Abstract
Background
Mitral valve prolapse (MVP) is a common disorder associated with mitral regurgitation (MR), endocarditis, heart failure and sudden death. In the familial context, prior studies have described non-diagnostic mitral valve morphologies (‘prodromal forms’ and ‘minimal superior displacement’ [MSD]) that may represent early expression of MVP in those genetically predisposed. Our objective was to explore the spectrum of MVP abnormalities in the community and compare their clinical and echocardiographic features.
Methods
Phenotypic heterogeneity of MVP was assessed by measuring annular diameter (D), leaflet displacement (Dis), thickness (T), anterior/posterior leaflet projections (A, P) onto the annulus, coaptation height (C or P/D), and MR jet height (JH) in a sample of 296 individuals of the Framingham Offspring Study who were identified as having MVP (n=77) or its prodromal form (N=11) or MSD (N=57), with 151 controls with no feature of MVP or its non-diagnostic forms.
Results
The prodromal form did not meet diagnostic criteria but resembled fully diagnostic MVP with regards to D, T and JH (all p > 0.05); they were similar to individuals with posterior MVP with regard to leaflet asymmetry and coaptation height (p = 0.91). Compared to MSDs and controls, prodromals had greater C, T, D and JH (all p < 0.05). MSDs shared the posterior leaflet asymmetry with classic MVP, but their coaptation point was more posterior (C = 31% versus 42%, p<0.0001).
Conclusion
Non-diagnostic morphologies of MVP are observed in the community and share the common feature of posterior leaflet asymmetry with fully affected individuals. Prodromal morphology and MSD may represent early expressions of MVP and additional studies are warranted to elucidate the natural history of these phenotypes.
Keywords: mitral valve prolapse, echocardiography
Introduction
Mitral valve prolapse (MVP) is a common disorder (2-5%)1-3 characterized by fibromyxomatous changes in one or both of the mitral leaflets that cause displacement of the leaflets into the left atrium.4, 5 MVP is associated with mitral regurgitation (MR), endocarditis, heart failure and sudden death.6-11 Despite being the most common valvular pathology requiring surgery,12 little is known about the genetic mechanisms underlying the genesis and progression of MVP. To date, three loci for autosomal dominant, non-syndromic MVP have been described on chromosomes 11, 16 and 13.13-15 While filamin A has been identified as causing an X-linked form of MVP,16, 17 the genes for the more common form of autosomal dominant MVP are unknown. In the family linked to chromosome 13 and in other families,15 we have shown that previously non-diagnostic morphologies of MVP may represent mild or early stages of phenotypic expression in gene carriers. These morphologies include the ‘prodromal form’ and ‘minimal systolic displacement’ (MSD). Both the prodromal phenotype and MSD share features of excessive leaflet motion with fully affecteds, as demonstrated by superior motion towards the left atrium, bulging of the posterior leaflet relative to the anterior (albeit not diagnostic by quantitative assessment) and asymmetry of coaptation. In addition, in the prodromal form, leaflet excess can also manifest itself by anterior motion and a shift of the coaptation point towards the septum and the aortic root (Figure 1) as detailed below (see Methods). Prodromal members and individuals with MSD shared either the complete or a major portion of the haplotype with fully diagnostic MVP in our genetics studies.15 These non-diagnostic forms may, therefore, represent early expression of MVP in those genetically predisposed.
Figure 1.
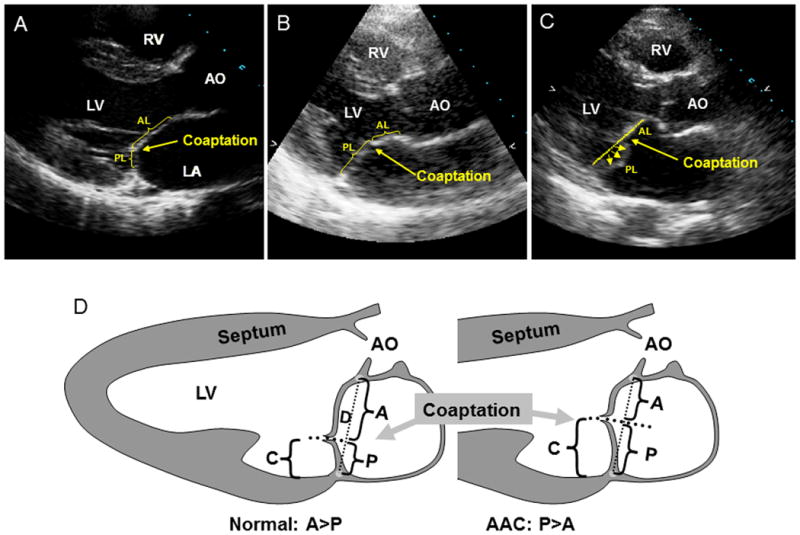
Echo examples of posteriorly coapting leaflets (anterior leaflet [AL]; posterior leaflet [PL]) in a normal subject (A) versus increased coaptation height in a prodromal individual and an elongated posterior leaflet (B) and in a subject with classic posterior MVP into the left atrium (LA) (C). D, Schematics showing projections of anterior (A) and posterior (P) leaflets onto the mitral annular diameter (D). C indicates the coaptation height relative to the annulus and is calculated as P/D. AO, aorta; LV, left ventricle; and RV, right ventricle.
The spectrum of early MVP abnormalities noted above has been described to date only in families. We hypothesized that non-diagnostic forms of MVP exist in the general population. The Framingham Heart Study (FHS) represents an ideal setting to explore the phenotypic heterogeneity of MVP in the community with a focus on prodromal forms and MSD.
Recognizing these so-called ‘early forms’ of MVP may be important because MVP often manifests clinically in the 5th or 6th decade of life as a severe cardiac event. Early recognition may facilitate newer therapeutic approaches, analogous to those currently being investigated in the Marfan syndrome, in which angiotensin I receptor blockade leads to down regulation of TGF-beta and limitation of clinical disease progression.18-20
Methods
Participants
Individuals who participated in the fifth examination cycle of the FHS Offspring cohort (1991-1995), constituted the sampling frame for this investigation. The examination protocol was approved by the institutional review board of Boston Medical Center, and all subjects provided written informed consent.
At this examination cycle, all attendees underwent routine transthoracic echocardiography (see below). As previously described,3 the echocardiograms of all subjects who had previously been identified as possibly having MV leaflet displacement suggesting MVP in any two-dimensional view or on M-mode echocardiography at any prior examination of the Offspring cohort were reviewed to ensure that no cases of MVP were missed among 3491 attendees at the fifth examination cycle. This broad approach identified 518 individuals with possible MVP. First, the echocardiograms obtained at the fifth examination of these subjects were assessed to identify those in whom qualitative superior displacement of the MV leaflets during systole warranted a quantitative evaluation to determine whether MVP was actually present. The 151 individuals identified in this way were paired with controls (1:1) matched for age and sex who were also drawn from the fifth examination cycle but who were initially coded as having no evidence of prolapse. Of this original sample of 302 individuals, echocardiographic images of 6 individuals were deemed of inadequate quality for detailed analysis. Therefore, the final sample for this investigation consisted of 296 individuals (151 controls). Also, for the purpose of this investigation, the total number of offspring individuals in the fifth generation was 3485 (3491-6). Prevalence of MVP in the Offspring cohort was calculated based on this denominator.
Clinical characteristics
At the fifth examination cycle, all attendees underwent a routine medical history, targeted physical examination for cardiovascular disease, anthropometry and laboratory assessment of cardiovascular disease risk factors. Clinical variables used in the present investigation included: age, sex, history of smoking, treatment for hypertension, systolic and diastolic blood pressure, body mass index (BMI, calculated as weight (kg) / [height (m)]2), and the presence of a precordial systolic murmur on auscultation.
Echocardiographic characteristics
All subjects underwent standard two-dimensional echocardiography with a commercially available system (Sonos 1000, Hewlett–Packard, Andover, Mass.) that used a 2.5-MHz transducer. Images included complete parasternal, apical, and subcostal views and color Doppler assessment of valvular regurgitation. All measurements were performed with a DigiView Off-line Cardiac Analysis System.
Echocardiograms were examined blinded to previous MVP diagnosis3 and clinical history. Using current two-dimensional echocardiographic criteria,21, 22 the diagnosis of MVP was made by measurement of maximal MV leaflet superior systolic displacement (Dis) relative to the line connecting the annular hinge points (annular dimension or D). The projections of the anterior and posterior MV leaflets onto the mitral annulus (A, P) were also assessed at end-systole (Figure 1D).15 The meeting point of the MV leaflets relatively to the annulus was quantified by the leaflet coaptation height (C or P/D) (See Figure 1). Normally, the MV leaflets meet posteriorly within the 25-30% of the left ventricular cavity because the posterior leaflet is shorter than the anterior (Figure 1A). In patients with MVP, coaptation is typically displaced anteriorly, consistent with elongation of the posterior MV leaflet, which can produce excessive leaflet motion not only into the left atrium but also toward the aortic root. Finally, MR severity was quantified by color Doppler as the maximum systolic proximal MR jet height (JH). Previous studies have indicated that proximal jet size basically reflects the size of the vena contracta, a fundamental measure of lesion severity, and correlates well with independent invasive and noninvasive measures of regurgitant volume and fraction.23-26 A jet height of 2 mm effectively separates trace physiologic backflow from mild MR, while 5 mm or more indicate moderate severity.23, 25, 26 All of the above echocardiographic parameters were measured in the parasternal or apical long-axis views at end-systole. MV leaflet thickness (T) was measured at end-diastole in the same views as the leading to trailing edge of the thickest area of the mid-portion of the leaflet, excluding focal areas of thickness and chordae.
Based on prior clinical and prognostic studies,8, 9, 21, 22, 27, 28 MVP was diagnosed if leaflet displacement exceeded 2 mm. Subjects were classified as having classic MVP (displacement >2 mm, thickness ≥5 mm) or non-classic MVP (displacement >2 mm, thickness <5 mm).8, 21, 22, 27 Non-diagnostic forms of uncertain clinical importance were described based on the common feature of posterior leaflet asymmetry, frequent in fully affected members. Subjects with borderline degrees of displacement of the MV leaflets (≤2 mm) but posterior coaptation were designated as having MSD (Figure 2). Prodromal individuals didn't have diagnostic leaflet displacement beyond the annulus, but their pattern of leaflet closure or coaptation resembled fully expressed MVP. Compare Figure 1C, in which classic MVP leaflets meet halfway up the dotted annular line, with Figure 1B, which shows a subject with no displacement of leaflets into the left atrium beyond the annulus but with an anterior shift (> 40%) of the coaptation point. This shift has been correlated quantitatively with posterior leaflet length (see Discussion). Finally, controls were defined as individuals: 1) who did not meet the echocardiographic criteria described for MVP, prodromals, or MSDs, and 2) who had no other MV disease such as mitral stenosis of congenital mitral pathology.
Figure 2.
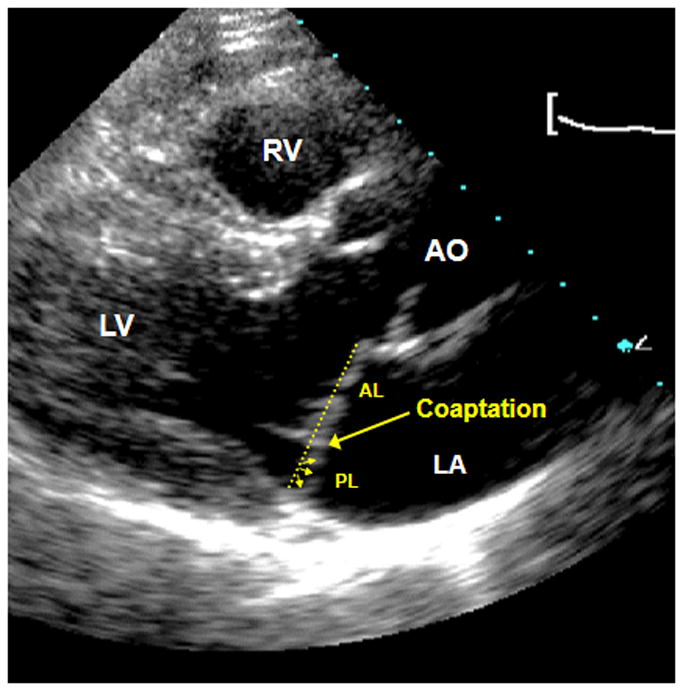
Example of minimal systolic displacement (MSD) with 1) posterior coaptation 2) posterior leaflet asymmetry which resembles that seen in fully affected individuals BUT with 3) borderline degree of displacement (≤ 2 mm, involving the posterior leaflet).
Statistical methods
Clinical (sex, systolic murmur, smoking history, treatment for hypertension, BMI, systolic and diastolic blood pressure) and echocardiographic characteristics (D, T, Dis, A, P, C, and JH) were compared in the four groups (MVP, MSD, prodromals, and controls) by ANOVA for continuous variables and by logistic regression analysis for categorical variables. All echocardiographic parameters were adjusted for age and sex. Correlation coefficients among observations made by the same reader on different occasion or among different observers reading the same images were used to estimate inter and intra-observer variability for select MV measurements. All analyses were conducted using SAS version V9.3 on a SUN Ultrasparc workstation. A two-sided p value <0.05 was the criterion for statistical significance. A post-hoc Duncan test was used to correct for multiple comparisons.
Results
The clinical characteristics of the participants are summarized in Table 1. Age, sex distribution and BMI were similar in all groups (MVP, MSD, prodromals, and controls, p > 0.05 for all comparisons). There was no statistically significant difference among the four groups in regards to blood pressure, history of smoking and treatment of hypertension (p > 0.05 for all comparisons). Patients with MVP had a higher prevalence of a precordial systolic murmur compared to the other three categories (all p < 0.05).
Table 1. Clinical characteristics.
|
MVP (n=77) |
MSD (n=57) |
AAC (n=11) |
Referents (n=151) |
|
|---|---|---|---|---|
| Demographics and Lifestyle | ||||
| Age (years) | 55.9 ± 10.7 | 57.4 ± 9.8 | 59.5 ± 7.1 | 55.4 ± 10.1 |
| Sex, n (% women) | 42 (54) | 40 (70) | 7 (64) | 96 (63) |
| History of ever smoking, n (%) | 28 (36.3) | 21 (36.8) | 2 (18.1) | 68 (45.0) |
|
| ||||
| Clinical | ||||
| Body mass index (kg/m2) | 24.7 ± 3.2 | 24.3 ± 3.4 | 26.4 ± 6.0 | 26.2 ± 4.8 |
| Systolic blood pressure (mmHg) | 122 ± 17 | 125 ± 15 | 125 ± 17 | 123 ± 19 |
| Diastolic blood pressure (mmHg) | 72 ± 10 | 74 ± 7 | 74 ± 4 | 73 ±10 |
| Treatment for hypertension, n (%) | 10 (13) | 9 (16) | 2 (18) | 28 (18) |
| Systolic murmur, n (%) | 22 (28)* | 7 (12) | 1 (9) | 18 (12) |
MVP = mitral valve prolapse; MSD = minimal superior systolic displacement of the mitral leaflets; AAC = abnormal anterior coaptation. Values are expressed as mean ± SD (%)
p<0.05.
The echocardiographic characteristics of the participants with classic MVP and its non-classic forms are summarized in Table 2. The overall prevalence of MVP (including its non classic forms) was 2.2% of the offspring generation (77/3485). The majority of cases of MVP was non-classic (41/77 [53%]). Prolapse more commonly involved only the posterior MV leaflet (34/77 [44%]). All the participants with MSD except one had involvement of the posterior MV leaflet only.
Table 2. Mitral valve and left chamber characteristics by echocardiography.
|
MVP (n=77) |
MSD (n=57) |
AAC (n=11) |
Referents (n=151) |
|
|---|---|---|---|---|
| Annular size, D (mm) | 30.9 ± 5.6a | 28.6 ± 4.1b | 30.1 ± 4.1a | 27.3 ± 3.8b |
| Anterior projection, A (mm) | 17.6 ± 4.8a | 19.3 ± 3.9a | 15.4 ± 1.7b | 19.3 ± 3.6a |
| Posterior projection, P (mm) | 13.0 ± 4.4a | 8.9 ± 1.9b | 15.4 ± 1.7c | 7.8 ± 1.8b |
| Coaptation height, C or P/D (%) | 42 ± 12a | 31 ± 6b | 49 ± 3c | 28 ± 2b |
| Anterior thickness (mm) | 4.3 ± 0.9a | 3.4 ± 0.5b | 4.0 ± 0.6a,c | 3.1 ± 0.7d |
| Posterior thickness (mm) | 4.8 ± 0.9a | 3.6 ± 0.6b | 4.2 ± 0.9a,c | 3.1 ± 0.7d |
| Anterior displacement (mm) | 1.5 ± 1.4a | 0.0 ± 0.1b | 0.0 ± 0.0b | 0.0 ± 0.0b |
| Posterior displacement (mm) | 2.2 ± 1.2a | 1.5 ± 0.2b | 1.1 ± 0.6c | 0.0 ± 0.0d |
| LA ant-post diameter (mm) | 3.2 ± 0.3a | 3.0 ± 0.2b | 3.1 ± 0.2a | 3.0 ± 0.4c |
| LV fractional shortening (%) | 37 ± 5a | 36 ± 4a | 37 ± 3a | 36 ± 4a |
MVP = mitral valve prolapse; MSD = minimal systolic displacement; AAC = abnormal anterior coaptation; LA = left atrium; ant-post = antero-posterior; LV = Left ventricular. In each row, means with different superscript letters are significantly different, p<0.05, otherwise p≥0.05.
Morphological heterogeneity
Review of echocardiographic images revealed a wide spectrum of phenotypic morphologies in MVP. Of the 296 individuals that we evaluated, 77 had fully diagnostic MVP and varying leaflet involvement as described in Table 2. Nevertheless, the majority of individuals with MVP had asymmetric prolapse of the posterior MV leaflet relative to the anterior, a common pattern described in other MVP series.21, 29 Sixty-eight individuals were designated as having forms not meeting current diagnostic criteria: 11 with the prodromal morphology, and 57 with MSD of the MV posterior leaflet.
The echocardiographic differences between the four groups are shown in Table 3. The prodromal morphology did not meet diagnostic criteria (mean posterior displacement beyond the annulus of 1.1 ± 0.6 mm, no anterior leaflet displacement) but resembled the 77 MVPs with regards to D, posterior/anterior T and JH (all p > 0.05). Interestingly, prodromals were similar to posterior MVP relatively to leaflet asymmetry (evidence of posterior leaflet bulging compared to the anterior by qualitative assessment, not quantified if not beyond the annulus). Coaptation height was higher in prodromals compared to all MVPs (bileaflet + monoleaflet), likely due to the contribution of anterior MVP (C = 49% versus 42% in all MVP, p = 0.007). It is hypothesized that a longer anterior leaflet in anterior MVP may lower the coaptation point (i.e., shift it posteriorly), leading to a smaller coaptation height value in the overall MVP group. For similar reasons, anterior leaflet projection was smaller and posterior leaflet projection was greater in prodromals compared to all MVPs (p = 0.007 and p = 0.05, respectively). A sub-analysis comparing prodromals to bileaflet, anterior, and posterior MVP by ANOVA was pursued to account for type of leaflet involvement IFigure 3). The sub-analysis showed that prodromals had an anteriorly shifted coaptation height, similarly to posterior MVP (C = 49% versus 50% in posterior MVP, p = 0.9). Coaptation height was higher than bileaflet and monoleaflet anterior MVP (C = 49% in prodromals versus 27% and 42%, p = 0.007 and p < 0.0001, respectively). Compared to the 57 individuals with MSD, and the 151 controls, prodromals had greater C, T, D and JH (all p < 0.05). MSDs shared the posterior leaflet asymmetry with MVP, but similar to controls, their MV leaflet coaptation point was more posterior (C = 31% in MSDs and 28% in controls versus 42% in MVPs, both p<0.0001). When adjusted for age and sex, annular size and MR proximal JH were slightly greater in MSDs when compared to controls (p = 0.02 and p = 0.004, respectively; this difference was statistically non- significant in the unadjusted analysis), possibly reflecting different cavity size and filling pressures in younger females compared to older males. Annular size and degree of MR remained less than in individuals with MVP and prodromal morphology after sex/age adjustment. The other results described above did not differ when the analysis was adjusted to age and sex.
Figure 3.
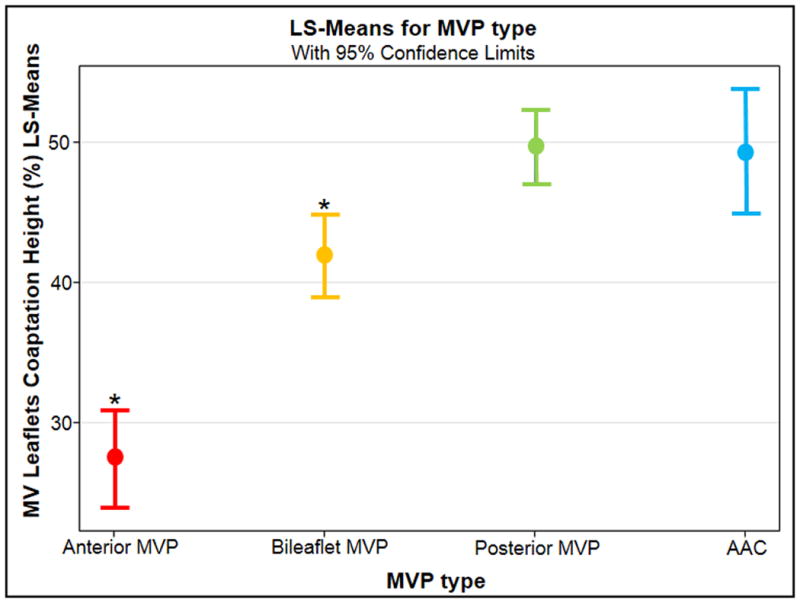
Comparison between prodromals and MVP type relative to coaptation height. MV= mitral valve. LS = least square.
Patterns of mitral regurgitation
Except for MVP, the amount of mitral regurgitation was trivial (JH < 2 mm) in all categories. Even among individuals with MVP, only 5/77 had more than moderate (JH ≥ 5 mm) mitral regurgitation, thus confirming the known low rates of clinically apparent disease in the FHS MVP population.3 Interestingly, the 9 individuals with a prodromal morphology and quantifiable MR all showed a late-systolic (albeit trivial) jet of regurgitation similar to classic MVP, as previously described in families.15
Reproducibility of Echocardiographic Findings
The intraobserver and interobserver correlations for mitral-leaflet displacement, leaflet thickness, and the degree of mitral regurgitation in 20 subjects exceeded 0.97 as previously reported.3 Reproducibility for the novel echocardiographic parameters of anterior/posterior leaflet projections and leaflet coaptation height was also very good with correlations exceeding 0.94.
Discussion
Our investigation demonstrates that non-diagnostic morphologies previously recognized only in the familial context are not infrequent in the community and share features indicating excessive MV leaflet motion with fully diagnostic MVP. Specifically, we have recognized two ‘early’” or non-diagnostic phenotypes of MVP in the FHS: the ‘prodromal morphology’ and MSD. Prodromal individuals lack diagnostic MV leaflet displacement into the atrium but have two salient features: anterior displacement of the coaptation point >40%, and a leaflet coaptation pattern of bulging of the posterior leaflet relative to the anterior similar to that seen in classic posterior leaflet MVP (the most common MVP type in the FHS), and in many individuals with bileaflet MVP. Therefore, leaflet elongation can manifest itself not only by superior motion into the LA but also by anterior motion that shifts the coaptation point of the MV leaflets toward the aortic root and septum. Quantitatively, we previously observed that the height of coaptation relative to the annulus (P/D in Figure 1D) correlated well with the ratio of anterior to posterior leaflet length (r =0.83) in the chromosome 11 family.15 This association has been recognized during surgical repair of MVP in patients who have long posterior MV leaflets that are more prone to having their coapted leaflets shift anteriorly and obstruct the LV outflow tract,30 an abnormality that is reducible by Carpentier's ‘sliding’ of the posterior leaflet downward.31 In the current study, we have also shown for the first time that prodromals resemble fully diagnostic MVP relatively to other features such as having thicker MV leaflets, a larger MV annulus, and with regard to the degree of mitral regurgitation on color flow imaging. These additional features may apply to prodromals found in the general population only, or simply may not have been adequately quantified in the families previously evaluated. In MSD, the coaptation point is posterior (similarly to normal individuals), but the presence of excessive leaflet motion is demonstrated by posterior MV leaflet asymmetry and borderline leaflet displacement. In individuals with MSD, MV leaflets are thinner and the amount of MR is trivial, suggesting that MSD may represent a milder non-diagnostic phenotype compared to the prodromal morphology.
Our study not only explores the phenotypic spectrum of MVP in the community, but also confirms the low prevalence of significant MVP-related MR in the general population in comparison with referral-biased series.3, 32 Specifically, the degree of MR was on average trivial in both MSD and prodromals (although in the latter case closer to the mild MR found in the majority of MVPs), with only 5 fully diagnostic MVP cases showing greater than moderate MR. Our study also confirms that MVP is not a disease of young women as previously reported,33 but has similar age and sex distribution compared to normal individuals.3
The clinical significance of non-diagnostic MV morphologies is intuitive in the familial context. Specifically, prodromal members and individuals with MSD shared either the complete or a major portion of the haplotype with fully diagnostic MVP in the pedigree linked to chromosome 13.15 This same prodromal morphology was also observed in the family linked to chromosome 11. When we reviewed all echocardiograms in that family blinded to haplotype status, we discovered 5 individuals with a prodromal morphology who turned out to be carriers of the haplotype, as did another with MSD.15 In the general population, progression studies are needed to fully understand the clinical significance of non-diagnostic MV morphologies. If non-diagnostic forms are truly early or mild MVP phenotypes, they could provide an opportunity for tailored interventions to limit clinical disease progression and/or reveal modifying genes or environmental factors. The novel echocardiographic parameter of coaptation height is essential for better understanding of MVP mechanisms and progression: MVP is not just excessive superior but also anterior motion, an innovative concept that can lead to discovery. Similarly to Marfan syndrome, in which angiotensin I receptor blockade leads to down regulation of TGF-beta and limitation of clinical disease progression, targeted medical intervention on early stages of MVP may improve clinical outcomes such as development of MR or heart failure.
Study limitations
The true number of non-diagnostic morphologies, particularly of the prodromal individuals, may have been underestimated in the current study. Specifically, the study design was based on the initial qualitative identification of MVP cases followed by age-sex matching and generation of a limited sample. Review and quantification of echocardiographic parameters was simply not possible for all 3845 individuals of the offspring generation, leading to possible under diagnosis of prodromal morphologies. In addition, the offspring generation was assessed at echo 5 when the average age of the participants was approximately 60 years. Prodromal morphologies are mostly found in younger individuals in the familial context, mainly in children of affected parents.
Conclusions
As in the familial context, non-diagnostic morphologies exist in the community and share the common feature of posterior leaflet asymmetry with MVP. Prodromal morphology and MSD may represent early expressions of MVP and potential targets for tailored interventions to limit clinical disease progression.
Acknowledgments
This work was funded by a Founders Affiliate American Heart Association Clinical Research grant (F.N.D.) and by a … (R.S.V.)
Senior authorship is acknowledged for both R.S.V. and E.J.B.
References
- 1.Devereux RB, Jones EC, Roman MJ, Howard BV, Fabsitz RR, Liu JE, Palmieri V, Welty TK, Lee ET. Prevalence and correlates of mitral valve prolapse in a population-based sample of American Indians: the Strong Heart Study. Am J Med. 2001;111(9):679–685. doi: 10.1016/s0002-9343(01)00981-0. [DOI] [PubMed] [Google Scholar]
- 2.Freed LA, Benjamin EJ, Levy D, Larson MG, Evans JC, Fuller DL, Lehman B, Levine RA. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J Am Coll Cardiol. 2002;40(7):1298–1304. doi: 10.1016/s0735-1097(02)02161-7. [DOI] [PubMed] [Google Scholar]
- 3.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341(1):1–7. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 4.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104(21):2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 5.Tamura K, Fukuda Y, Ishizaki M, Masuda Y, Yamanaka N, Ferrans VJ. Abnormalities in elastic fibers and other connective-tissue components of floppy mitral valve. Am Heart J. 1995;129(6):1149–1158. doi: 10.1016/0002-8703(95)90397-6. [DOI] [PubMed] [Google Scholar]
- 6.Avierinos JF, Detaint D, Messika-Zeitoun D, Mohty D, Enriquez-Sarano M. Risk, determinants, and outcome implications of progression of mitral regurgitation after diagnosis of mitral valve prolapse in a single community. Am J Cardiol. 2008;101(5):662–667. doi: 10.1016/j.amjcard.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Devereux RB, Kramer-Fox R, Shear MK, Kligfield P, Pini R, Savage DD. Diagnosis and classification of severity of mitral valve prolapse: methodologic, biologic, and prognostic considerations. Am Heart J. 1987;113(5):1265–1280. doi: 10.1016/0002-8703(87)90955-0. [DOI] [PubMed] [Google Scholar]
- 8.Marks AR, Choong CY, Sanfilippo AJ, Ferre M, Weyman AE. Identification of high-risk and low-risk subgroups of patients with mitral-valve prolapse. N Engl J Med. 1989;320(16):1031–1036. doi: 10.1056/NEJM198904203201602. [DOI] [PubMed] [Google Scholar]
- 9.Perloff JK, Child JS. Clinical and epidemiologic issues in mitral valve prolapse: overview and perspective. Am Heart J. 1987;113(5):1324–1332. doi: 10.1016/0002-8703(87)90961-6. [DOI] [PubMed] [Google Scholar]
- 10.Zuppiroli A, Mori F, Favilli S, Barchielli A, Corti G, Montereggi A, Dolara A. Arrhythmias in mitral valve prolapse: relation to anterior mitral leaflet thickening, clinical variables, and color Doppler echocardiographic parameters. Am Heart J. 1994;128(5):919–927. doi: 10.1016/0002-8703(94)90590-8. [DOI] [PubMed] [Google Scholar]
- 11.Zuppiroli A, Rinaldi M, Kramer-Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol. 1995;75(15):1028–1032. doi: 10.1016/s0002-9149(99)80718-8. [DOI] [PubMed] [Google Scholar]
- 12.Waller BF, Morrow AG, Maron BJ, Del Negro AA, Kent KM, McGrath FJ, Wallace RB, McIntosh CL, Roberts WC. Etiology of clinically isolated, severe, chronic, pure mitral regurgitation: analysis of 97 patients over 30 years of age having mitral valve replacement. Am Heart J. 1982;104(2 Pt 1):276–288. doi: 10.1016/0002-8703(82)90204-6. [DOI] [PubMed] [Google Scholar]
- 13.Disse S, Abergel E, Berrebi A, Houot AM, Le Heuzey JY, Diebold B, Guize L, Carpentier A, Corvol P, Jeunemaitre X. Mapping of a first locus for autosomal dominant myxomatous mitral-valve prolapse to chromosome 16p11.2-p12.1. Am J Hum Genet. 1999;65(5):1242–1251. doi: 10.1086/302624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freed LA, Acierno JS, Jr, Dai D, Leyne M, Marshall JE, Nesta F, Levine RA, Slaugenhaupt SA. A locus for autosomal dominant mitral valve prolapse on chromosome 11p15.4. Am J Hum Genet. 2003;72(6):1551–1559. doi: 10.1086/375452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nesta F, Leyne M, Yosefy C, Simpson C, Dai D, Marshall JE, Hung J, Slaugenhaupt SA, Levine RA. New locus for autosomal dominant mitral valve prolapse on chromosome 13: clinical insights from genetic studies. Circulation. 2005;112(13):2022–2030. doi: 10.1161/CIRCULATIONAHA.104.516930. [DOI] [PubMed] [Google Scholar]
- 16.Kyndt F, Gueffet JP, Probst V, Jaafar P, Legendre A, Le Bouffant F, Toquet C, Roy E, McGregor L, Lynch SA, Newbury-Ecob R, Tran V, Young I, Trochu JN, Le Marec H, Schott JJ. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation. 2007;115(1):40–49. doi: 10.1161/CIRCULATIONAHA.106.622621. [DOI] [PubMed] [Google Scholar]
- 17.Kyndt F, Le Scouarnec S, Jaafar P, Gueffet JP, Legendre A, Trochu JN, Jousseaume V, Chaventre A, Schott JJ, Le Marec H, Probst V. Genetic aspects of valvulopathies. Arch Mal Coeur Vaiss. 2007;100(12):1013–1020. [PubMed] [Google Scholar]
- 18.Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332(6027):361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312(5770):117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radonic T, de Witte P, Baars MJ, Zwinderman AH, Mulder BJ, Groenink M. Losartan therapy in adults with Marfan syndrome: study protocol of the multi-center randomized controlled COMPARE trial. Trials. 2010;11:3. doi: 10.1186/1745-6215-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine RA, Stathogiannis E, Newell JB, Harrigan P, Weyman AE. Reconsideration of echocardiographic standards for mitral valve prolapse: lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J Am Coll Cardiol. 1988;11(5):1010–1019. doi: 10.1016/s0735-1097(98)90059-6. [DOI] [PubMed] [Google Scholar]
- 22.Levine RA, Triulzi MO, Harrigan P, Weyman AE. The relationship of mitral annular shape to the diagnosis of mitral valve prolapse. Circulation. 1987;75(4):756–767. doi: 10.1161/01.cir.75.4.756. [DOI] [PubMed] [Google Scholar]
- 23.Tribouilloy C, Shen WF, Rey JL, Adam MC, Lesbre JP. Mitral to aortic velocity-time integral ratio. A non-geometric pulsed-Doppler regurgitant index in isolated pure mitral regurgitation. Eur Heart J. 1994;15(10):1335–1339. doi: 10.1093/oxfordjournals.eurheartj.a060390. [DOI] [PubMed] [Google Scholar]
- 24.Grayburn PA, Fehske W, Omran H, Brickner ME, Luderitz B. Multiplane transesophageal echocardiographic assessment of mitral regurgitation by Doppler color flow mapping of the vena contracta. Am J Cardiol. 1994;74(9):912–917. doi: 10.1016/0002-9149(94)90585-1. [DOI] [PubMed] [Google Scholar]
- 25.Hall SA, Brickner ME, Willett DL, Irani WN, Afridi I, Grayburn PA. Assessment of mitral regurgitation severity by Doppler color flow mapping of the vena contracta. Circulation. 1997;95(3):636–642. doi: 10.1161/01.cir.95.3.636. [DOI] [PubMed] [Google Scholar]
- 26.Mele D, Vandervoort P, Palacios I, Rivera JM, Dinsmore RE, Schwammenthal E, Marshall JE, Weyman AE, Levine RA. Proximal jet size by Doppler color flow mapping predicts severity of mitral regurgitation. Clinical studies. Circulation. 1995;91(3):746–754. doi: 10.1161/01.cir.91.3.746. [DOI] [PubMed] [Google Scholar]
- 27.Perloff JK, Child JS. Mitral valve prolapse. Evolution and refinement of diagnostic techniques. Circulation. 1989;80(3):710–711. doi: 10.1161/01.cir.80.3.710. [DOI] [PubMed] [Google Scholar]
- 28.Levine RA, Handschumacher MD, Sanfilippo AJ, Hagege AA, Harrigan P, Marshall JE, Weyman AE. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989;80(3):589–598. doi: 10.1161/01.cir.80.3.589. [DOI] [PubMed] [Google Scholar]
- 29.Grayburn PA, Berk MR, Spain MG, Harrison MR, Smith MD, DeMaria AN. Relation of echocardiographic morphology of the mitral apparatus to mitral regurgitation in mitral valve prolapse: assessment by Doppler color flow imaging. Am Heart J. 1990;119(5):1095–1102. doi: 10.1016/s0002-8703(05)80240-6. [DOI] [PubMed] [Google Scholar]
- 30.Maslow AD, Regan MM, Haering JM, Johnson RG, Levine RA. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease. J Am Coll Cardiol. 1999;34(7):2096–2104. doi: 10.1016/s0735-1097(99)00464-7. [DOI] [PubMed] [Google Scholar]
- 31.Jebara VA, Dervanian P, Acar C, Grare P, Mihaileanu S, Chauvaud S, Fabiani JN, Deloche A, Carpentier A. Mitral valve repair using Carpentier techniques in patients more than 70 years old. Early and late results. Circulation. 1992;86(5 Suppl):II53–59. [PubMed] [Google Scholar]
- 32.Avierinos JF, Gersh BJ, Melton LJ, 3rd, Bailey KR, Shub C, Nishimura RA, Tajik AJ, Enriquez-Sarano M. Natural history of asymptomatic mitral valve prolapse in the community. Circulation. 2002;106(11):1355–1361. doi: 10.1161/01.cir.0000028933.34260.09. [DOI] [PubMed] [Google Scholar]
- 33.Devereux RB, Brown WT, Kramer-Fox R, Sachs I. Inheritance of mitral valve prolapse: effect of age and sex on gene expression. Ann Intern Med. 1982;97(6):826–832. doi: 10.7326/0003-4819-97-6-826. [DOI] [PubMed] [Google Scholar]


