Abstract
During ischemia and heart failure, there is an increase in cardiac glycolysis. To understand if this is beneficial or detrimental to the heart, we chronically elevated glycolysis by cardiac-specific overexpression of phosphatase-deficient 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2) in transgenic mice. PFK-2 controls the level of fructose-2,6-bisphosphate (Fru-2,6-P2), an important regulator of phosphofructokinase and glycolysis. Transgenic mice had over a threefold elevation in levels of Fru-2,6-P2. Cardiac metabolites upstream of phosphofructokinase were significantly reduced, as would be expected by the activation of phosphofructokinase. In perfused hearts, the transgene caused a significant increase in glycolysis that was less sensitive to inhibition by palmitate. Conversely, oxidation of palmitate was reduced by close to 50%. The elevation in glycolysis made isolated cardiomyocytes highly resistant to contractile inhibition by hypoxia, but in vivo the transgene had no effect on ischemia-reperfusion injury. Transgenic hearts exhibited pathology: the heart weight-to-body weight ratio was increased 17%, cardiomyocyte length was greater, and cardiac fibrosis was increased. However, the transgene did not change insulin sensitivity. These results show that the elevation in glycolysis provides acute benefits against hypoxia, but the chronic increase in glycolysis or reduction in fatty acid oxidation interferes with normal cardiac metabolism, which may be detrimental to the heart. glycolysis; transgenic
In a healthy heart, glucose accounts for a small percentage of the energy supply, but glucose comes to play a more important role in cardiac pathology. Glycolysis increases in congestive heart failure (CHF) and cardiac hypertrophy (7). Glycolysis also rises during cardiac ischemia (28). In acute ischemia, glycolysis can become the only source of ATP and is therefore needed for the survival of cardiomyocytes. However, chronically elevated glycolysis, as occurs during long-term CHF, may not be so beneficial. Rather than being an adaptive response to heart failure, the elevation of cardiac glucose metabolism may be one more example of the prominent reversion to fetal gene expression that occurs during CHF (34). Unlike the adult heart, glycolysis is the primary energy source of the fetal heart (35). In the adult heart, glucose and fat metabolism are tightly and inversely regulated, as illustrated by the Randle cycle (27). Impairment of fat uptake or fat metabolism will increase cardiac glucose metabolism, and it has been shown that impaired fat metabolism is detrimental to the heart (2). In a Type 2 diabetic environment (4), increased cardiac glucose uptake improves cardiac function. However, the effect of increased cardiac glucose metabolism has not been tested in healthy animals.
Metabolic flux analysis (17) has demonstrated that the control of cardiac glycolysis is shared by several reactions. One of the control reactions is carried out by 6-phosphofructo-1-kinase (PFK-1) (12, 29), which catalyzes the phosphorylation of fructose-6-phosphate (F6P) to fructose-1,6-bisphosphate (Fru-1,6-P2). F6P is in equilibrium with glucose-6-phosphate (G6P), and these two sugars initiate glycogen synthesis, the hexosamine pathway, and the hexose monophosphate shunt. Thus, PFK-1 not only has an important role in regulating glycolysis but, by controlling the metabolism of F6P, PFK-1 can also modulate several important reactions branching off of glycolysis. PFK-1 activity is tightly controlled and negatively regulated by products of fat metabolism, including ATP and citrate. The most important positive regulator of PFK-1 is fructose-2,6-bisphosphate (Fru-2,6-P2). The bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2) catalyses the synthesis and degradation of Fru-2,6-P2. PFK-2 is, in turn, regulated by a complex network of kinases, phosphatases, and metabolites (31). In this study, we used a cardiac-specific transgene for a mutant form of liver PFK-2 to directly elevate cardiac Fru-2,6-P2 and increase glycolysis. This produced multiple effects on the heart, both beneficial and detrimental.
EXPERIMENTAL PROCEDURES
Development of transgenic mice
The bifunctional enzyme PFK-2 controls levels of F-2,6-P2 by catalyzing two opposing reactions: F6P + ATP → Fru-2,6-P2 + ADP and Fru-2,6-P2 → F6P + Pi. The development of the kinase-active/bisphosphatase-deficient PFK-2 mutant has been previously described (19, 38, 39). Cardiac-specific expression was obtained by ligating a 1.6-kb KpnI/HindIII fragment of kinase-active liver PFK-2 (38, 39) behind the α-myosin heavy chain (MHC) promoter (10). The transgene was designated Mk (for the MHC promoter and the kinase-active PFK-2 gene). Transgenic mice were produced on a FVB background and maintained as heterozygotes by breeding to FVB mice. Standard embryo microinjection procedures were used for producing transgenic animals. All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and were approved by the United State Department of Agriculture-certified institutional animal care committee. Male mice aged 90 –130 days were used in the following experiments.
Measurement of metabolites
For the assays of G6P, F6P, and Fru-1,6-P2, hearts were homogenized in 1 M ice-cold perchloric acid and centrifuged. Supernatants were neutralized with 2 M KHCO3. The supernatant from neutralized tissue extracts was used for the estimation of these metabolites by the fluorometric method (23).
Cardiac Fru-2,6-P2 was extracted from fresh heart tissue in 10–20 volumes of 50 mM NaOH and kept at 80°C for 5 min. The extract was cooled and neutralized at 0°C by the addition of ice-cold 1 M acetic acid in the presence of 20 mM HEPES. After centrifugation at 8,000 g for 10 min, the supernatant was collected and assayed for Fru-2,6-P2 by the PFK-1 activation method (36).
For the assay of glycogen, snap-frozen tissue was weighed, homogenized in 9 volumes of 30% KOH at 0°C, and then heated at 70°C for 30 min. Glycogen was precipitated with absolute alcohol and saturated sodium sulfate and redissolved in 0.1 M acetate buffer (pH 4.7). Homogenate glycogen (10 μl) was then hydrolyzed to glucose by the addition of 50 ng amyloglucosidase (Boehringer-Mannheim) in 100 μl of 0.1 M acetate buffer. Glucose was then measured by a fluorometric assay using hexokinase and G6P dehydrogenase as previously reported (22).
Cardiac perfusion for measurements of glycolysis, palmitate oxidation, and lactate production
Langendorff perfusions were carried out as previously described (21, 22). The heart was rapidly canulated through the aorta and retrogradely perfused at 2 ml/min with Krebs-Henseleit (KH) buffer, which consisted of 120 mM NaCl, 20 mM NaHCO3, 4.6 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgCl2, 1.25 mM CaCl2, and 5 mM glucose. Throughout the perfusion, KH buffer was continuously equilibrated with 95% O2-5% CO2, which maintained a pH of 7.4, and temperature was maintained at 37°C. The heart was paced throughout the procedure at 6 Hz (6 V, 3 ms). Perfusion pressure and contractility were monitored continuously during the perfusion, as previously described (21, 22). To study the effect of insulin, baseline glycolysis was determined for the first 30 min followed by 50 min in the presence of 200 μU/ml insulin. Glycolysis in the presence of palmitate was measured by bringing the perfusate to 0.4 mM palmitate (5) by the addition of 1/20th volume of 8 mM palmitate bound to 10% BSA in KH buffer.
Lactate production and glycolysis measurements with [5-3H]glucose were performed with perfusion buffer containing 5 mM glucose as previously described (8). Palmitate oxidation was measured by perfusion with 5 mM glucose plus 0.4 mM palmitate containing 0.75 μCi/ml [9, 10-3H] palmitate. Tritiated water produced from tritiated glucose or palmitate during cardiac perfusion was measured by diffusion and scintillation counting. Effluent from each time point of the perfusion was assayed in duplicate. For each experiment, background counts were determined by performing the same equilibration on perfusion buffer that had not passed through the heart. Diffusion efficiency was measured in each experiment using tritiated water. Lactate concentration was measured in sixfold diluted effluent using Eaton Bioscience Kit-11112.
Murine in vivo myocardial ischemia-reperfusion and infarct size determination
Male FVB and Mk mice were subjected to in vivo left coronary artery occlusion and reperfusion as previously described (11, 13–16). Briefly, mice were anesthetized with intraperitoneal injections of ketamine hydrochloride (50 mg/kg) and pentobarbital sodium (50 mg/kg). Animals were then attached to a surgical board with their ventral side up. Mice were orally intubated with polyethylene (PE)-90 tubing connected to a Hugo Sachs mouse ventilator. The tidal volume and respiratory rate were set for each mouse based on body weight and according to standard mammalian allometric equations. Mice were supplemented with 100% O2 via the ventilator side port. Throughout anesthesia, body temperature was monitored with a rectal thermometer and held constant between 36 and 37°C with an infrared heating lamp. A median sternotomy was performed using an electric cautery, and the proximal left main coronary artery was visualized and completely occluded for 40 min with 7-0 silk suture mounted on a tapered needle (BV-1, Ethicon).
At 24 h of reperfusion, mice were anesthetized as previously described, intubated, and connected to a mouse ventilator. A catheter (PE-10 tubing) was placed in the common carotid artery to allow for Evans blue dye injection. A median sternotomy was performed, and the left main coronary artery was religated in the same location as before. Evans blue dye (~1.2 ml of a 2% solution) was injected into the carotid artery catheter into the heart to delineate the ischemic zone from the nonischemic zone. The heart was rapidly excised and serially sectioned perpendicular to the long axis in 1-mm-thick sections, which were then incubated in 1.0% 2,3,5-triphenyltetrazolium chloride for 5 min at 37°C to demarcate the viable and nonviable myocardium within the risk zone. Each of the five 1-mm-thick myocardial slices was weighed. A blinded observer assessed the areas of infarct, risk, and nonischemic zone using computer-assisted planimetry (Image J, version 1.38x). All of the procedures for area at risk (AAR) and infarct size determination have been previously described (13–15).
Histological experiments
Collagen accumulation in heart sections was determined as previously described (1). Heart sections (5 μm) were placed on slides, deparaffinized, and incubated with a saturated solution of picric acid containing 0.1% Sirius red for staining collagen and 0.1% fast green, which stains noncollagen proteins. Sections were kept in the dark and incubated for 30 min. Sections were rinsed with distilled water, dehydrated, and then mounted with permount. Sections were visualized and photographed, and the interstitial fibrosis was quantified by a blinded observer using a scale of 0–2 based on the severity of the collagen accumulation, as previously described (8).
Cardiomyocyte assay
Cardiomyocytes were isolated, and myocyte contractility was measured as previously described (41). For the oxygenated assay, myocytes were placed in an open chamber at 22°C in HEPES-buffered KH buffer [135 mM NaCl, 4 mM KCl, 1 mM MgCl2, 10 mM HEPES, 0.33 mM NaH2PO4, 10 mM glucose, 10 mM 2,3-butanedione monoxime (BDM), and 1.2 mM CaCl2; pH 7.4] for 2 h. BDM was included in the contractility assay buffer because it preserves rod-shaped myocytes during metabolic inhibition (18). We (40) have previously described the use of BDM during hypoxic assays of myocyte contractility. For hypoxia, cardiomyocytes were perfused in the same buffer saturated with N2 for 15 min and maintained and assayed in a sealed chamber for 2 h at 22°C. Myocytes were stimulated at 1 Hz using platinum electrodes attached to the chamber. The mechanical properties of ventricular myocytes were assessed using a video-based edge detection system (IonOptix, Milton, MA) as previously described (41). Cell shortening and relengthening were assessed using the following indexes: peak shortening, time to 90% relengthening, and maximal velocities of shortening and relengthening (±dL/dt).
PFK-2 expression analysis
PFK-2 protein was analyzed by Western blot analysis as previously reported (8). In brief, PFK-2 was extracted from fresh hearts by homogenization, and the PFK-2 enzyme was precipitated with 40% PEG. Pellets were redissolved, and the concentration of extracted protein was measured by the BCA method (Pierce Chemical, Rockford, IL). Equal amounts of extracted protein (100 μg) were used for Western blots. Rabbit anti-rat liver PFK-2 serum was used as the primary antibody at a 1:1,000 dilution. Bands were visualized using horseradish peroxidase-conjugated goat anti-rabbit secondary antibody and ECL (Amersham Life Science, Buckinghamshire, UK).
Quantitative RT-PCR
The RNA expression of cardiac pathological markers brain natriuretic peptide (BNP) and β-MHC was measured by real-time quantitative PCR using an ABI 7300 instrument (Applied Biosystems, Foster City, CA) with a modified protocol (6). In brief, total RNA was purified from the frozen heart and reverse transcribed to cDNA. RNA-derived cDNA (10 ng) was mixed with Taqman universal PCR Master Mix and the appropriate primers and probe and run at 50°C for 2 min, 95°C for 10 min, and 95°C for 15 s followed by 60°C for 1 min for 40 cycles. The assay identification numbers for the specific primers and probe of each gene were Mm01255779_g1 for BNP and Mm00600555_m1 for β-MHC (Applied Biosystems). The results for β-MHC and BNP were normalized to the mitochondrial-encoded NADPH dehydrogenase (complex I) subunit 4 (ND4) mRNA level.
Statistical analysis
Comparisons were performed by two-way ANOVA and a post hoc test. Single comparisons were performed by Student’s t-test. The accepted level of significance was 0.05.
RESULTS
Transgenic lines
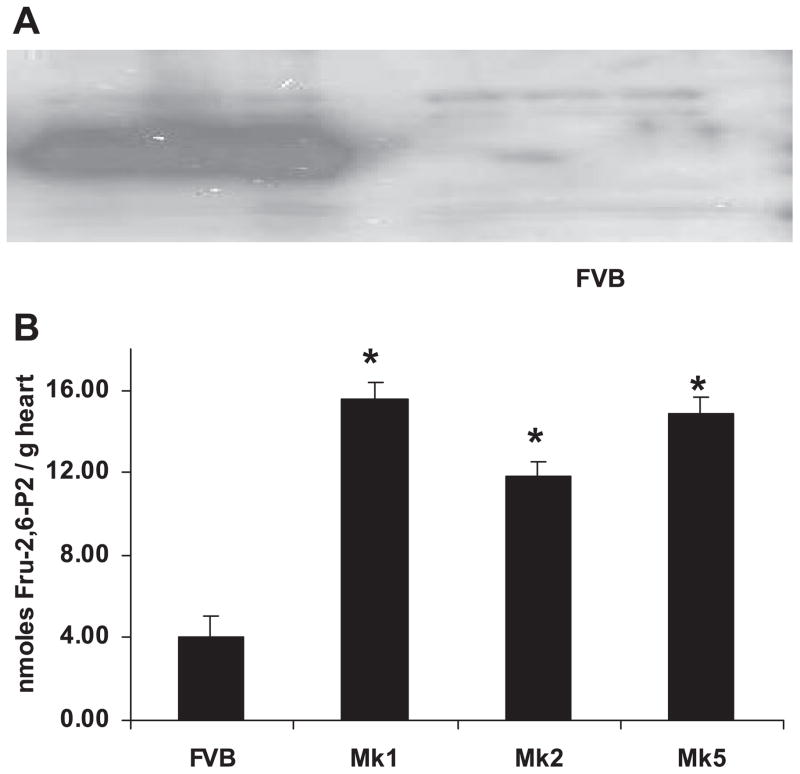
Transgenic mice were generated with the transgene Mk, which contained a kinase-active/bisphosphatase-deficient mutant of liver PFK-2 (37) regulated by the cardiac-specific α-MHC promoter (10). Six lines of Mk transgenic mice were generated on a FVB background and were designated as lines Mk1 to Mk6. The founders of lines 1, 2, 4, and 5 produced litters. Western blots with antibody made against liver PFK-2 (Fig. 1A) demonstrated obvious overexpression of the transgene in Mk mouse hearts. To confirm the increase in PFK-2 activity, we measured the level of Fru-2,6-P2 in transgenic and control hearts. Cardiac Fru-2,6-P2 was elevated by three- to fourfold in all transgenic lines (Fig. 1B). Mk1 and Mk2 mice were produced first and had indistinguishable phenotypes for gene expression and Fru-2,6-P2 levels, and they were used interchangeably in experiments concerning phenotype.
Fig. 1.
Transgenic hearts overexpress 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2) protein and have elevated fructose-2,6-bisphosphate (Fru-2,6-P2). A: for the Western blot, cardiac protein was analyzed using rabbit anti-rat liver PFK-2 antibody. Extracts from 3 Mk1 hearts are shown. Similar results were obtained in all Mk transgenic lines. B: for the measurement of Fru-2,6-P2, hearts were extracted with 50 mM NaOH and estimated using pyrophosphate-dependent fructose-6-phosphate 1-phosphotransferase. Transgenic Fru-2,6-P2 values were significantly higher than those in FVB hearts (*P < 0.01 by ANOVA). No significant differences were obtained within Mk groups. The number of animals used were 4 FVB, 4 Mk1, 4 Mk2, and 3 Mk5 mice.
Cardiac metabolite concentrations
By modifying PFK activity, the Mk transgene is expected to alter the concentration of metabolites of the glycolytic pathway. Levels of several metabolites were measured in flash-frozen hearts from FVB and Mk transgenic mice that had been fasted for 3 h. Table 1 shows these results. G6P was reduced by ~1.6 fold in Mk transgenic hearts. Coincident with the reduction in G6P levels, glycogen was decreased by more than eightfold in Mk hearts. Two metabolites downstream of PFK, Fru-1,6-P2 and pyruvate, were increased in concentration; however, the increase did not reach statistical significance. Fru-1,6-P2 was measured as the combined pool of Fru-1,6-P2, dihydroxyacetone phosphate, and glyceraldehyde-3-phosphate since these metabolites are in equilibrium and are most accurately measured together (23).
Table 1.
Concentrations of glycolytic intermediates in FVB and Mk hearts
| Glucose-6-Phosphate, nmol/g | Fructose-1,6-Bisphosphate, nmol/g | Fructose-2,6-Bisphosphate, nmol/g | Pyruvate, nmol/g | Glycogen, μmol/g | |
|---|---|---|---|---|---|
| FVB | 225±67 | 277±93 | 5.12±0.88 | 133±44 | 2.4±1.94 |
| Mk | 140±17 | 356±108 | 10.4±1.34 | 227±110 | 0.27±0.22 |
| P value | 0.01 | 0.25 | 0.01 | 0.15 | 0.01 |
Values are means ± SE for at least 5 mice/group after a 3-h fast. P values were determined by Student’s t-test.
Effect of kinase-active PFK-2 on cardiac metabolism
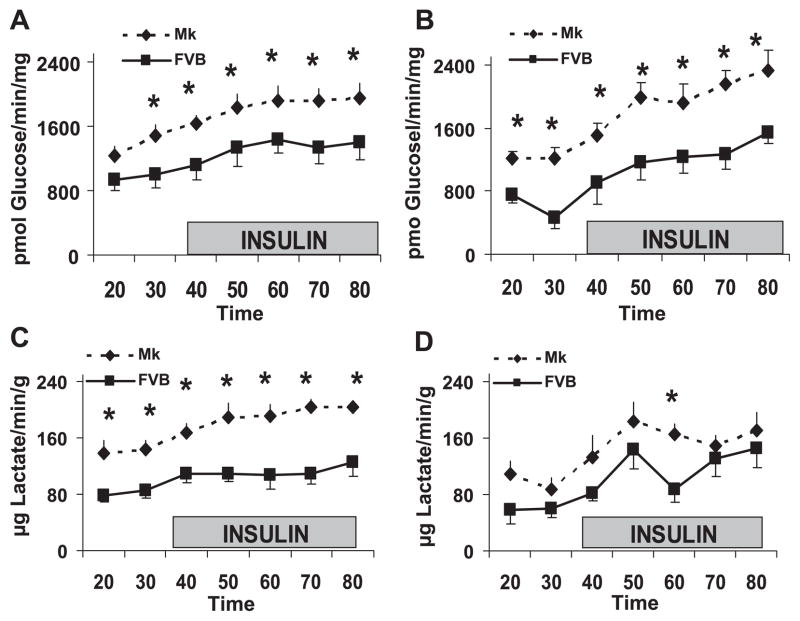
The effect of the Mk transgene on glycolysis was assessed in Langendroff-perfused hearts by measuring the metabolism of 5-tritiated glucose. The release of tritiated water measures the glycolytic steps through the triose phosphate isomerase reaction. The amount of lactate produced during the perfusion was also measured. As shown in Fig. 2, in both the presence and absence of palmitate, the release of tritiated water and lactate were significantly elevated in Mk transgenic hearts compared with control hearts. The Mk effect on glycolysis was evident both before and after the addition of insulin. The increase in glycolysis did not appear to be related to contractility or perfusion pressure since no significant differences were observed for contractility between transgenic and control hearts at any time point (data not shown). Also, in both groups, the perfusion pressure averaged 65 mmHg while the flow rate was maintained at 2 ml/min.
Fig. 2.
The kinase-active PFK-2 transgene increases glycolysis in Langendorff-perfused hearts. A and C: glycolysis (A) and lactate release (C) without palmitate. B and D: glycolysis (B) and lactate release (D) in the presence of 0.4 mM palmitate. The shaded box shows the addition of 200 μU/ml insulin. Values for FVB and Mk hearts were compared by two-way ANOVA (*P < 0.05). Values are means ± SE for at least 5 mice/time point.
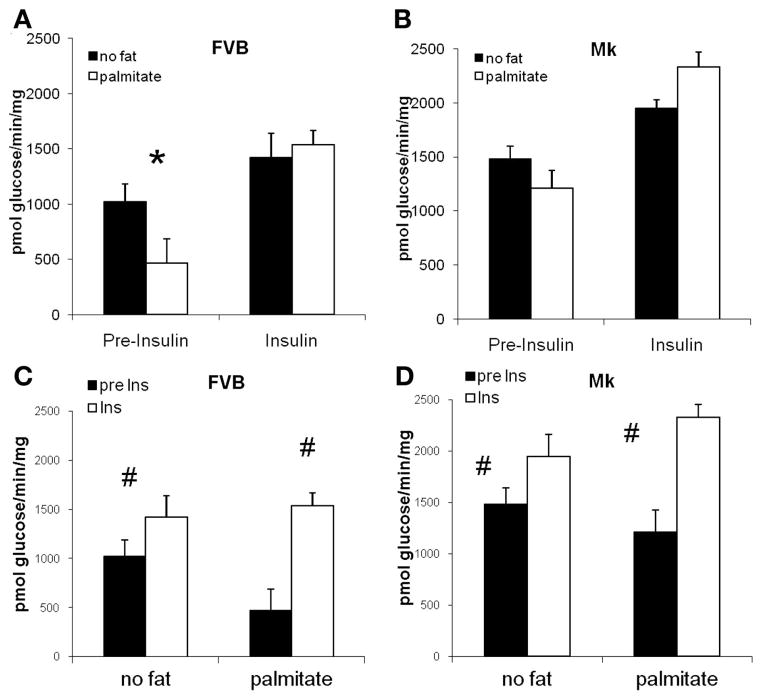
To determine if the transgene altered the effect of palmitate and insulin, the results for tritiated water release were replotted in Fig. 3. Before the addition of insulin, we found palmitate to be a more potent inhibitor of glycolysis in FVB mice (55% inhibition) than in Mk mice (18% inhibition). In the presence of insulin, palmitate did not reduce glycolysis in FVB or Mk hearts.
Fig. 3.
Effect of palmitate and insulin (Ins) on glycolysis in FVB mice and Mk mice. Preinsulin glycolysis was reduced by 55% (*P < 0.02) in FVB mice (A) but only by 18% in Mk mice (B; P = 0.24). In the presence of insulin, palmitate did not reduce glycolysis in FVB or Mk hearts. C and D: insulin elevated glycolysis in FVB (C) and Mk (D) hearts with or without palmitate (#P < 0.02, preinsulin vs. insulin by paired t-test). The relative effect of insulin was greater in the presence of palmitate because insulin decreased palmitate inhibition of glycolysis. Data are from Fig. 2 at 30 and 80 min.
Our laboratory (8) has previously reported that a transgene that reduced cardiac Fru-2,6-P2 blunted the insulin response in perfused hearts. However, the present study (Fig. 3, C and D) failed to support the converse: compared with FVB hearts, there was no increase in the insulin response in Mk hearts with increased Fru-2,6-P2. In the absence of palmitate, insulin increased glycolysis by 39% and 31%, respectively, in FVB and Mk hearts. In the presence of palmitate, the insulin response was markedly increased, by 230% of basal in FVB mice and by 93% of basal in Mk hearts. The very large insulin response in palmitate-perfused FVB hearts was due primarily to the potent inhibition of basal glycolysis by palmitate (Fig. 3A), which was eliminated by the addition of insulin. Folmes et al. (9) have also reported that insulin has a greater effect on glycolysis in the presence of palmitate.
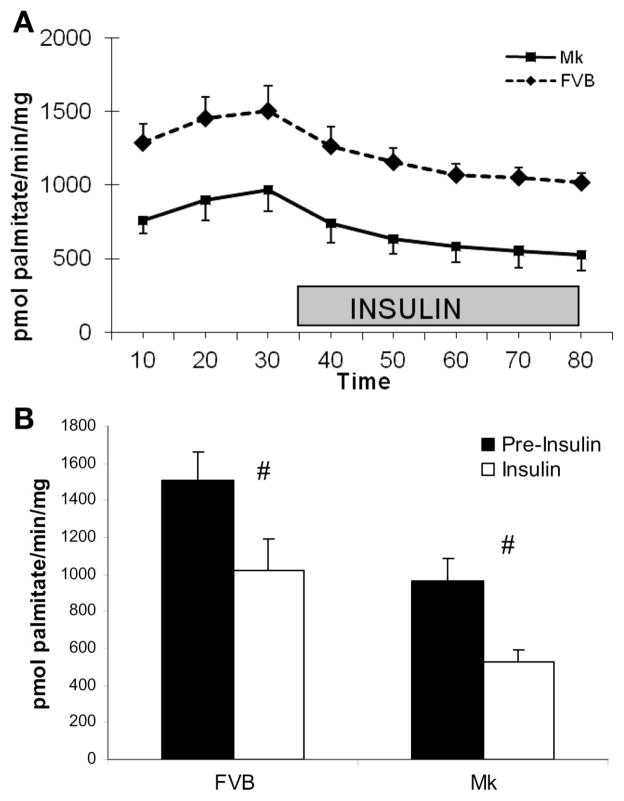
In addition to the Mk-induced increase in glycolysis, we found a 30–50% significant reduction in palmitate oxidation in Mk hearts that was true throughout the duration of the perfusion (Fig. 4A). Insulin produced a significant reduction (P < 0.01 by paired t-test) in palmitate oxidation in both FVB and Mk hearts. The average decrease produced by insulin was 32 ± 1% and 42 ± 6% for FVB and Mk hearts, respectively (Fig. 4B), which was not significantly different in the two types of hearts (FVB vs. Mk, P = 0.13).
Fig. 4.
The Mk transgene reduces palmitate oxidation in Langendorff-perfused hearts. A: FVB palmitate oxidation values were greater than Mk values at all time points (P < 0.01 by two-way ANOVA, n = 5 FVB and 7 Mk hearts). B: effect of insulin on palmitate oxidation by replotting the 30- and 80-min results from A. Insulin reduced palmitate oxidation by 32% in FVB hearts and by 42% in Mk hearts (#P < 0.01 by paired t-test).
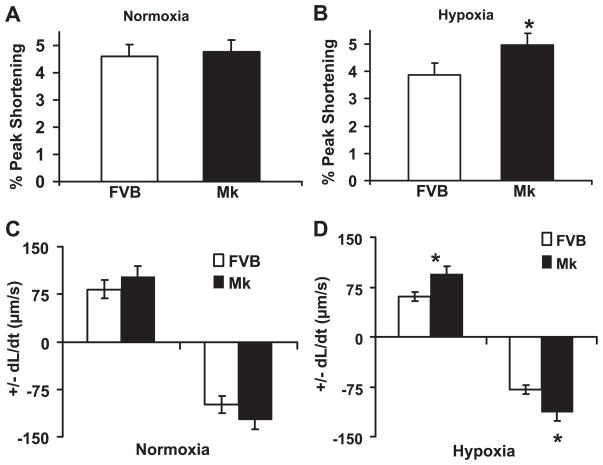
Myocyte contractility in hypoxia
To determine the effect of increased glycolysis on cardiomyocyte contractility and the response to hypoxia in Mk transgenic hearts, myocytes were isolated from Mk and FVB hearts and incubated with oxygenated or nitrogen-bubbled Krebs buffer. Myocyte contractility was measured using a video-based edge detection system, and the results are shown in Fig. 5. Under normoxic conditions, there were no significant differences in contractility between Mk and FVB myocytes, as assessed by ±dL/dt and the percentage of peak shortening. However, under hypoxic condition, Mk myocytes showed significantly increased contractility compared with FVB control myocytes for all three parameters. In FVB myocytes, hypoxia reduced all of the parameters of contractility by 20% to >30%, but in Mk myotyes, hypoxia had a 0–10% effect. These results indicate that the Mk transgene provided almost complete protection from hypoxia-induced contractile deficits in our culture conditions.
Fig. 5.
Overexpression of kinase-active PFK-2 improves contractility under hypoxia. Cardiomyocytes were isolated and incubated under normoxic or hypoxic conditions. The contractile properties of ventricular myocytes from FVB control and Mk transgenic mouse hearts was measured by video-based edge detection. Graphs show the percentage of shortening under normoxic (A) and hypoxic (B) conditions as well as the maximal velocities of cell shortening and relengthening (±dL/dt) under normoxia (C) and hypoxia (D). Values are means ± SE for 60 – 80 cells from 4 mice/group. *P < 0.02, Mk vs. FVB by Student’s t-test.
Myocardial infarct size
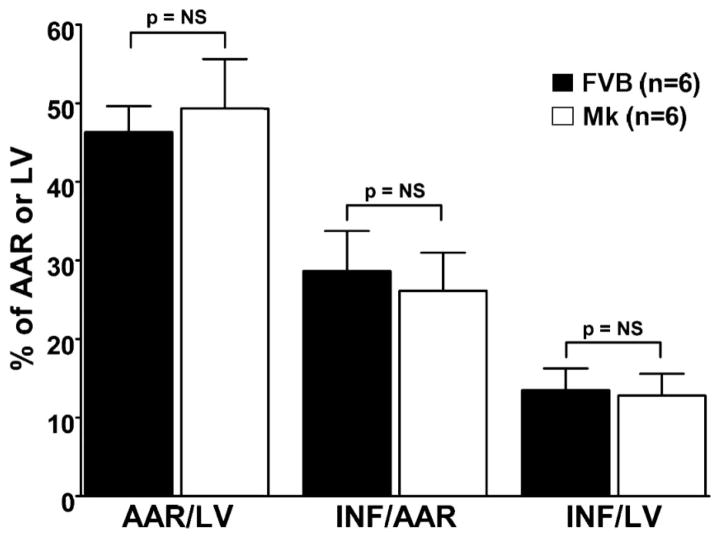
To establish whether the cytoprotective effects associated with Mk transgenesis in isolated myocytes extended to the in vivo setting, we subjected FVB and Mk mice to experimental myocardial infarction. As shown in Fig. 6, the AARs for infarction with respect to left ventricular (LV) size were not significantly different between FVB (46.3 ± 3.3%) and Mk (49.3 ± 6.3%) groups. Infarct sizes expressed relative to the AAR were also not different between FVB (28.7 ± 5.1%) and Mk (26.2 ± 4.8%) group. Likewise, infarct sizes expressed relative to the entire LV were not different between FVB (13.5 ± 2.8%) and Mk (12.8 ± 2.8%) groups.
Fig. 6.
Myocardial infarct size (INF) was determined after 40 min of in vivo coronary occlusion and 24 h of reperfusion. The area at risk (AAR) with respect to the left ventricle (LV) was not significantly different between FVB wild-type and Mk transgenic mice, indicating consistent execution of the surgical protocol between the two groups. The amount of nonviable myocardium, according to triphenyltetrazolium negativity, was also similar between the two groups. This was true whether INF was expressed relative to the AAR (INF/AAR) or to the entire LV (INF/LV). P = not significant (NS) for all comparisons. n = 6 mice/group.
Cardiac hypertrophy in transgenic hearts
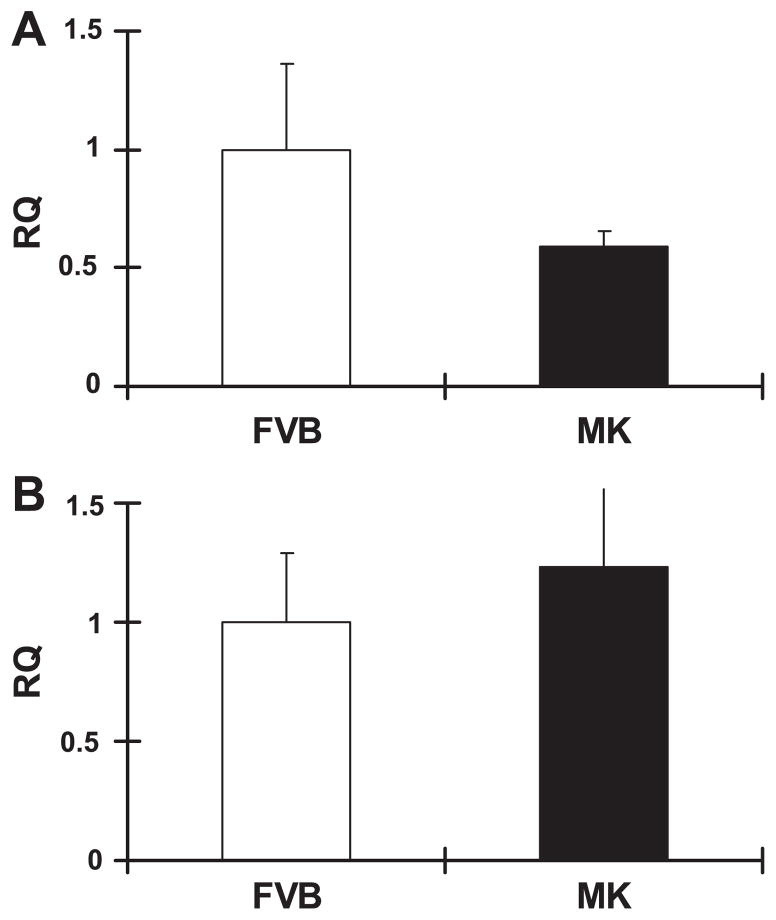
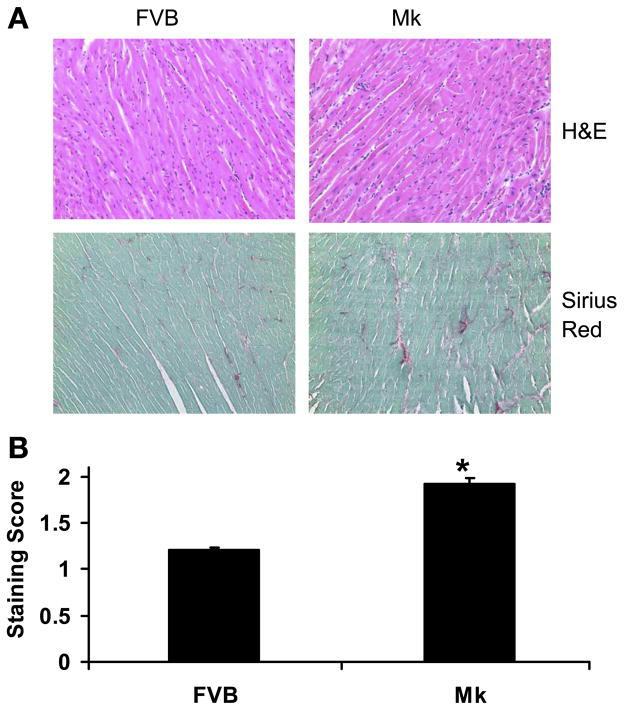
Despite the fact that the Mk transgene was beneficial to hypoxic cardiomyocyte function, we noted an increase in cardiac size. When measured, we found that the heart weight and heart weight-to-body weight ratio were significantly increased by ~17% compared with FVB control mice, whereas body weights were similar between Mk mice and FVB mice (Table 2). To determine if this corresponded to a change in cardiomyocyte size, we also compared isolated cardiomyocyte length (Table 2) using video-based edge detection. Cardiomyocyte length in Mk transgenic hearts was significantly longer than that in FVB hearts. The cardiac hypertrophy raised the possibility that elevated glycolysis was detrimental to the heart. The expression of two markers of cardiac stress, β-MHC and BNP, were unchanged (Fig. 7), and hematoxylin and eosin staining (Fig. 8A) of Mk hearts appeared normal. However, Sirius red staining for fibrosis appeared to be consistently increased in Mk hearts (Fig. 8A), and this was confirmed by semiquantitative analysis (Fig. 8B).
Table 2.
Mk hearts and myocytes are enlarged
| Age, days | Body weight, g | Heart Weight, mg | Heart Weight/Body Weight | Myocyte Length, μm | |
|---|---|---|---|---|---|
| FVB | 138±2 | 29.9±0.6 | 132.7±3.3 | 4.44±0.07 | 126±3 |
| Mk | 131±4 | 29.8±0.6 | 155.3±2.5* | 5.21±0.08* | 151±3* |
Values are means ± SE; n = 13 male Mk mice and 27 male FVB mice. Myocyte length was the average of 52 myocytes from 4 mice/group.
P < 0.001 by Student’s t-test.
Fig. 7.
Real-time PCR showed no elevation of brain natriuretic peptide (A) and β-myosin heavy chain (B) mRNA in MK transgenic mice. RQ, relative quantity of each mRNA normalized to the standard mRNA, ND4. Values are means ± SE for 4 mice/group. Values were not significantly different by Student’s t-test.
Fig. 8.
Fibrosis in Mk hearts. Cardiac morphology was visualized by hematoxylin and eosin (H&E) staining, and collagen accumulation was visualized by Sirius red staining at a magnification of ×40. A: representative staining of FVB and Mk hearts. B: average (±SE) scores for Sirius red staining from 60 photographs taken from 3 FVB and 3 Mk mouse hearts. Staining was rated by a blinded observer on a scale of 0–2, where 0 indicates mild interstitial accumulation of collagen, 1 indicates increased interstitial accumulation of collagen, and 2 severe interstitial accumulation of collagen. Values are means ± SE and were analyzed by Student’s t-test (*P < 0.01).
DISCUSSION
Lipid metabolism provides most energy for cardiac contraction, but under some pathological conditions, such as hypertrophy and ischemia-reperfusion (1), glucose metabolism increases. It is not clear whether this increase in glucose metabolism is a beneficial, adaptive response of the heart or a detrimental, maladaptive response. To test this, we developed transgenic mice designed to have a specific and permanent increase in cardiac glycolysis independent of hypertrophy or ischemia. Multiple lines of mice were produced with a cardiac-specific, bisphosphatase-deficient mutant of the enzyme PFK-2, designated transgene Mk. The transgene produced a three- to fourfold increase in cardiac levels of Fru-2,6-P2, which is a potent stimulator of the glycolytic regulatory enzyme PFK-1. In the present study, we show multiple effects of the transgene. It increased glycolysis, reduced palmitate oxidation, and made glycolysis less susceptible to inhibition by palmitate. The transgene markedly improved myocyte contractility during hypoxia, but it did not alter the infarct size in response to ischemia in vivo. Despite some beneficial actions, the Mk transgene promoted hypertrophy and cardiac fibrosis.
For the effects produced by the Mk transgene to provide relevant information about the role of Fru-2,6-P2 in cardiac pathology, it is important that the increase in Fru-2,6-P2 concentration in Mk transgenic hearts be similar to the concentrations achieved in known conditions of cardiac pathology. Pressure overload hypertrophy induces a two- to sixfold increase in Fru-2,6-P2 (3, 26). Anoxia or ischemia results in a two- to threefold elevation in cardiac Fru-2,6-P2 content (25). And stimulation of isolated cardiomyocytes with insulin produces a fourfold increase in Fru-2,6-P2 content (20). Thus, the three- to fourfold increase in Fru-2,6-P2 seen in our transgenic mice is similar to the increases produced by insulin, hypertrophy, and anoxia or ischemia in the heart (3, 20, 25, 26).
An elevation in Fru-2,6-P2 levels should stimulate activity of PFK-1. If this is true, glycolytic metabolites upstream of PFK-1 should be reduced and metabolites downstream of PFK-1 should be increased in transgenic hearts. Our data showed that the concentration of G6P was significantly reduced by 1.6-fold. This reduction was likely the result of increased disposal of Fru-6-P by greater flux through PFK-1, creating a drain on G6P concentrations. Glycogen, the production of which branches off upstream of PFK-1, was diminished by almost ninefold in transgenic hearts. The large magnitude of the glycogen effect may be due to the fact that G6P has two roles in the control of glycogen content: G6P is a substrate for glycogen production and G6P is a positive regulator of glycogen synthase (30). In addition, the increased PFK-1 activity promotes greater use of glycogen to fuel glycolysis. The expected increase in metabolites downstream of PFK-1 was not as clear as the decrease in upstream metabolites. The concentrations of Fru-1,6-P2 and pyruvate increased 50–70%, but the changes were not statistically significant. This smaller downstream effect may reflect cardiac adaptation to increased PFK-1 flux. This could be due to greater lactate disposal, as we saw in perfused transgenic hearts, or to greater mitochondrial oxidation of pyruvate obtained from glucose (15–18).
The conversion of F6P to Fru-1,6-P2 by PFK is one of the rate-limiting, regulated steps of cardiac glycolysis. Therefore, we anticipated an increase of glycolytic flux in perfused Mk hearts. Even in the absence of lipid, glycolytic flux in Langendorff-perfused Mk hearts was consistently 20% higher than in nontransgenic hearts. This was not secondary to elevated contractility or to altered coronary vascular resistance since we detected no effect of the transgene on these parameters. The most likely cause was the increased activity of PFK. These results support a significant role for Fru-2,6-P2 even in the absence of lipid. The addition of 0.4 mM palmitate to the perfusate produced an apparent twofold increase in the impact of the transgene on glycolytic flux. This was because palmitate depressed glycolysis less in Mk hearts than in FVB control hearts. Fatty acids inhibit glycolysis in large part by inhibiting PFK via the PFK inhibitors citrate and ATP. Fru-2,6-P2 antagonizes this inhibition (12). In addition, we found that palmitate oxidation was about twofold lower in Mk hearts; consequently, palmitate perfusion produced less citrate and ATP in Mk hearts than in FVB hearts. Therefore, we suggest that a combination of elevated Fru-2,6-P2 and reduced production of citrate and ATP minimized the effect of fatty acids to inhibit glycolysis in Mk hearts. The mechanism behind the Fru-2,6-P2-reduced palmitate oxidation is less clear. In previous studies, upregulation of glycolysisis with a glucose transporter (Glut)1 transgene increased fatty acid oxidation (24), whereas upregulation of glycolysis with a Glut4 transgene had no effect on fatty acid oxidation (5). It is also possible that Fru-2,6-P2 may have unrecognized direct or indirect effects on lipid metabolism.
In our previous transgenic model with reduced cardiac levels of Fru-2,6-P2, sensitivity to insulin was sharply reduced (8). This led us to expect an increase in cardiac insulin sensitivity in our new Mk model. However, this was not the case for several parameters of insulin action including stimulation of glycolysis, inhibition of palmitate oxidation, and phosphorylation of Akt (data not shown). Our previous (8) and present results suggest that a basal level of Fru-2,6-P2 is required for a normal insulin response but that further elevation of Fru-2,6-P2 beyond normal levels does not produce a further increase in insulin sensitivity.
Basal contractility was unchanged in Mk hearts when assayed either during Langendorff perfusion or in isolated cardiomyocytes. This is consistent with results we have obtained with two other transgenic models of increased or decreased glycolysis (8, 22). The results from all of these transgenic lines show that altering glycolysis for many months does not change myocyte contractility when assayed in oxygenated conditions. During hypoxia, glycolysis becomes more important to myocyte function. Consistent with this, cultured Mk myocytes were much less susceptible to hypoxia. In fact, under hypoxic conditions that significantly impaired control myocytes in our assays, we saw no detectable decline in Mk cardiomyocyte contractility. Resistance to hypoxia is the opposite of what we previously found in myocytes with impaired glycolytic capacity (8). This confirms the essential role of glycolysis during hypoxic conditions. However, isolated myocyte experiments do not allow for extrapolation to in vivo conditions. In vivo, multiple fuel substrates are available, in contrast to the sole substrate of glucose used for in vitro myocyte culture. Also, excess lactate from increased glycolysis in Mk hearts may accumulate in poorly perfused myocardium of ischemic intact hearts (33). In addition, fibrosis and hypertrophy in Mk mice could increase cardiac work during in vivo ischemia, and the reduced glycogen content (Table 1) of Mk hearts reduced carbohydrate reserves. Therefore, it was not a complete surprise that the protection from hypoxia in isolated myocytes was not replicated in vivo, where infarct damage following ischemia-reperfusion was similar in transgenic and control hearts. A more complete analysis of ischemia resistance in Mk hearts should be undertaken using low-flow and stop-flow perfusion of isolated hearts to distinguish what components contribute to resistance to hypoxia of cardiomyocytes in culture but not in vivo.
Heart weight, heart weight-to-body weight ratio, and cardiomyocyte length were all significantly elevated in Mk transgenic mice, demonstrating hypertrophy. To test if this mild hypertrophy indicated cardiac pathology, we examined two other signs of pathology: upregulation of “fetal state cardiac genes” (29–30) and induction of fibrosis. There was no significant effect of the Mk transgene on the expression of β-MHC and BNP. Since these genes are upregulated by stress, this did not support the conclusion that Mk hearts were undergoing severe pathological stress (32). On the other hand, we found that cardiac fibrosis was increased, and fibrosis is a cardiac stress marker (6). Overall, the weight of the evidence, fibrosis and hypertrophy imply that the Mk transgene induces a mild state of pathology. By constitutively elevating Fru-2,6-P2, the Mk transgene chronically elevated glycolysis and blocked the normal mechanisms controlling carbohydrate usage. The increased glycolysis of chronic heart failure may ultimately be maladaptive. It is striking that our present transgene, which elevated Fru-2,6-P2, and our previous transgene (8), which reduced Fru-2,6-P2, both produced mild hypertrophy and fibrosis. This demonstrates the sensitivity of the heart to disruption of normal metabolic control.
In summary, we produced mice with chronic and stable elevation of cardiac Fru-2,6-P2. This resulted in significant changes in cardiac metabolite concentrations, increased glycolysis, reduced palmitate oxidation, and protection of isolated myocytes from hypoxia. Finally, chronic elevation of glycolysis produced detrimental effects, suggesting that the elevation of glycolysis in failing hearts could be injurious to an already compromised heart.
Acknowledgments
GRANTS
This work was supported by National Institutes of Health Grants HL-62892 and DK-073586 (to P. N. Epstein) and HL-83320 (to S. P. Jones) and by American Heart Association National Scientist Development Grant 0535270N (to S. P. Jones).
References
- 1.Angyalosi G, Neveu R, Wolowczuk I, Delanoye A, Herno J, Auriault C, Pancre V. HLA class II polymorphism influences onset and severity of pathology in Schistosoma mansoni-infected transgenic mice. Infect Immun. 2001;69:5874–5882. doi: 10.1128/IAI.69.9.5874-5882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augustus AS, Buchanan J, Park TS, Hirata K, Noh HL, Sun J, Homma S, D’Armiento J, Abel ED, Goldberg IJ. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem. 2006;281:8716–8723. doi: 10.1074/jbc.M509890200. [DOI] [PubMed] [Google Scholar]
- 3.Beauloye C, Marsin AS, Bertrand L, Vanoverschelde JL, Rider MH, Hue L. The stimulation of heart glycolysis by increased workload does not require AMP-activated protein kinase but a wortmannin-sensitive mechanism. FEBS Lett. 2002;531:324–328. doi: 10.1016/s0014-5793(02)03552-4. [DOI] [PubMed] [Google Scholar]
- 4.Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- 5.Belke DD, Larsen TS, Gibbs EM, Severson DL. Glucose metabolism in perfused mouse hearts overexpressing human GLUT-4 glucose transporter. Am J Physiol Endocrinol Metab. 2001;280:E420–E427. doi: 10.1152/ajpendo.2001.280.3.E420. [DOI] [PubMed] [Google Scholar]
- 6.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop SP, Altschuld RA. Increased glycolytic metabolism in cardiac hypertrophy and congestive failure. Am J Physiol. 1970;218:153–159. doi: 10.1152/ajplegacy.1970.218.1.153. [DOI] [PubMed] [Google Scholar]
- 8.Donthi RV, Ye G, Wu C, McClain DA, Lange AJ, Epstein PN. Cardiac expression of kinase-deficient 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase inhibits glycolysis, promotes hypertrophy, impairs myocyte function, and reduces insulin sensitivity. J Biol Chem. 2004;279:48085–48090. doi: 10.1074/jbc.M405510200. [DOI] [PubMed] [Google Scholar]
- 9.Folmes CD, Clanachan AS, Lopaschuk GD. Fatty acids attenuate insulin regulation of 5′-AMP-activated protein kinase and insulin cardioprotection after ischemia. Circ Res. 2006;99:61–68. doi: 10.1161/01.RES.0000229656.05244.11. [DOI] [PubMed] [Google Scholar]
- 10.Gulick J, Subramaniam A, Neumann J, Robbins J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J Biol Chem. 1991;266:9180–9185. [PubMed] [Google Scholar]
- 11.Hoffmeyer MR, Scalia R, Ross CR, Jones SP, Lefer DJ. PR-39, a potent neutrophil inhibitor, attenuates myocardial ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2000;279:H2824–H2828. doi: 10.1152/ajpheart.2000.279.6.H2824. [DOI] [PubMed] [Google Scholar]
- 12.Hue L, Beauloye C, Marsin AS, Bertrand L, Horman S, Rider MH. Insulin and ischemia stimulate glycolysis by acting on the same targets through different and opposing signaling pathways. J Mol Cell Cardiol. 2002;34:1091–1097. doi: 10.1006/jmcc.2002.2063. [DOI] [PubMed] [Google Scholar]
- 13.Jones SP, Girod WG, Palazzo AJ, Granger DN, Grisham MB, Jourd’Heuil D, Huang PL, Lefer DJ. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol Heart Circ Physiol. 1999;276:H1567–H1573. doi: 10.1152/ajpheart.1999.276.5.H1567. [DOI] [PubMed] [Google Scholar]
- 14.Jones SP, Greer JJ, Kakkar AK, Ware PD, Turnage RH, Hicks M, van HR, de CR, Kawashima S, Yokoyama M, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H276–H282. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 15.Jones SP, Hoffmeyer MR, Sharp BR, Ho YS, Lefer DJ. Role of intracellular antioxidant enzymes after in vivo myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H277–H282. doi: 10.1152/ajpheart.00236.2002. [DOI] [PubMed] [Google Scholar]
- 16.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marbán E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–1182. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 17.Kashiwaya Y, Sato K, Tsuchiya N, Thomas S, Fell DA, Veech RL, Passonneau JV. Control of glucose utilization in working perfused rat heart. J Biol Chem. 1994;269:25502–25514. [PubMed] [Google Scholar]
- 18.Koyama T, Boston D, Ikenouchi H, Barry WH. Survival of metabolically inhibited ventricular myocytes is enhanced by inhibition of rigor and SR Ca2+ cycling. Am J Physiol Heart Circ Physiol. 1996;271:H643–H650. doi: 10.1152/ajpheart.1996.271.2.H643. [DOI] [PubMed] [Google Scholar]
- 19.Kurland IJ, El Maghrabi MR, Correia JJ, Pilkis SJ. Rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Properties of phospho-and dephospho forms and of two mutants in which Ser32 has been changed by site-directed mutagenesis. J Biol Chem. 1992;267:4416–4423. [PubMed] [Google Scholar]
- 20.Lefebvre V, Mechin MC, Louckx MP, Rider MH, Hue L. Signaling pathway involved in the activation of heart 6-phosphofructo-2-kinase by insulin. J Biol Chem. 1996;271:22289–22292. doi: 10.1074/jbc.271.37.22289. [DOI] [PubMed] [Google Scholar]
- 21.Liang Q, Carlson EC, Donthi RV, Kralik PM, Shen X, Epstein PN. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes. 2002;51:174–181. doi: 10.2337/diabetes.51.1.174. [DOI] [PubMed] [Google Scholar]
- 22.Liang Q, Donthi RV, Kralik PM, Epstein PN. Elevated hexokinase increases cardiac glycolysis in transgenic mice. Cardiovasc Res. 2002;53:423–430. doi: 10.1016/s0008-6363(01)00495-3. [DOI] [PubMed] [Google Scholar]
- 23.Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic; 1993. pp. 111–228. [Google Scholar]
- 24.Luptak I, Balschi JA, Xing Y, Leone TC, Kelly DP, Tian R. Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-alpha-null hearts can be rescued by increasing glucose transport and utilization. Circulation. 2005;112:2339–2346. doi: 10.1161/CIRCULATIONAHA.105.534594. [DOI] [PubMed] [Google Scholar]
- 25.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den BG, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 26.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, Tian R. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–667. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- 27.Neely JR, Denton RM, England PJ, Randle PJ. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem J. 1972;128:147–159. doi: 10.1042/bj1280147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neely JR, Rovetto MJ. Techniques for perfusing isolated rat hearts. Methods Enzymol. 1975;39:43–60. doi: 10.1016/s0076-6879(75)39008-3. [DOI] [PubMed] [Google Scholar]
- 29.Newsholme EA, Randle PJ. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964;93:641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen JN, Richter EA. Regulation of glycogen synthase in skeletal muscle during exercise. Acta Physiol Scand. 2003;178:309–319. doi: 10.1046/j.1365-201X.2003.01165.x. [DOI] [PubMed] [Google Scholar]
- 31.Okar DA, Manzano A, Navarro-Sabate A, Riera L, Bartrons R, Lange AJ. PFK-2/FBPase-2: maker and breaker of the essential biofactor fructose-2,6-bisphosphate. Trends Biochem Sci. 2001;26:30–35. doi: 10.1016/s0968-0004(00)01699-6. [DOI] [PubMed] [Google Scholar]
- 32.Pandya K, Kim HS, Smithies O. Fibrosis, not cell size, delineates beta-myosin heavy chain reexpression during cardiac hypertrophy and normal aging in vivo. Proc Natl Acad Sci USA. 2006;103:16864–16869. doi: 10.1073/pnas.0607700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–173. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz K, Boheler KR, de la BD, Lompre AM, Mercadier JJ. Switches in cardiac muscle gene expression as a result of pressure and volume overload. Am J Physiol Regul Integr Comp Physiol. 1992;262:R364–R369. doi: 10.1152/ajpregu.1992.262.3.R364. [DOI] [PubMed] [Google Scholar]
- 35.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 36.Van Schaftingen E, Lederer B, Bartrons R, Hers HG. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982;129:191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- 37.Wu C, Okar DA, Newgard CB, Lange AJ. Overexpression of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase in mouse liver lowers blood glucose by suppressing hepatic glucose production. J Clin Invest. 2001;107:91–98. doi: 10.1172/JCI11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu C, Okar DA, Stoeckman AK, Peng LJ, Herrera AH, Herrera JE, Towle HC, Lange AJ. A potential role for fructose-2,6-bisphosphate in the stimulation of hepatic glucokinase gene expression. Endocrinology. 2004;145:650–658. doi: 10.1210/en.2003-1290. [DOI] [PubMed] [Google Scholar]
- 39.Wu CD, Okar DA, Peng L, Lange AJ. Decreasing fructose-2,6-bisphosphate leads to diabetic phenotype in normal mice. Diabetes. 2002;51:A319–A319. [Google Scholar]
- 40.Ye G, Donthi RV, Metreveli NS, Epstein PN. Overexpression of hexokinase protects hypoxic and diabetic cardiomyocytes by increasing ATP generation. Cardiovasc Toxicol. 2005;5:293–300. doi: 10.1385/ct:5:3:293. [DOI] [PubMed] [Google Scholar]
- 41.Ye G, Metreveli NS, Ren J, Epstein PN. Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes. 2003;52:777–783. doi: 10.2337/diabetes.52.3.777. [DOI] [PubMed] [Google Scholar]