t), was related to the concentration of norepinephrine via an ‘Emax’ model
| (5) |
where:
ΔMAP = MAPmax − MAP0. MAPmax and MAP0 are respectively the maximal, basal MAP values and EC50MAP the concentration that induces 50% of the maximal effect on MAP.
Population pharmacokinetic–pharmacodynamic analysis
Drug concentrations and responses were analyzed using a population approach, i.e. a non-linear mixed effect modelling approach. Data were analyzed using the MONOLIX software (version 4.13s, http://www.lixoft.com/) along with the SAEM algorithm 19,20. Differential equations were written in a MLXTRAN script file in MONOLIX to estimate the parameters. Residual variabilities were described by additive, proportional or exponential error models depending on the observation. An exponential model was used for between-subject variabilities (BSV). The effect of a covariate on a structural parameter was retained if it caused a decrease in the Bayesian information criterion (BIC) and/or reduced the corresponding BSV with P < 0.05. Only covariates with a plausible effect on pharmacokinetic and pharmacodynamic parameters were investigated. The main covariates of interest in this paediatric population were BW, post-natal age and post-conceptional age (PCA).
Visual predictive check (VPC) evaluation
Plasma norepinephrine concentration and MAP time courses were simulated from their respective final population model and compared with the observed data to evaluate the predictive performance of the model.
The vector of pharmacokinetic parameters from 400 replicates of the database was simulated using the final model. Each vector parameter was drawn in a log-normal distribution with a variance corresponding to the previously estimated BSV. A simulated residual error was added to each simulated concentration. The 5th, 50th and 95th percentiles of the simulated dependent variables at each time point were then overlaid on the observed data and a visual inspection was performed. Because the patients received different norepinephrine regimens, the prediction-corrected VPC was used to produce the VPC plots 21.
Evaluation and validation
Diagnostic graphics were used for evaluation of the goodness-of-fit. Concentration and effects profiles were simulated and compared with the observed data with the aid of the visual predictive check in order to validate the model.
Results
Patient data
Fifty patients were enrolled in this study, 12 of whom were excluded because of incomplete parental consent in five cases, use of dopamine or variation in dosing of other associated catecholamines in five cases and sample haemolysis in two other cases.
Hence, 38 children were included in the study. C0 samples were obtained in 22 patients, C1 in 38 children and C2 in 16 children for a total of 76 observations. Haemodynamic data (HR, MAP) were available in the 38 children with 422 and 431 observations, respectively.
Among the 38 children, 11 neonates including seven premature children with a gestational age < 37 weeks (32 weeks n = 1, 34 weeks n = 1, 35 weeks n = 3, 36 weeks n = 2) were recorded. Respiratory (n = 16), neurological (n = 11), cardiovascular (n = 9), hepatic (n = 9), haematological (n = 8), genetic (n = 6), gastrointestinal (n = 3), metabolic (n = 3) and renal (n = 1) chronic or congenital diseases were present at admission. Malnutrition (<−2SD) was recorded in 14 children. Two children were without any medical history.
Aetiologies of systemic arterial hypotension were as follows: septic shock (n = 16), non-traumatic cerebral injury (n = 6), heavy sedation (n = 8), severe congenital diaphragmatic defect (n = 8).
Mechanical invasive ventilation was performed in 37 children and nitric oxide was necessary for pulmonary hypertension in 12 children. Red blood cell and/or platelet and/or fresh frozen plasma transfusion was needed in 17 cases. Four patients had continuous renal replacement therapy. Hydrocortisone was administered in 18 patients. Respiratory dysfunction was observed in 31 children, renal dysfunction in 14, hepatic dysfunction in 15, neurological dysfunction in 14 and haematological dysfunction in 14 others. Seventeen children (45%) died during the ICU stay. Baseline patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Patient characteristics (n = 38) | |
|---|---|
| Demographics | |
| Age, months (range) | 7.6 (0−182) |
| Gender, male, n (%) | 27 (71) |
| Body weight, kg (range) | 6.7 (2–85) |
| Physiological profile | |
| Factor V activity, % (range) | 72 (30–140) |
| Creatinine clearance, ml min−1 1.73 m−2 (range) | 53.5 (5–300) |
| PELOD score (range) | 31 (13–73) |
| SNAP-II (range) | 58 (21–79) |
| Hepatic dysfunction, n (%) | 15 (39) |
| Renal dysfunction, n (%) | 14 (37) |
| Organ dysfunction (n > 3), n | 14 (37) |
| Baseline kinetic and dynamic data | |
| Plasma norepinephrine concentration, μg l−1 (range) | 0.54 (0.03–2.16) |
| Heart rate, beats min−1 (range) | 136 (80–170) |
| Mean arterial pressure, mmHg (range) | 46 (25–57) |
| Urine output, ml kg−1 h−1 (range) | 1.9 (0–4.9) |
| Plasma lactate concentration, mmol l−1 (range) | 1.85 (0.4–20) |
| Plasma glucose concentration, mmol l−1 (range) | 7 (0.8–15) |
PELOD score: Paediatric Logistic Organ Dysfunction; SNAP-II, Score for Neonatal Acute Physiology.
Saline fluid expansion was performed in 22 children before or during norepinephrine infusion. Associated catecholamine treatment initiated prior to norepinephrine infusion and without dosing variation were epinephrine (n = 5) and dobutamine (n = 6). Norepinephrine was infused at a dose of 0.5 μg kg−1 min−1 (0.05–2) for a duration of 1.5 days (1–13). pH, plasma HCO3− (mmol l−1), plasma ionized calcium (mmol l−1) and base deficit levels at onset of norepinephrine infusion were: 7.30 (6.77–7.49); 23 (10–42); 1.2 (0.94–1.52) and −4 (−15–16), respectively.
Norepinephrine pharmacokinetics
The increase in norepinephrine concentration during infusion was significant: 3.75 μg l−1 (0.88−46) compared with the baseline norepinephrine concentration, 0.54 μg l−1 (0.03−2.16) (P < 0.001). A one compartment open model with linear elimination adequately described the norepinephrine time courses. The pharmacokinetic parameters were V, CL and q0. The residual variability was ascribed to a proportional model. BW was the main covariate influencing CL and q0 (P < 0.001). Both PWR values were fixed to ¾ according to the BW-based allometric rule. The BW effect improved the model. Both CL and q0 BSVs were decreased from 1.09 and 1.38 to 0.62 and 1.06 respectively. Concomitantly, BIC decreased from 350 to 301. Because V could not be accurately estimated and due to the hydrophilic nature of norepinephrine, V was assumed to be equal to the circulating volume.
No other covariate (gender, pH, temperature, PELOD, SNAP-II, number of organ dysfunctions, use of other catecholamines, requirement for renal replacement therapy, creatinine clearance, factor V activity, malnutrition) influenced the pharmacokinetics. The final relationship for norepinephrine CL and q0 was: CL(BWi) = θCL × (BWi)3/4 and q0(BWi) = θq0 × (BWi)3/4, then θCL (l h−1 kg−1) = 6.6, θq0 (μg h−1 kg−1) = 3.12 where θCL and θq0 are typical unit clearance and endogenous production rate, respectively. For a patient weighing 10 kg, norepinephrine CL, q0, V and t1/2 were CL(10 kg) = 6.6 × 103/4 = 37.1 l h−1, q0 (10 kg) = 3.12 × 103/4 = 17.5 μg h−1, V(10 kg) = 0.08 × 10 = 0.8 l and t1/2(10 kg) = [0.693 × V(10 kg) /CL(10 kg)] × 60 = 0.9 min.
Table 2 summarizes the final population estimates. All parameters were estimated with good accuracy. Figure 1 depicts population and individual norepinephrine predicted concentrations vs. observed concentrations. Figure 2 depicts the results of the normalized prediction distribution error (NPDE) analysis for norepinephrine concentration while Figure 3 depicts the VPC plots which show that the observed concentrations were well centred around the simulated median predictions.
Table 2.
Population pharmacokinetic parameters
| Pharmacokinetic parameters | Estimate | RSE (%) |
|---|---|---|
| θCL (l h−1 kg−1) | 6.6 | 11 |
| θBW (CL(BWi) = θCL × BWi3/4) | 0.75 (fixed) | NA |
| θq0 (μg h−1 kg−1) | 3.12 | 23 |
| θBW (q0(BWi) = θq0 × BWi3/4) | 0.75 (fixed) | NA |
| V (l) for a 10 kg individual | 0.8 | NA |
| t1/2 (min), for a 10 kg individual | 0.9 | NA |
| ηCL (square root of ω2CL) | 0.6 | 14 |
| ηq0 (square root of ω2q0) | 1.1 | 17 |
| Residual variability (proportional) | 0.25 | 17 |
The volume of distribution of norepinephrine was ascribed to the circulating volume, estimated as a function of bodyweight, V = 0.08 × BW CL, elimination clearance; q0, endogenous production rate; V, volume of distribution; θCL, typical unit clearance; θq0, typical unit endogenous production rate; θBW, bodyweight influential parameter; t1/2, half-life; RSE (%), relative standard error; η, between subject variability (BSV); BW, bodyweight; NA, not applicable.
Figure 1.
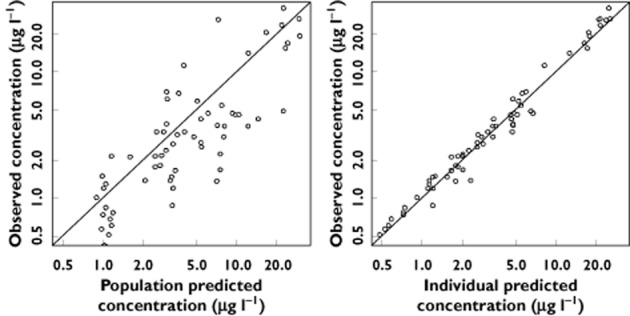
Population and individual predicted norepinephrine concentrations vs. observed concentrations
Figure 2.
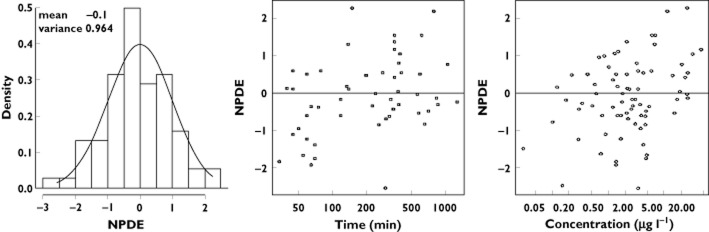
Results of the normalized prediction distribution error (NPDE) analysis for norepinephrine concentration. The NPDE distributions for norepinephrine are shown in the histogram on the left. The solid line depicts a normal distribution, with values in inset specifying the mean and variance of the observed NPDE distribution in the histogram. On the right, the NPDE distributions over time after initiation of norepinephrine and against the observed concentrations are also shown
Figure 3.
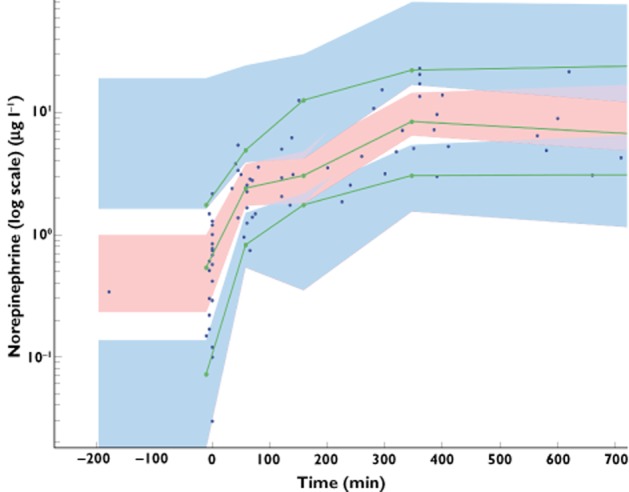
Prediction corrected-visual predictive check (PC-VPC) for norepinephrine concentrations vs. time in minutes. The green lines depict the 5th, 50th and 95th percentiles of observed data; the areas represent the 95% confidence interval around the simulated percentiles. The blue colour represents the 5th and 95th percentile of the predicted concentration vs. time while the pink colour represents the median predicted concentration vs. time. Time 0 min represents the starting time of norepinephrine infusion
Norepinephrine pharmacodynamics
After initiation of norepinephrine infusion, MAP values increased significantly from 46 mmHg (26–57) to 50 mmHg (25–92) (P < 0.001), while HR remained stable from 136 beats min−1 (80–170) to 137 beats min−1 (68–219) (P = 0.33). Variation from basal values for urine output (ml kg−1 h−1), plasma glucose (mmol l−1) and lactate concentrations (mmol l−1) were not significant during norepinephrine infusion: 1.9 (0–4.9) vs. 2 (0.4–10.9) P = 0.5, 7 (0.8–15) vs. 7.2 (2.5–15.5) P = 0.7 and 1.85 (0.4–20) vs. 2 (0.7–19) P = 0.9, respectively (Figure 4).
Figure 4.
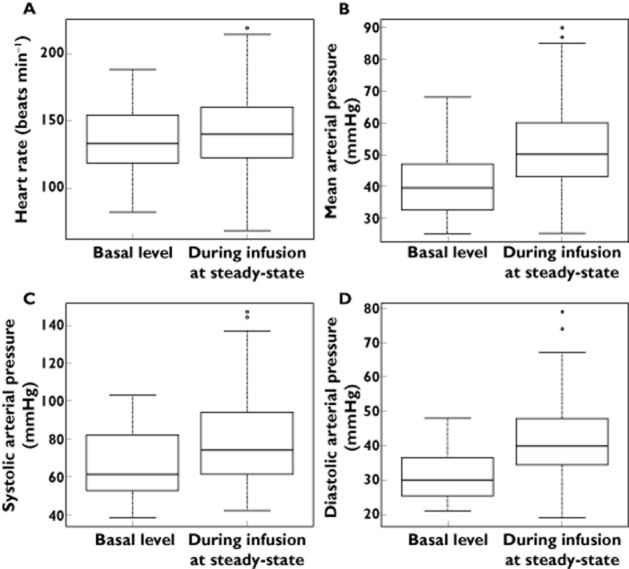
Box and whisker plots of heart rate (A) (P = 0.33), mean arterial pressure (B) (P < 0.001), systolic arterial pressure (C) (P < 0.001) and diastolic arterial pressure levels (D) (P < 0.001) before and during norepinephrine infusion
The Emax models, expressed by equation 5 well explained the variations in MAP as a function of norepinephrine concentration. The residual variability was ascribed to a proportional model. BSVs could be estimated for MAP0, and ΔMAP. Post-conceptional age (PCA) was the main covariate influencing MAP0 (P < 0.001) where MAP0i = MAP0 × PCA/9i 0.166. Including PCA in the model dramatically decreased the BIC and improved the curve fitting of the model. In addition, the number of organ dysfunctions was found significant (P < 0.001) in the estimation of ΔMAP: 32 mmHg or 12 mmHg for a number of organ dysfunctions ≤3 or ≥4, respectively. The BSVs for MAP0 and ΔMAP varied from 0.18 and 0.57 (model including PCA influencing MAP0), to 0.17 and 0.32 (final model). The BIC also decreased from 2622 (when including age) to 2609 (final model).
No other covariate improved the model (including gender, PELOD, SNAP-II, pH, base deficit, use of other catecholamines, temperature and causes of hypotension). The final population parameters are summarized in Table 3. Figure 5 depicts the population and individual predicted MAP concentrations vs. observed MAP. Figure 6 depicts the results of the NPDE analysis for MAP and Figure 7 depicts the VPC plots which show that the observed HR and MAP values were well centred around the predicted median of the model.
Table 3.
Haemodynamic population parameters
| Haemodynamic parameters | Estimate | RSE (%) |
|---|---|---|
| MAP0 (mmHg) | 34 | 5 |
| θPCA (MAP0i = MAP0 × PCA/9i0.166) | 0.166 | 19 |
| ΔMAP (mmHg); (n) organ dysfunction 1 to 3 | 32 | 24 |
| ΔMAP (mmHg); (n) organ dysfunction 4 to 6 | 12 | 24 |
| EC50MAP (μg l−1) | 4.11 | 43 |
| ηMAP0 (square root of ω2MAP0) | 0.17 | 15 |
| ηΔMAP (square root of ω2C50MAP) | 0.3 | 36 |
| Residual variability (proportional) | ||
| – MAP | 0.14 | 4 |
EC50MAP, NorEp concentration producing 50% of MAPmax; MAP0, Basal MAP; ΔMAP = MAPmax−MAP0 RSE (%), relative standard error; η, between subject variability (BSV); PCA, post-conceptional age; θPCA, PCA influential parameter.
Figure 5.
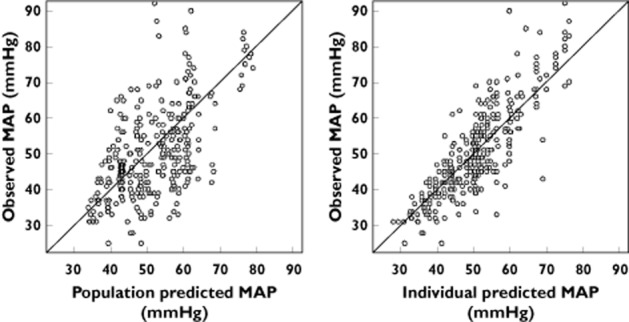
Population and individual predicted mean arterial pressure vs. observed mean arterial pressure
Figure 6.
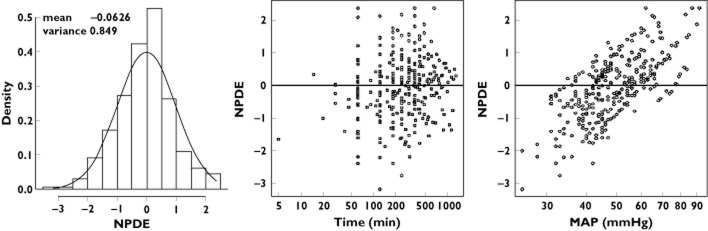
Results of the NPDE analysis for mean arterial pressure. The NPDE distributions for mean arterial pressure are shown in the histogram on the left. The solid line depicts a normal distribution, with values in inset specifying the mean and variance of the observed NPDE distribution in the histogram. On the right, the NPDE distributions over time after initiation of norepinephrine and against the observed mean arterial pressure are also shown
Figure 7.
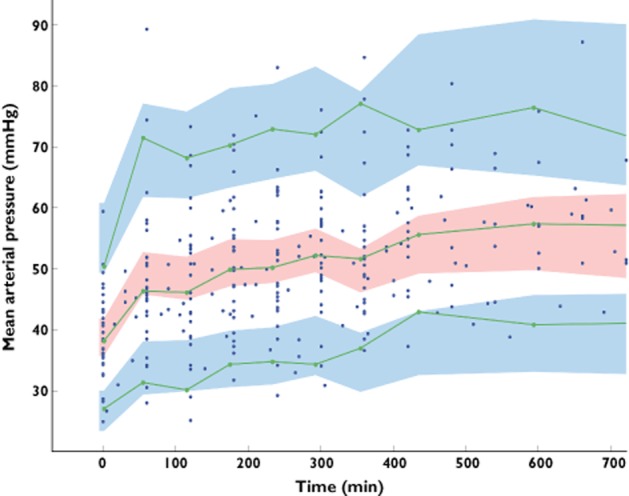
Prediction corrected-visual predictive check (PC-VPC) for mean arterial pressure observations vs. time. The green lines depict the 5th, 50th and 95th percentiles of observed data; the areas represent the 95% confidence interval around the simulated percentiles. The blue colour represents the 5th and 95th percentile of the predicted mean arterial pressure vs. time while the pink colour represents the median predicted mean arterial pressure vs. time. Time 0 min represents the starting time of norepinephrine infusion.
Norepinephrine dosing simulations
Using the pharmacokinetic and haemodynamic model, the effects of various infusion rates of norepinephrine on MAP were assessed as a function of age and BW according to the two organ dysfunction groups (number of dysfunctions ≤3 and ≥4, respectively).
As shown in Figure 8, the increase in norepinephrine concentration vs. infusion rate was linear, while the increases in MAP were curvilinear, due to the norepinephrine concentration-Emax model.
Figure 8.
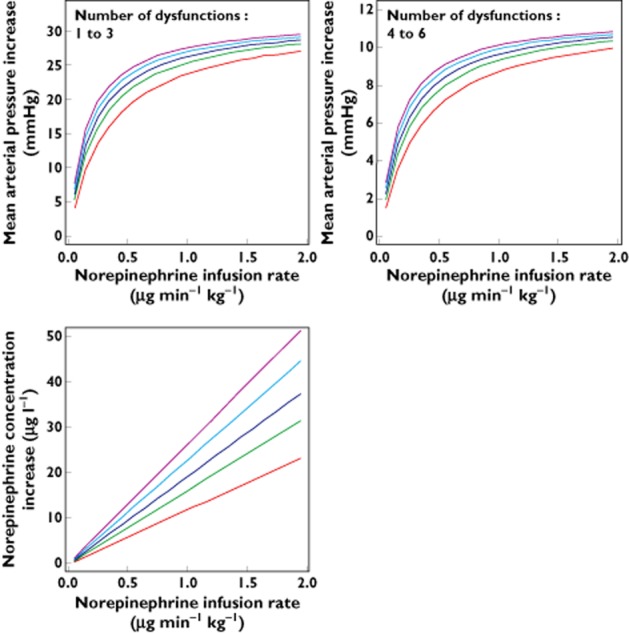
Dosing simulations depicting the increases in norepinephrine concentration and haemodynamic responses as a function of infusion rate in children of different bodyweights and ages for patients with a number of organ dysfunctions ≤3 or ≥4, respectively. Body weight (kg):  3, age (months): 0.462; body weight (kg):
3, age (months): 0.462; body weight (kg):  10, age (months): 12; body weight (kg):
10, age (months): 12; body weight (kg):  20, age (months): 72; body weight (kg):
20, age (months): 72; body weight (kg):  40, age (months): 144; body weight (kg):
40, age (months): 144; body weight (kg):  70, age (months): 300
70, age (months): 300
Discussion
The present study gathered experimental data which allowed for the first time a satisfactory description of norepinephrine population pharmacokinetics and pharmacodynamics in hypotensive critically ill children. The main findings were that (i) norepinephrine kinetics were well described using a one-compartment linear model, (ii) MAP was related to norepinephrine concentration using an Emax model and (iii) pharmacokinetic and pharmacodynamic between-subject variabilities were related to BW, age and severity of illness.
Norepinephrine pharmacokinetics
A one compartment open model with linear elimination adequately described the data as previously reported while saturation kinetics were not observed since norepinephrine is metabolized by two major redundant intracellular enzymes 22. The effect of BW using the allometric scale on clearance and endogenous norepinephrine production improved the model and partly explained the between-subject variability. This was not unexpected since endogenous rates of production and clearance of norepinephrine are dependent on enzymatic maturation, both of which are related to age and BW 23.
Although norepinephrine can be eliminated by the liver and partly by the kidney, we did not find any effect of creatinine clearance or factor V activity on clearance possibly because of the small sample size. Likewise, in contrast to the study of Beleoil et al., severity of illness was not found significant possibly due to differences in patient age and illness between the two studies 11.
The volume of distribution could not be adequately estimated since norepinephrine concentration was measured only under steady-state. However, adjusting the volume of distribution to the circulating volume is justified considering the hydrophilic nature of norepinephrine.
Norepinephrine pharmacodynamics
The increase in MAP was adequately fitted to an Emax model incorporating the kinetic parameters estimated at the first stage. HR was unchanged during infusion even if norepinephrine had some degree of β1-adrenergic and chronotropic effect. However, the increase in MAP can enhance a baroreflex, thereby decreasing HR 24,25. These two opposite phenomena resulted in an unchanged HR. Moreover, the absence of change in plasma glucose concentration was not surprising since norepinephrine does not usually have a major β2-adrenergic effect except in pulmonary arteries, thus decreasing vascular tone 26. Hence, only MAP was related to norepinephrine pharmacokinetics, which was not unexpected since major norepinephrine effects are mediated by α-adrenergic receptors which increase vascular tone 27. Basal MAP is known to be related to age, which was further confirmed in our pharmacodynamics modelling. Indeed, including PCA dramatically improved the model 28.
Finally, ΔMAP decreased in instances where the number of organ dysfunctions was > 3, suggesting a role of illness severity. This may be explained by the lower vascular response to norepinephrine in severe shock 29.
Since other catecholamines were infused in some children, this could have had a confounding effect on the haemodynamic responses. However, in the present study, none of the parameters of the haemodynamic model was found to be influenced by the infusion of the other catecholamines.
Norepinephrine dosing simulations
Using the final model, it was possible to highlight the different responses to a same infusion rate according to age, BW and severity of illness. Therefore, these simulations suggest implementing an a priori initial dosing schedule for specific BW, age and number of organ dysfunctions, in order to produce a suitable increase in MAP. Interestingly, the obtained plots clearly show that the amplitude of the increase in MAP following various norepinephrine infusion rates is related to the child's BW and severity of illness, i.e. the lower the BW, the younger the age and the higher the number of organ dysfunctions, the smaller the amplitude of MAP increase.
Limitations of the study
The small sample size, the high proportion of neonates and the heterogeneous causes of hypotension likely limited the identification of other significant covariates that could affect either the pharmacokinetics or the responses to norepinephrine. While a theoretical sample number of 50 children was initially deemed necessary to reach a good estimate of pharmacokinetic parameters (RSE < 50%), only 38 children with 76 observed concentrations could be included. Neverthless, this albeit reduced sample size allowed us to model effectively the pharmacokinetics and pharmacodynamics with good accuracy for all of the estimates (RSE < 50%). Moreover, this study was conducted in real-life conditions, e.g. critically ill children, thus making it difficult to include more patients within a reasonable time frame in this setting.
Even though the catecholamine dosing regimen should be adjusted based on haemodynamic monitoring in real time, this study highlights potential differences in MAP increases according to individual characteristics. Indeed, our results suggest using higher dosing regimens at the initiation of norepinephrine in the most critically ill and youngest children. These findings are in keeping with observational data reported by Lampin et al. 3 who highlighted the use of higher doses of norepinephrine in septic children (0.5 ± 0.4 μg kg−1 min−1 starting dose up to 2.5 ± 2.2 μg kg−1 min−1 maximum dose) than those typically recommended in the literature which suggest a starting dose at 0.3 μg kg−1 min−1 1,2. However, this latter starting dose may be insufficient for certain patients and thus our model could help clinicians to reach the target MAP more quickly.
Finally, the present norepinephrine dosing simulations are limited (i) to the initiation and the first hours of infusion and (ii) to dosing not exceeding 2 μg kg−1 min−1. Indeed, while our model would enable to optimize starting doses by using patient characteristics to establish starting treatment, it is likely less applicable during the titration regimen per se. To this end, we are currently developing an algorithm for starting regimens adjusted to the individual characteristics of the patients (PCA, weight) and severity of illness (number of organ dysfunctions). This algorithm will be tested in our paedriatric ICU to validate its feasibility in real life.
In conclusion, this original study on pharmacokinetics and haemodynamic effects of norepinephrine in hypotensive critically ill children clearly showed that between-subject variability was related to the substantial role of age, BW and severity of illness. Taking into account these individual characteristics may help clinicians in determining an appropriate initial a priori dosing regimen.
Acknowledgments
The authors thank Marie Godard, Saphia Faked, Sandra Colas, Agnès Cimerman Mame Diagne, Agnes Mougenet, Isabelle Drouet, Brigitte Hubert, Olivier Bustarret, Jérome Rambaud, Sandrine Jean, Christelle Joffre, Elsa Kermorvant, Anne Armengaud, Jean Bergounioux, Agnès Giuseppi, Mikaël Capelo and Mathilde Plançon for their help in collecting the data.
Authors' contributions
MO collected, analyzed the data and drafted the manuscript. SU analyzed the data and drafted the manuscript. OSV made substantial contributions to the analysis and interpretation of the data. FL, LD, LSB collected the data and were involved in revising the manuscript and PH was involved in critically revising the manuscript for important intellectual content.
JMT conceived the study, participated in its design and coordinated and drafted the manuscript. All authors read and approved the final manuscript.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare MO had a support from the PHRC régional (programme hospitalier de recherche clinique) AOR 11 162, 2011, « pharmacocinétique et pharmacodynamie de population des catécholamines chez l'enfant et le nouveau-né », no financial relationship with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
We confirm that we have all necessary and appropriate consent from each child's parents involved in the study, including consent to participate in the study and consent to publish.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Definitions
References
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, Doctor A, Davis A, Duff J, Dugas MA, Duncan A, Evans B, Feldman J, Felmet K, Fisher G, Frankel L, Jeffries H, Greenwald B, Gutierrez J, Hall M, Han YY, Hanson J, Hazelzet J, Hernan L, Kiff J, Kissoon N, Kon A, Irazuzta J, Lin J, Lorts A, Mariscalco M, Mehta R, Nadel S, Nguyen T, Nicholson C, Peters M, Okhuysen-Cawley R, Poulton T, Relves M, Rodriguez A, Rozenfeld R, Schnitzler E, Shanley T, Kache S, Skippen P, Torres A, von Dessauer B, Weingarten J, Yeh T, Zaritsky A, Stojadinovic B, Zimmerman J, Zuckerberg A. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lampin ME, Rousseaux J, Botte A Sadik A, Cremer R, Leclerc F. Noradrenaline use for septic shock in children: doses, routes of administration and complications. Acta Paediatr. 2012;101:e426–430. doi: 10.1111/j.1651-2227.2012.02725.x. [DOI] [PubMed] [Google Scholar]
- 4.Tourneux P, Rakza T, Abazine A, Krim G, Storme L. Noradrenaline for management of septic shock refractory to fluid loading and dopamine or dobutamine in full-term newborn infants. Acta Paediatr. 2008;97:177–180. doi: 10.1111/j.1651-2227.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 5.Leuenberger U, Gleeson K, Wroblewski K, Prophet S, Zelis R, Zwillich C, Sinoway L. Norepinephrine clearance is increased during acute hypoxemia in humans. Am J Physiol. 1991;261:H1659–1664. doi: 10.1152/ajpheart.1991.261.5.H1659. [DOI] [PubMed] [Google Scholar]
- 6.Johnston AJ, Steiner LA, O'Connell M, Chatfield DA, Gupta AK, Menon DK. Pharmacokinetics and pharmacodynamics of dopamine and norepinephrine in critically ill head-injured patients. Intensive Care Med. 2004;30:45–50. doi: 10.1007/s00134-003-2032-4. [DOI] [PubMed] [Google Scholar]
- 7.Eldadah MK, Schwartz PH, Harrison R, Newth CJ. Pharmacokinetics of dopamine in infants and children. Crit Care Med. 1991;19:1008–1011. doi: 10.1097/00003246-199108000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Fisher DG, Schwartz PH, Davis AL. Pharmacokinetics of exogenous epinephrine in critically ill children. Crit Care Med. 1993;21:111–117. doi: 10.1097/00003246-199301000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz PH, Eldadah MK, Newth CJ. The pharmacokinetics of dobutamine in pediatric intensive care unit patients. Drug Metab Dispos. 1991;19:614–619. [PubMed] [Google Scholar]
- 10.Abboud I, Lerolle N, Urien S, Tadié JM, Leviel F, Fagon JY, Faisy C. Pharmacokinetics of epinephrine in patients with septic shock: modelization and interaction with endogenous neurohormonal status. Crit Care. 2009;13:R120. doi: 10.1186/cc7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beloeil H, Mazoit JX, Benhamou D, Duranteau J. Norepinephrine kinetics and dynamics in septic shock and trauma patients. Br J Anaesth. 2005;95:782–788. doi: 10.1093/bja/aei259. [DOI] [PubMed] [Google Scholar]
- 12.Lacroix J, Gauthier M, Hubert P, Leclerc F, Gaudreault P. 2007. pp. 3–33. Surveillance cardiorespiratoire In: Urgences et soins intensifs pédiatriques, (2ème édition) Masson CHU Sainte-Justine.
- 13.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 14.Guillemin A, Troupel S, Galli A. Determination of catecholamines in plasma by high-performance liquid chromatography. Clin Chem. 1988;34:1913–1914. [PubMed] [Google Scholar]
- 15.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 16.Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, Lacroix J, Leclerc F. Validation of the pediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 17.Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 18.Riley AA, Arakawa Y, Worley S, Duncan BW, Fukamachi K. Circulating blood volumes: a review of measurement techniques and a meta analysis in children. ASAIO J. 2010;56:260–264. doi: 10.1097/MAT.0b013e3181d0c28d. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal. 2005;49:1020–1030. [Google Scholar]
- 20.Chan PL, Jacqmin P, Lavielle M, McFadyen L, Weatherley B. The use of the SAEM algorithm in MONOLIX software for estimation of population pharmacokinetic-pharmacodynamic-viral dynamics parameters of maraviroc in asymptomatic HIV subjects. J Pharmacokinet Pharmacodyn. 2011;38:41–61. doi: 10.1007/s10928-010-9175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacol Ther. 2001;91:35–62. doi: 10.1016/s0163-7258(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg C, Notterman DA. Pharmacokinetics of cardiovascular drugs in children. Inotropes and vasopressors. Clin Pharmacokinet. 1994;27:345–367. doi: 10.2165/00003088-199427050-00003. [DOI] [PubMed] [Google Scholar]
- 24.Chu CA, Sindelar DK, Neal DW, Cherrington AD. Hepatic and gut clearance of catecholamines in the conscious dog. Metabolism. 1999;48:259–263. doi: 10.1016/s0026-0495(99)90044-6. [DOI] [PubMed] [Google Scholar]
- 25.Martin C, Viviand X, Leone M, Thirion X. Effect of norepinephrine on the outcome of septic shock. Crit Care Med. 2000;28:2758–2765. doi: 10.1097/00003246-200008000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Tourneux P, Rakza T, Bouissou A, Krim G, Storme L. Pulmonary circulatory effects of norepinephrine in newborn infants with persistent pulmonary hypertension. J Pediatr. 2008;153:345–349. doi: 10.1016/j.jpeds.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrine, dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit Care Med. 2011;39:450–455. doi: 10.1097/CCM.0b013e3181ffe0eb. [DOI] [PubMed] [Google Scholar]
- 28.Aneja R, Carcillo J. Differences between adult and pediatric septic shock. Minerva Anestesiol. 2011;77:986–992. [PubMed] [Google Scholar]
- 29.Macarthur H, Westfall TC, Riley DP, Misko TP, Salvemini D. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc Natl Acad Sci U S A. 2000;97:9753–9758. doi: 10.1073/pnas.97.17.9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definitions


