Abstract
Ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist, has been shown to be effective not only for its anesthetic properties but also for the analgesic and opiate-sparing effects. However, data on efficacy and safety of oral ketamine for the treatment of neuropathic or cancer pain syndromes is limited with most of the evidence based on small clinical trials and anecdotal experiences. In this review, we will analyze the clinical data on oral ketamine in the palliative care setting. After an extensive search using five major databases, a total of 19 relevant articles were included. No official clinical guidelines for the use of oral ketamine in this patient population were found. Studies on oral ketamine for cancer and neuropathic pain have shown mixed results which could be partially due to significant differences in hepatic metabolism. In addition, we will include a case report of a 38-year-old female with neurofibromatosis type 1 (NF1) with history of chronic, severe pain in her fingertips secondary to multiple glomus tumors which evolved into CRPS resistant to multiple therapies but responsive to oral ketamine. Based on our experience with oral ketamine, this drug should be administered after an intravenous trial to monitor response and side effects in patients with an adequate functional status. However, patients in the palliative care and hospice setting, especially the one at the end of their lives, may also benefit from oral ketamine even if an intravenous trial is not feasible.
Keywords: oral ketamine, palliative care, cancer pain, neuropathic pain, neurofibromatosis type 1, complex regional pain syndrome
Introduction
As the palliative care field continues to grow, it moves further from the initial concept of only treating patients with active, progressive, far-advanced diseases at the end of their lives to treating patients with chronic medical conditions. This, in turn, presents new challenges for palliative care clinicians. Certain pain syndromes such as cancer pain and neuropathic pain, either from central or from peripheral etiology, are among the most challenging and difficult to effectively treat. Treatment of these conditions more commonly requires high-dose opiates which may be associated with an increase in significant side effects, which can preclude adequate analgesia. The addition of adjuvant therapies in pain management has allowed clinicians to treat pain in a multimodal approach, using medications with different mechanisms of action, in order to improve efficacy and minimize side effects. Ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist, has been shown to be effective in the treatment of opiate-resistant pain syndromes of different etiologies and is being increasingly used in the palliative care setting due to its opiate-sparing effects. The efficacy and safety of parenteral ketamine for the treatment of these syndromes has been well reviewed.1–3 Oral administration of ketamine may be as effective as the parenteral route for treating chronic pain in the palliative care population, allowing patients to continue their normal activities and adding to quality of life.
Ketamine is a general anesthetic that antagonizes the activity of glutamate on (NMDA) receptors.4 When glutamate binds to the NMDA receptor, a nonselective ion channel is opened which allows Na+ and Ca2+ ions into the cell and K+ out of the cell. This results in increased excitatory synaptic transmission due to the depolarizing effects of ion movement along their respective concentration gradients.5–7 Ketamine binds to the phencyclidine (PCP) binding site of the NMDA receptor and inhibits excitatory neurotransmission.8 At higher doses, ketamine produces general anesthesia. At lower doses, ketamine may inhibit the activation of NMDA receptors in secondary afferent neurons, which is thought to be essential for increased pain sensitivity (windup) caused by repeated nociceptive stimulation.9
When used for anesthesia, ketamine is usually administered intravenously; however, when administered at lower doses for pain management, ketamine may be administered orally. There are limited data that detail the pharmacokinetics of oral ketamine. When given orally, ketamine undergoes extensive first-pass metabolism in the liver.10 As a result, bioavailability of oral ketamine ranges from 16.5% to 24%, depending on the dosage form used.10–12 The T1/2 of oral ketamine is reported to be 5.1 to 5.6 hours, which does not differ from the T1/2 of ketamine administered intravenously.10 After oral administration, peak plasma concentrations are achieved in approximately 30 minutes.13 Ketamine is primarily metabolized to norketamine by CYP3A4 and CYP2B6. Norketamine is an active metabolite, although it is far less potent than ketamine, and is renally excreted.10,13
Adverse effects are commonly reported after the oral administration of ketamine and are frequently severe enough to result in the discontinuation of therapy. Ketamine-associated adverse effects are most commonly related to altered function of the central nervous system and include vivid dreams or nightmares, anxiety, hallucinations, dizziness, and paranoid ideations. Gastrointestinal adverse affects are also associated with oral ketamine use and include nausea, vomiting, loss of appetite, and abdominal pain.14 Additionally, there are rare case reports of cystitis which is temporally related to the initiation and discontinuation of oral ketamine therapy.15,16 Unfortunately, the numbers in the studies investigating the use of oral ketamine are currently too small to allow for incidence of these various adverse affects to be estimated.
In this article, the authors will review the existing evidence on the use of oral ketamine for the treatment of neuropathic pain (central or peripheral) and cancer pain in the palliative care population. In addition, 3 case reports of pain are presented from a wide variety of medical conditions that have been successfully treated with oral ketamine.
Methods
An extensive search was completed using 5 major biomedical databases: PubMed, Embase, Scopus, Web of Science, and Biosis, for English-language literature on oral ketamine in the palliative care setting. The keywords searched were “oral ketamine,” “palliative care,” “cancer pain,” and “neuropathic pain.” A total of 80 articles were found. Inclusion criteria were as follows: study performed in patients (adults and children) with medical conditions that are routinely seen in a palliative care setting; the indication for ketamine use was neuropathic pain or cancer pain; ketamine was administered orally; and, complete information about ketamine dosages and pain relief. Case reports and series were also included. Exclusion criteria were as follows: review articles; ketamine was administered via subcutaneous or intravenous route; indication for treatment was chronic benign pain (ie, postoperative, restless leg syndrome, or posttrauma); or study was performed in healthy volunteers. A total of 19 articles were relevant for the use of oral ketamine in the palliative care setting. In all, 12 articles on neuropathic pain are included—8 case reports, 3 case series, and 1 placebo-controlled trial. For cancer pain, a total of 6 articles are included—3 case reports, 2 randomized controlled trials, and 1 case series. No randomized controlled studies were found for the pediatric population.
Results
Cancer Pain
A prospective study conducted by Mercadante et al17 reported significant pain relief when sublingual ketamine was added as breakthrough analgesia. Twelve patients with refractory pain secondary to advanced cancer, previously treated with different opiate regimens, were included in this study. A permanent intrathecal device was placed according to the principal pain site. The infusion pump was filled with a combination of morphine, levobupivacaine, and clonidine, with the latter added only in the presence of a neuropathic pain component. The patients were offered either additional intrathecal doses of levobupivacaine 0.25% (1.5 mg) or sublingual ketamine (25 mg) for breakthrough pain. The patients had been receiving a mean of 36 mg of intravenous morphine per episode. Of the 12 patients, 3 received sublingual ketamine that was well tolerated over 12 episodes, resulting in adequate pain control and was well tolerated. Lauretti et al18 have also analyzed the role of oral ketamine as an adjuvant therapy for cancer pain. A total of 60 patients were randomized to a control arm (20 mg of oral morphine, 10 mg every 12 hours), nitroglycerin patch (5 mg/d), dipyrone group (500 mg, every 6 hours), or oral ketamine (0.5 mg/kg, every 12 hours). Patients received initial daily oral morphine dosages of up to 80 to 90 mg/d to keep the visual analog scale (VAS) less than 4. After the study drug was introduced, patients were allowed to increase their oral morphine daily consumption as needed. Despite a decrease in VAS pain scores after the introduction of the study drug, there was no statistical difference among the 4 groups. However, daily consumption of oral morphine on the control group was significantly higher on days 15, 20, and 30 when compared to the oral ketamine group. Sedation was also significantly higher on the morphine and dipyrone groups. A second double-blind, randomized study conducted by Ishizuka et al19 evaluated the association of oral ketamine and morphine in the treatment of cancer pain. A total of 30 patients were randomized to a combination of oral morphine (10 mg, every 6 hours) and oral ketamine (10 mg, every 8 hours) or oral morphine (10 mg, every 6 hours) and placebo. There was no statistical difference between both arms in terms of pain relief, number of times that the morphine needed to be adjusted, and side effects. Further randomized controlled trials are needed to confirm these results.
Oral ketamine can be use in a broad variety of clinical scenarios, from a highly functional patient with pain refractory to opiate treatment, to a patient with pain at the end of life. Several case studies have reported a significant benefit from subcutaneous and oral ketamine infusions for cancer pain at the end of life with a variable dosage ranging from 70 mg/d as initial dose up to 3.2 g/d final dose and 30 to 150 mg/d initial dose up to 60 to 375 mg/d final dose, respectively.20–23
Neuropathic Pain
The use of oral ketamine as an adjuvant to opiate analgesia in neuropathic pain syndromes has received increased interest. Despite the increasing use of oral ketamine for neuropathic pain of central or peripheral origin, there are only a limited number of studies and case reports which have shown conflicting results. Several case reports have demonstrated clinical benefit of oral ketamine in a variety of pain syndromes of central origin such as the complex regional pain syndrome (CRPS), multiple sclerosis (MS), poststroke pain (PSP), and postamputation stump pain (PASP), and postherpetic neuralgia (PHN).24–27 The majority of patients had previously been treated with various modalities including multidrug analgesic regimens and interventional approaches such as spinal cord stimulators and epidural steroid injections. In the case of the patient with CRPS, oral ketamine was shown to have efficacy but for a short duration.24 The initial dose of oral ketamine was 30 mg every 8 hours with the highest dose achieved being 60 mg every 6 hours (240 mg/d). There was a decrease in the VAS pain score from 10/10 initially to 3 to 4/10 over a period of approximately 5 months. Nausea and vomiting were the only side effects; these were controlled with haloperidol. In a placebo-controlled trial conducted by Furuhashi-Yonaha et al,28 8 patients with chronic neuropathic pain of central and peripheral origin including CRPS, PHN, and phantom limb pain were randomly assigned to receive oral ketamine (0.5 mg/kg, every 6 hours) or placebo for a week after an initial trial of intravenous ketamine. The severity of pain and allodynia was reduced within 15 minutes of administration lasting from 6 to 8 hours. Ketamine plasma levels were undetectable despite good pain relief. In all, 50% of the patients continued the treatment with oral ketamine (initial dose, 26–136 mg/d) for up to 54 months, with a significant reduction in VAS pain scores. No serious adverse events were observed.
The effectiveness of oral ketamine has also been studied in a patient with MS having paroxysmal and persistent pain and allodynia in the right thoracic region (T5-T8).25 Initially, the patient received a trial of intravenous ketamine of 15 mg (0.2 mg/kg) which decreased the pain to 0/10, then switched to oral dextromethorphan, an NMDA receptor antagonist, which was poorly tolerated due to severe dizziness. Subsequently, oral ketamine was started at 20 mg/d, increased to twice a day, and continued for up to a year. There was a significant decrease in paroxysmal and persistent pain and allodynia with a decrease in VAS score by more than 5 points. The addition of oral keta-mine showed a significant improvement in the quality of life with mild dizziness as the only reported side effect. It has also been used in a patient with central PSP following an intracerebral hemorrhage.26 The pain was localized in the right-hand side of the body and associated with allodynia and hyperalgesia. Oral ketamine was used as adjuvant therapy to opiates, tricyclic antidepressants, and anticonvulsants. A trial of intravenous ketamine was performed at an initial dose of 7 mg (0.1 mg/kg) with partial response, administering a second 14 mg (0.2 mg/kg) bolus after 30 minutes with a marked reduction in pain, allodynia, and hyperalgesia. Following the intravenous trial, the patient was started on oral ketamine 50 mg at bedtime, later titrated to 50 mg every 8 hours in combination with diazepam 5 mg every 8 hours to prevent the psychomimetic effects from ketamine. The patient experienced a significant improvement in the pain within 3 weeks of initiating therapy with a remarkable improvement in quality of life for up to 9 months. Opiates and anticonvulsants were tapered down and discontinued without any evidence of withdrawal or worsening pain. In another case report, a patient with PASP experienced adequate pain relief after a trial of intravenous ketamine (loading dose 0.1 mg/kg followed by a continuous infusion at 7 μμg/kg/min).27 The McGill Pain Questionnaire scores were reduced from 43 to 2 and VAS scores were 0 at 4, 6, 18, and 24 hours after the initial intravenous infusion, but the pain returned in 31 hours. The patient was treated with oral ketamine at a dose of 50 mg, which provided adequate relief within 10 minutes and lasted for 6 hours. Subsequently, the patient was placed on an outpatient regimen of oral ketamine every 6 hours, which continued to provide adequate pain relief, even after 3 months of treatment.
A study conducted by Kannan et al29 evaluated the efficacy and safety of oral ketamine in patients with cancer having neuropathic pain. A total of 9 patients previously on opiates, anticonvulsants, or combinations of these drugs, and reporting a pain score of 6 or greater were included in this study. Oral ketamine was prescribed to all the patients at a dose of 0.5 mg/kg every 8 hours in addition to their existing analgesic regimen. Of the 9 patients,6 (67% with diagnosis of head and neck cancer) experienced a decrease in their numerical rating score (NRS) of more than 3 within the first 24 hours of starting oral ketamine. The most troublesome side effects were drowsiness, anorexia, and vomiting; however, the increase in the severity of nausea/vomiting was not statistically significant. Conversely, sedation during the first 2 weeks of treatment was reported by 89% of patients. There was no report of hallucinations either auditory or visual, vertigo, or restlessness. Fifty-six percent of the patients continued treatment with oral ketamine after the study was completed. In a case report, a patient with human T-cell lymphotropic virus type 1-associated myelopathy with intractable peripheral neuropathic pain received oral ketamine at a starting dose of 50 mg every 8 to 12 hours after an initial trial of intravenous ketamine infusion at 10 mg. The patient had significant pain reduction (VAS pain score, 86/100 to 20/100) and no side effects.30 Another report of a patient with history of hepatitis C, cirrhosis, and cryoglobulinemia with mixed nociceptive–neuropathic pain secondary to leg ulcerations described the benefits of oral ketamine for refractory pain.31 The patient was started on oral ketamine as an adjuvant to opiate therapy at 20 mg every 8 hours with improvement on the pain from 8/10 to 5/10. Within 4 months, the ketamine was adjusted to 15 mg every 8 hours with a subsequent decrease in opiate consumption and excellent pain control. Finally, a patient with a unilateral headache secondary to PHN received an intramuscular ketamine trial of 15 mg (0.2 mg/kg) for refractory symptoms.32 The pain relief lasted for 2 hours after the parenteral dose. A ketamine subcutaneous infusion of 5 mg/ h (0.06 mg/kg per h) was then started, which the patient was able to tolerate without any side effects. Subsequently, oral ketamine was started at 240 mg/d in 6 divided doses, titrated up to 1000 mg/d in 5 divided doses. Eventually, the pain resolved within 5 weeks and the patient was able to discontinue the ketamine 2 weeks later.
Importantly, numerous other studies have reported more limited benefits from oral ketamine. A randomized controlled trial (n = 1) conducted by Haines and Gaines33 determined the proportion of patients with chronic neuropathic pain responding to oral ketamine and differentiated the true treatment effects from nonspecific effects. A total of 21 patients were enrolled in this study. All the patients were submitted to the unblinded part of the study in which they were treated with oral ketamine for 1 week at a starting dose of 20 mg/d, titrated up to effect or to a maximum daily dose of 100 mg. Fifty-seven percent of the patients withdrew from the study due to intolerable side effects or persistent pain. Only 9 patients progressed to the n of 1 portion of the study where they were randomized to oral ketamine or placebo. There was no difference in therapeutic effect between the ketamine and the placebo weeks in 67% of the patients. The presence of intolerable side effects such as lightheadedness, dizziness, tiredness, headaches, bad dreams, and a floating feeling was a limiting factor that precluded the analgesic benefit of the drug. In a retrospective study, Enarson et al34 evaluated the analgesic efficacy and side effects of treatment with oral ketamine in patients with central and peripheral chronic neuropathic pain. A total of 21 patients with refractory pain were included in this study. Oral ketamine was added to their analgesic regimens at a starting dose of 100 mg in divided doses titrated to effect with median dose of 220 mg/d. In all, 43% of the patients discontinued the medication due to intolerable side effects. Thirty-three percent of the patients reported significant pain relief as evidenced by lower pain scores and decreased use of breakthrough medications, but only 14% decided to continue the medication for at least a year at daily doses ranging from 100 to 240 mg. Oral ketamine (maximum dose, 900 mg/d) was not effective in a case report by a patient with neuropathic pain secondary to end-stage metastatic adenocarcinoma of the cervix at the end of life.35
No official clinical guidelines for the conversion from parenteral to oral ketamine have been published. Benitez-Rosario et al23 presented a series of case reports in which an equianalgesic ratio of 1:1 was suggested. Four patients with neuropathic pain of different etiologies refractory to treatment with opiates and anticonvulsants were transitioned from a subcutaneous ketamine infusion (0.1–0.2 mg/kg per h) to oral ketamine (starting dose, 25 every 8 hours; maximum dose, 150–375 mg/d). These results are not consistent with the previous reports which have suggested a conversion ratio of 25% to 50% of parenteral dose.22
Pediatric Population
Ketamine, either oral36–38 or intravenous,39,40 has shown to be safe and effective for analgesia and sedation during invasive procedures in the pediatric oncology population. However, the literature on ketamine as an adjuvant therapy for cancer pain in children is limited. Several case reports have described the use of intravenous ketamine as adjuvant therapy to opiates, to reduce tolerance in children with cancer pain secondary to neuroblastoma,41,42 acute myelogenous leukemia,43 osteosarcoma,43 glioblastoma multiforme,44 and peripheral neuroectodermal tumor.45 In all these case reports, intravenous ketamine was administered in patients at the end of their lives for intractable pain.
A study conducted by Finkel et al46 described the use of sub-anesthetic doses of intravenous ketamine to treat children with uncontrolled cancer pain. A total of 11 children with advanced stage cancer and inadequate pain control despite escalation of opiates were enrolled in the study. All the patients had shown signs of opiate tolerance and/or intolerable side effects from previous analgesic regimen. The starting dose for ketamine was 0.1 to 0.2 mg/kg per h, and the duration of treatment ranged from 1 to 75 days. Seventy-three percent of the patients had a reduction in opiate doses (2 patients with 100% opiate-sparing effect) and subjective improvement in analgesic control. Most of the patients were discharged from the hospital and observed to be more interactive with parents and caregivers than when prescribed high-dose opiates.
There are no studies on the use of oral ketamine as adjuvant analgesic therapy in this patient population. To our knowledge, there is only one case study recently published in which oral ketamine was used in a 4-year-old patient with metastatic undifferentiated sarcoma with involvement of the retroperitoneum, spinal canal, and liver. The patient had been previously on morphine (0.2 mg/kg every 3 hours) for 7 months. Oral ketamine was started at an initial dose of 3 mg/kg per d in 3 divided doses, as adjuvant treatment. The patient’s pain decreased from 8 to 9/10 to 0 to 1/10 on VAS pain score, with a significant reduction in the daily morphine requirement. No side effects were reported and the patient was able to improve the quality of life for 2 weeks before death.47 The utilization of oral ketamine as adjuvant therapy to opiate analgesia could add a potential benefit in pain management in the pediatric palliative and end-of-life care setting. However, further clinical trials need to be conducted to justify approval of new uses of ketamine in these 2 patient populations.
Case Report
We report 3 sequential cases of severe neuropathic pain with positive clinical responses to oral ketamine. They span the spectrum of palliative care patients; from an older male with sickle cell disease, to a young male with acute lymphocytic leukemia (ALL), to a middle-aged female with a chronic pain secondary to NF1. Successful pain reduction was achieved in an older male who experienced acute pain post nonmyeloablative allogeneic stem cell transplant for sickle cell disease. He had obtained minimal benefit from adequate trials of gabapentin, pregabalin, and nortriptyline. His initial dose of oral ketamine was 10 mg every 12 hours with titration to 20 mg every 6 hours, along with continued long- and short-acting opiates. An increase in function was reported, requiring less short-acting opiate due to a decrease in pain intensity and being able to rest better at night, which added to his quality of life. No bothersome side effects were noted. The second case was a young male with relapsed refractory ALL with escalating pain at the end of life. He coincidently discovered the pain relief when given intravenous ketamine as a procedural anesthetic. He started at an oral dose of 7.5 mg every 8 hours and at the time of death had a dose of 40 mg every 6 hours in combination with an intravenous hydromorphone patient-controlled analgesia pump and steroids.
The main focus of discussion will be the case of a 38-year-old Hispanic female with neurofibromatosis type 1 (NF1) with a history of chronic, severe pain in her fingertips secondary to multiple glomus tumors (small but painful benign NF1-associated tumors of the glomus body, a thermoregulatory shunt located in the fingertips) which evolved into CRPS, resistant to multiple therapies. The molecular pathogenesis and clinical course of this patient have been previously reported (participant “NF1-G10” in Brems et al48 and patient number “NIH-1” in Stewart et al49). Here, we focus on her response to intravenous and oral ketamine.
Briefly, despite 3 surgeries to resect multiple histologically proven glomus tumors from multiple fingers, the patient continued to have progressive, severe pain in her right and left hands. In addition to NF1, her medical history included migraine headaches as well as neck and low back pain of unclear etiology. She had inadequate relief of her hand pain from multiple analgesic therapies including steroid injections in the right wrist, anticonvulsants (gabapentin and pregabalin), topical analgesic (lidocaine), opiates (hydromorphone and oxycodone), and digital nerve blocks. Approximately, 11 months after the third surgery, she started methadone (2.5 mg/day) for severe neuropathic pain in her hands and was diagnosed with glomus tumor-associated CRPS. In support of this diagnosis, she had previously benefited from multiple stellate ganglion blocks, each of which provided 2 to 3 weeks of relief.
Given the intractability of CRPS in both hands, she was admitted to the intensive care unit of the NIH Clinical Center for a 3-day infusion of continuous low-dose ketamine. Magnetic resonance imaging of both hands was performed first to evaluate for tumor recurrence; there were no changes compared to a previous study on the left hand. A small enhancing lesion in the right fourth digit was suspicious for a glomus tumor; however, no surgery was performed since this digit was among the least painful. The tip of the left fourth digit was visibly swollen, particularly in comparison to the other digits. Table 1 lists the NRS and treatment provided during the 72-hour admission. Digital blocks with bupivacaine (0.25%, 0.5 mL) were administered to her left fourth digit only. No digital blocks were administered in the right hand or her painful right great toe so that these could serve as controls. Ketamine infusion was started at 0.1 mg/kg per h and continued for 72 consecutive hours. The patient was monitored for adverse reactions during the infusion; however, none were reported. She developed a migraine headache approximately halfway through the ketamine infusion period that was relieved with sumatriptan. Upon discharge, she was instructed to taper methadone as tolerated.
Table 1.
Pain Level (Quantified by the Numerical Rating Score), Methadone Requirement, and Frequency of Bupivacaine Digital Blocks Before and During Administration of Intravenous (Weeks 1 and 20) and Oral Ketamine (Week 42) in a 38-Year-Old Female With NF1 and CRPS Secondary to Glomus Tumorsa
| Intervention | Pretreatment | 0–24 Hours | 25–48 Hours | 49–72 Hours |
|---|---|---|---|---|
| Treatment—start of week 1 | ||||
| Bupivacaine blocks (left hand) | None | F4 | F4 | F4 |
| Bupivacaine blocks (right foot) | None | None | None | None |
| Methadone | 10 mg q8h | 10 mg q8h | 10 mg qd | 10 mg qd |
| Pain level | ||||
| Left hand | 10 | 6 | <5 | 3 |
| Right hand | 5 | ND | ND | <3 |
| Right toe | ND | ND | ND | ND |
| Treatment—start of week 20 | ||||
| None | F3, F4, F5 | F4, F5 | F4, F5 | |
| None | None | None | None | |
| 15 mg q12h | 5 mg q12h | 5 mg q12 h | 5 mg q12h | |
| Pain level | ||||
| 9 | 1 | 1 | 0 | |
| 5 | 1 | 1 | 0 | |
| 4 | ND | ND | 0 | |
| Treatment—start of week 42 | ||||
| None | F3, F4, F5 | F3, F4, F5 | F3, F4, F5 | |
| None | T1 | T1 | None | |
| 10 mg q8h | 10 mg q8hr | 10 mg q12h | 10 mg qd | |
| Pain level | ||||
| 7.5 | 0 | 0 | 0 | |
| 4.5 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | |
Abbreviations: F4, fourth digit (ring finger); T1, large toe; ND, no data; q, every.
Pain level prior to treatment represents maximal pain in any digit. Pain level during treatment represents level at the end of each time period.
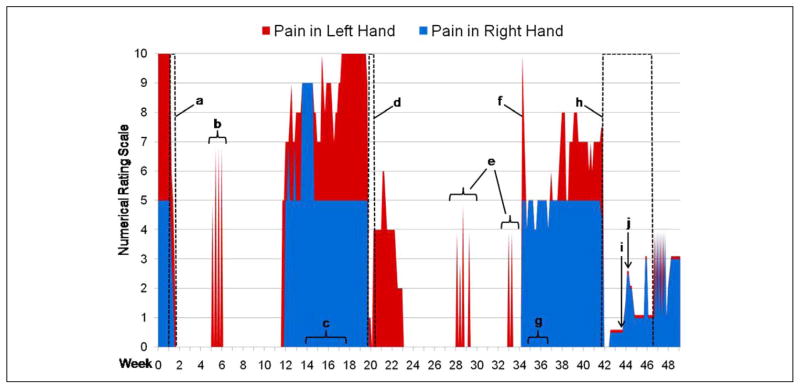
Within a week of discharge, the patient reported no pain in both hands at 0/10 (Figure 1). The allodynia and hyperalgesia improved dramatically. All the fingers of the patient’s (and large right toe) remained essentially pain free for 9 weeks, with the exception of 1 or 2 spasms per day of unclear etiology in her left third, fourth, and fifth digits, lasting a few seconds each, for a total of about 5 consecutive days. Notably, the patient’s frequent migraine headaches, neck pain, and low back pain also resolved during this 9-week period. She was able to discontinue methadone entirely. Subsequently, her pain returned, ultimately escalating to preketamine infusion levels over a period of approximately 6 weeks, again with the left hand more painful than the right. She again noted several episodes of numbness and paresthesias of both hands. Her right great toe pain, low back pain, and frequent migraine headaches also returned.
Figure 1.
Self-reported pain following infusion and oral ketamine. A, First ketamine infusion (0.1 mg/kg per h for 72 hours) with bupivacaine digital blocks as noted in text; methadone use was tapered and ultimately discontinued over the following week. B, Spasms of pain in left hand coincided with the unexpected death of a relative. Each spasm lasted a few seconds and occurred 2 to 3 times/d. C, New onset intermittent numbness in both hands of 2 to 3 minutes duration multiple times per day. D, Second ketamine infusion (0.1 mg/kg per h for 72 hours) with bupivacaine digital blocks as noted in text. E, Spasms of pain in left hand of similar duration as previously but unrelated to a plausible trigger. F, Patient described a day with the “worst ever pain, 30/10” prompting use of methadone for 3 days without significant relief. G, Intermittent numbness in both hands as described previously. H, First trial of oral ketamine (10 mg/d) with bupivacaine digital blocks as noted in text; methadone use was tapered. I, Methadone discontinued. J. Methadone resumed due to increase in pain.
Due to escalating symptoms, the patient returned to the NIH Clinical Center for a repeat ketamine infusion at week 20 (Figure 1). No changes were noted on a repeat MRI of the left hand. Prior to the second infusion of ketamine, the patient required 15 mg methadone every 12 hours and 8 mg hydromor-phone up to 3 times a day as needed. The pads of her left fourth and fifth digits were visibly swollen. Bupivacaine blocks (0.25%, 0.5 mL) were administered at the base of the left third, fourth, and fifth digits. The ketamine infusion was administered at 0.1 mg/kg per h. Bupivacaine digital blocks were repeated once on each of the next 2 days as before, for the left fourth and fifth digits only. At the end of 3 days, she reported a pain level of 0/10 in both hands and her right great toe (Figure 1 and Table 1). She denied any migraine headaches or low back pain. There were no ketamine-associated side effects.
As she returned home, she reported pain in her left third, fourth, and fifth digits. This lasted for 2 weeks and reached a maximum level of 6/10 before returning to 0/10. Her right hand was pain free during this time. Both hands then remained almost completely pain free for 11 weeks, except for 2 clusters of brief spasms in the third, fourth, and fifth fingers of both hands. These followed the same pattern as previously, occurring 1 or 2 times a day over a few consecutive days, with each spasm lasting only a few seconds. She continued to have migraine headaches but were reduced in frequency to a few times per month from a few times per week. The pain in the patient’s left hand then returned abruptly at a reported level of 10/10 for a brief period. She resumed taking methadone following this episode. For the next 7 weeks, she reported consistent pain in her left hand that oscillated between 4/10 and 8/10. The pain in her right hand was at a daily level of 4 to 5/10 over the same period of time. The patient also experienced recurrent pain in her right great toe. This occurred intermittently following the second ketamine infusion, lasting a week or more at a time at levels ranging from 3/10 to 7/10. Following the return of consistent pain in her fingers, she almost daily reported numbness in both hands, especially at nighttime.
She returned again to the NIH Clinical Center for a trial of oral ketamine and bupivacaine blocks. An MRI of the left hand showed only postsurgical changes. An MRI of the right foot did not show any lesions concerning for glomus tumor. Prior to treatment, the patient was taking 10 mg methadone every 8 hours. Immediately prior to initiation of the first bupivacaine digital blocks, the patient reported her pain at 7.5/10 in her left hand, 5/10 in her right toe, and 4.5/10 in her right hand (Table 1). Bupivacaine blocks were administered at the base of her left third, fourth, and fifth digits as previously; a block was also given at the base of the nail of her right toe with immediate relief. The intravenous ketamine solution was taken orally at an initial dose of 10 mg (10 mg/mL) every 8 hours. As previously, she was monitored for side effects, including hallucinations. By the morning following the initiation of therapy, the patient reported that the pain in both hands and her right big toe at 0/10. The patient developed a mild but persistent migraine headache shortly after admission for which sumatriptan did not provide adequate relief. She received right-hand side preauricular and masseter blocks with bupivacaine on the second and third day with relief of her headache. The pain in both hands and her right great toe continued to be at 0/10 for the remainder of her inpatient stay. On the last day of her inpatient stay, the patient had a panic attack. She denied a prior history of these. Following her return home, the patient reported the pain in both hands at 1/10 (Figure 1). She continued oral ketamine therapy at 10 mg every 12 hours. She continued to taper her methadone without experiencing an increase in her pain level until she stopped taking methadone completely a week and a half after her discharge. Over the next few days, however, she noted a gradual increase in pain in both hands. Once this reached a level of 2/10, she resumed methadone at 5 mg every 12 hours and gradually increased this to 10 mg every 8 hours. The pain in both hands gradually decreased to 1/10 over 3 or 4 days. About 5 weeks after her discharge, the patient had exhausted the supply of oral ketamine. Two days later, she began to experience spasms in the third, fourth, and fifth digits of both hands as before; each spasm lasted a few seconds. These spasms occurred for approximately 7 consecutive days before the pain then became constant at 3/10 in both hands. As of this writing, we were planning a return visit for her to NIH.
Summary and Recommendations
Despite the increasing use of oral ketamine in the palliative care setting, there are limited data regarding its efficacy in this patient population which make it more difficult to standardize this practice. Few randomized controlled trials have been published on efficacy and safety and the majority of the studies are based on anecdotal experience.
In patients with cancer pain, oral ketamine has been used as an adjuvant therapy in a wide variety of scenarios from patients with refractory pain syndromes with permanent intrathecal devices to patients at the end of their lives. Most of the studies have failed to demonstrate statistically significant pain relief. However, a study conducted by Lauretti et al18 did show a significant decrease in opiate consumption on the ketamine group when compared to oral morphine, which is consistent with the previously reported opiate-sparing effect. Other case studies have reported some benefit, with doses ranging from 25 mg for breakthrough pain in patients with intrathecal analgesic delivery systems, to 30 mg/d (in 3 divided doses), or 0.5 mg/kg every 12 hours.17–19 Based on the authors’ experience with cancer pain, effective analgesia may be achieved at doses of 80 mg/d (in 4 divided doses) in patients with cancer pain. In terms of the use of oral ketamine at the end of life, data published in several case studies20–23 have suggested a starting dose of 30 to 150 mg/d titrated up to 60 to 375 mg/d as final dose which falls within the range that was found to be effective in this setting (starting dose 22.5 mg/d, in 3 divided doses, final dose 160 mg/d in 4 divided doses). Differences in hepatic metabolism may account for the intraindividual variability. Interindividual differences have been also documented in patients with chronic orofacial pain as evidenced by lack of analgesic effect in patient with longer pain history.9
Studies of oral ketamine for neuropathic pain have shown mixed results. In case reports of patients with various neuropathic pain syndromes of central or peripheral etiologies (eg, plexopathy secondary to tumor compression, PHN, or peripheral neuropathy), oral ketamine seems to be most effective when used at an initial dose of 0.3 to 0.7 mg/kg per d, titrated up to every 6 hours.24–27 In several of these cases, intravenous ketamine (0.1–0.2 mg/kg) was given as a trial to assess for efficacy and response, and the patients were later transitioned to the oral route due to the short duration of the analgesic effects.
The authors’ experience using intravenous and oral ketamine in a patient with CRPS is consistent with the previously published literature. Following the administration of an intravenous ketamine infusion at a dose of 0.1 for 72 hours, we twice observed a significant reduction in the patient’s pain score (to 0/10) for up to 11 weeks, without the need of a loading or test dose. During these pain-free intervals, the patient was also able to discontinue methadone, illustrating the opiate-sparing properties of ketamine. We obtained similar results with the use of oral ketamine at a much lower conversion rate (5%–15% of the parenteral dose) that what has been previously reported.22,23 However, the pain-free interval was short-lived (only 2 weeks); a longer lasting analgesic effect may have been achieved had a conversion factor closer to 25% been used.
Intolerable adverse effects from oral ketamine are well documented in the literature and include sedation, lightheadedness, dizziness, tiredness, increased salivation, vivid dreams, dissociative feeling, hallucinations, nightmares, insomnia, among others. Such adverse effects have precluded an appropriate assessment of ketamine efficacy in several studies.33,34 Side effects are more commonly observed with intravenous rather than oral ketamine; this is likely due to the lower plasma concentrations obtained when using the latter route. Importantly, some studies have not reported any adverse effects with prolonged use of ketamine.28,29
No formal clinical guidelines for the administration of oral ketamine currently exist. Based on the review of literature and the authors’ institutional experience, the following steps are recommended when considering the addition of oral ketamine as adjuvant therapy for intractable neuropathic and/or cancer pain:
Optimize treatment with opiates and other adjuvant therapies (eg anticonvulsants, nonsteroidal anti-inflammatory drugs [NSAIDS]) to minimize side effects before starting ketamine.
Strongly recommend a trial with intravenous ketamine infusion before oral administration for a faster titration and monitoring of side effects. This should be ideally done in a monitor setting such as intensive or palliative care unit.
If the intravenous route if preferred then:
Start intravenous ketamine infusion at 0.1 mg/kg per h, titrate up by 0.1 mg/kg per h every 15 minutes as needed until adequate analgesia is achieved or side effects occur. Monitor vital signs (blood pressure, heart rate, and respiratory rate) every 15 minutes for the first hour, then every 30 minutes for the next 3 hours, and assess for pain and symptoms of dysphoria and excessive salivation.
Consider adding benzodiazepine (lorazepam or midazolam) on as needed basis for any signs of psychomimetic effects and/or antisialagogue therapy (glycopyrrolate and hyoscine) for increased secretions.
Continue intravenous infusion for at least 48 to 72 hours before oral administration.
Convert from intravenous to oral route using at least 15% of the total parenteral dose in up to 4 divided dose, having in consideration that the T1/2 of oral ketamine has been reported as 5.1 to 5.6 hours (eg, 70-kg patient, intravenous ketamine infusion 0.1 mg/kg per h = oral ketamine 20 mg every 12 hours).
After the intravenous infusion, reduce opiate by 25% daily, once adequate analgesia has been reached.
Titrate up by 0.3 mg/kg daily until adequate analgesia is achieved or side effects occur.
Patients in the palliative care setting, especially ones at the end of their lives, may also benefit from a trial of oral ketamine, even if an intravenous trial is not feasible. When using the oral route as a first option, monitoring for side effects with frequent pain assessments is recommended.
If the oral route is preferred then:
Start oral ketamine at doses as low as 0.3 to 0.5 mg/kg in up to 4 divided doses per day, titrate up by 0.3 mg/kg daily until adequate analgesia is achieved or side effects occur.
Future Directions
The best role for oral ketamine in the palliative care setting is uncertain. It remains, at best, a third- or fourth-line agent primarily as an opiate-sparing adjunct for patients with intractable cancer-related pain or neuropathy. As a prerequisite for more widespread clinical use, additional randomized controlled trials investigating efficacy and side effect profile of oral ketamine are needed. Such studies are predicated on the development of an oral formulation for outpatient use. Variations in clinical response and intolerable side effects, especially at higher doses, are obvious limiting factors. These are likely related to inter and intraindividual variability in drug metabolism and may be conducive to pharmacogenetic and pharmacogenomic prediction.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported in part by the Division of Intramural Research of the National Human Genome Research Institute (DRS and SLR) and the Division of Cancer Epidemiology and Genetics, National Cancer Institute (DRS).
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Mercadante S, Arcuri E, Tirelli W, Cascuccio A. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: a randomized, controlled, double blind, crossover, double-dose study. J Pain Symptom Manage. 2000;20(4):246–252. doi: 10.1016/s0885-3924(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 2.Eichenberger U, Neff F, Sveticic G, et al. Chronic phantom limb pain: the effects of calcitonin, ketamine, and their combination on pain and sensory thresholds. Anesth Analg. 2008;106(4):1265–1273. doi: 10.1213/ane.0b013e3181685014. [DOI] [PubMed] [Google Scholar]
- 3.Kiefer RT, Rohr P, Ploppa A, et al. Efficacy of ketamine in anesthetic dosage for the treatment of refractory complex regional pain syndrome: an open-label phase II study. Pain Med. 2008;9(8):1173–1201. doi: 10.1111/j.1526-4637.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- 4.Rabben T, Skjelbred P, Oye I. Prolonged analgesic effect of ketamine, an N-Methyl-D aspartate receptor inhibitor, in patients with chronic pain. J Pharmacol Exp Ther. 1998;289(2):1060–1066. [PubMed] [Google Scholar]
- 5.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11(3):327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 6.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–62. [PubMed] [Google Scholar]
- 7.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7(2):39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Lodge D, Johnson KM. Noncompetitive excitatory amino acid receptor antagonists. Trends Pharmacol Sci. 1990;11(2):81–86. doi: 10.1016/0165-6147(90)90323-z. [DOI] [PubMed] [Google Scholar]
- 9.Rabben T, Oye I. Interindividual differences in the analgesic response to ketamine in chronic orofacial pain. Eur J Pain. 2001;5(3):233–240. doi: 10.1053/eujp.2001.0232. [DOI] [PubMed] [Google Scholar]
- 10.Chong C, Schug SA, Page-Sharp M, Jenkins B, Ilett KF. Development of a sublingual/oral formulation of ketamine for use in neuropathic pain: preliminary findings from a three-way randomized, crossover study. Clin Drug Invest. 2009;29(5):317–324. doi: 10.2165/00044011-200929050-00004. [DOI] [PubMed] [Google Scholar]
- 11.Grant IS, Nimmo WS, Clements JA. Pharmacokinetics and analgesic effects of IM and oral ketamine. Br J Anaesth. 1981;53(5):805–810. doi: 10.1093/bja/53.8.805. [DOI] [PubMed] [Google Scholar]
- 12.Yanagihara Y, Ohtani M, Kariya S, et al. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Bio-pharm Drug Dispos. 2003;24(1):37–43. doi: 10.1002/bdd.336. [DOI] [PubMed] [Google Scholar]
- 13.Hagelberg NM, Peltoniemi MA, Saari TI, et al. Clarithromycin, a potent inhibitor of CYP3A, greatly increases exposure to oral S-ketamine. Eur J Pain. 2010;14(6):625–629. doi: 10.1016/j.ejpain.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Blonk MI, Koder BG, Van Den Bemt PM, Huygen FJ. Use of oral ketamine in chronic pain management: a review. Eur J Pain. 2010;14(5):466–472. doi: 10.1016/j.ejpain.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Storr T, Quibell R. Can ketamine prescribed for pain cause damage to the urinary tract? Palliat Med. 2009;23(7):670–672. doi: 10.1177/0269216309106828. [DOI] [PubMed] [Google Scholar]
- 16.Grégoire MC, MacLellan DL, Finley GA. A pediatric case of ketamine-associated cystitis. Urology. 2008;71(6):1232–1233. doi: 10.1016/j.urology.2007.11.141. [DOI] [PubMed] [Google Scholar]
- 17.Mercadante S, Arcuri E, Ferrera P, Villari P, Mangione S. Alternative treatments of breakthrough pain in patients receiving spinal analgesics for cancer pain. J Pain Symptom Manage. 2005;30(5):485–491. doi: 10.1016/j.jpainsymman.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Lauretti G, Lima I, Reis M, Prado W, Pereira N. Oral ketamine and transdermal nitroglycerin as analgesic adjuvants to oral morphine therapy for cancer pain management. Anesthesiology. 1999;90(6):1528–1533. doi: 10.1097/00000542-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Ishizuka P, Santos-Garcia JB, Kimito-Sakata R, Machado-Issy A, Mulich SL. Assessment of oral S(+) ketamine associated with morphine for the treatment of oncologic pain. Rev Bras Aneste-siol. 2007;57(1):19–31. doi: 10.1590/s0034-70942007000100003. [DOI] [PubMed] [Google Scholar]
- 20.Kotlińska-Lemieszek A. Subanesthetic ketamine: an essential adjuvant for intractable cancer pain. J Pain Symptom Manage. 2004;28(2):100–101. doi: 10.1016/j.jpainsymman.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Williams M. Ketamine for cancer pain. J Pain Symptom Manage. 2000;19(2):79–80. doi: 10.1016/s0885-3924(99)00142-6. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgibbon EJ, Hall P, Schroder C, Seely J, Viola R. Low dose ketamine as an analgesic adjuvant in difficult pain syndromes: a strategy for conversion from parenteral to oral ketamine. J Pain Symptom Manage. 2002;23(2):165–170. doi: 10.1016/s0885-3924(01)00393-1. [DOI] [PubMed] [Google Scholar]
- 23.Benitez-Rosario MA, Feria M, Salinas-Martin A, Martinez-Castillo LP, Martin-Ortega JJ. A retrospective comparison of the dose ratio between subcutaneous and oral ketamine. J Pain Symptom Manage. 2003;25(5):400–402. doi: 10.1016/s0885-3924(03)00072-1. [DOI] [PubMed] [Google Scholar]
- 24.Villanueva-Perez VL, Cerda-Olmedo G, Asensio-Samper JM, et al. Oral ketamine for the treatment of type I complex regional pain syndrome. Pain Pract. 2007;7(1):39–43. doi: 10.1111/j.1533-2500.2007.00109.x. [DOI] [PubMed] [Google Scholar]
- 25.Sakai T, Tomiyasu S, Ono T, Yamada H, Sumikawa K. Multiple sclerosis with severe pain and allodynia alleviated by oral ketamine. Clin J Pain. 2004;20(5):375–376. doi: 10.1097/00002508-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Vick PG, Lamer TJ. Treatment of central post-stroke pain oral ketamine. Pain. 2001;92(1–2):311–313. doi: 10.1016/s0304-3959(00)00488-7. [DOI] [PubMed] [Google Scholar]
- 27.Nikolajsen L, Hansen PO, Jensen TS. Oral ketamine therapy in the treatment of postamputation stump pain. Acta Anaesthesiol Scand. 1997;41(3):427–429. doi: 10.1111/j.1399-6576.1997.tb04710.x. [DOI] [PubMed] [Google Scholar]
- 28.Furuhashi-Yonaha A, Iida H, Asano T, et al. Short- and long-term efficacy of oral ketamine in eight chronic pain patients. Can J Anaesth. 2002;49(8):886–887. doi: 10.1007/BF03017431. [DOI] [PubMed] [Google Scholar]
- 29.Kannan TR, Saxena A, Bhatnagar S, Barry A. Oral ketamine as an adjuvant to oral morphine for neuropathic pain in cancer patients. J Pain Symptom Manage. 2002;23(1):60–65. doi: 10.1016/s0885-3924(01)00373-6. [DOI] [PubMed] [Google Scholar]
- 30.Kubota T, Miyata A. Successful use of ketamine for intractable burning pain of HTLV-1-associated myelopathy. J Pain Symptom Manage. 2005;30(5):397–399. doi: 10.1016/j.jpainsymman.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Okon T. Ketamine: an introduction for the pain and palliative medicine physician. Pain Physician. 2007;10(3):493–500. [PubMed] [Google Scholar]
- 32.Hoffmann V, Coppejans H, Vercauteren M, Adriaensen H. Successful treatment of postherpetic neuralgia with oral ketamine. Clin J Pain. 1994;10(3):240–242. doi: 10.1097/00002508-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Haines DR, Gaines SP. N of 1 randomised controlled trials of oral ketamine in patients with chronic pain. Pain. 1999;83(2):283–287. doi: 10.1016/s0304-3959(99)00117-7. [DOI] [PubMed] [Google Scholar]
- 34.Enarson MC, Hays H, Woodroffe MA. Clinical experience with oral ketamine. J Pain Symptom Manage. 1999;17(5):384–386. doi: 10.1016/s0885-3924(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 35.Gordon DB, Sehgal N, Schroeder ME, Cleary J. Treatment of pain crisis at end of life from severe lower extremity venous outflow obstruction with hyperalgesia and allodynia. J Pain. 2002;3(3):244–248. doi: 10.1054/jpai.2002.122948. [DOI] [PubMed] [Google Scholar]
- 36.Tobias JD, Phipps S, Smith B, Mulhern RK. Oral ketamine pre-medication to alleviate the distress of invasive procedures in pediatric oncology patients. Pediatrics. 1992;90(4):537–541. [PubMed] [Google Scholar]
- 37.Ozdemir D, Kayserili E, Arslanoglu S, Gulez P, Vergin C. Ketamine and midazolam for invasive procedures in children with malignancy: a comparison of routes of intravenous, oral, and rectal administration. J Trop Pediatr. 2004;50(4):224–228. doi: 10.1093/tropej/50.4.224. [DOI] [PubMed] [Google Scholar]
- 38.Gutstein HB, Johnson KL, Heard MB, Gregory GA. Oral ketamine preanesthetic medication in children. Anesthesiology. 1992;76(1):28–33. doi: 10.1097/00000542-199201000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Tammiga RY, Noordhoek M, Kroon J, Faber-Nijholt R. Ketamine anesthesia with or without diazepam premedication for bone marrow punctures in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2000;17(5):383–388. doi: 10.1080/08880010050034319. [DOI] [PubMed] [Google Scholar]
- 40.Pellier I, Monrigal JP, Le Moine P, et al. Use of intravenous ketamine-midazolam association for pain procedures in children with cancer: a prospective study. Paediatr Anaesth. 1999;9(1):61–68. [PubMed] [Google Scholar]
- 41.Tsui BC, Davies D, Desai S, Malherbe S. Intravenous ketamine infusion as an adjuvant to morphine in a 2-year-old with severe cancer pain from metastatic neuroblastoma. J Pediatr Hematol Oncol. 2004;26(10):678–680. [PubMed] [Google Scholar]
- 42.Anghelescu DL. Ketamine use for radiation of opioid tolerance in a 5-year-old girl with end-stage abdominal neuroblastoma. J Pain Symptom Manage. 2005;30(1):1–3. doi: 10.1016/j.jpainsymman.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Conway M, White N, St Jean C, Zempsky WT, Steven K. Use of continuous intravenous ketamine for end-stage cancer pain in children. J Pediatr Oncol Nurs. 2009;26(2):100–106. doi: 10.1177/1043454208328768. [DOI] [PubMed] [Google Scholar]
- 44.Klepstad P, Borchgrevink P, Hval B, Fleet S, Kaasa S. Long term treatment with ketamine in a 12-year-old girl with severe neuropathic pain caused by a cervical spinal tumor. J Pediatr Hematol Oncol. 2001;23(9):616–619. doi: 10.1097/00043426-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Fine PG. Low-dose ketamine in the management of opioid nonresponsive terminal cancer pain. J Pain Symptom Manage. 1999;17(4):296–300. doi: 10.1016/s0885-3924(98)00144-4. [DOI] [PubMed] [Google Scholar]
- 46.Finkel JC, Pestieau SR, Quezado ZM. Ketamine as an adjuvant for treatment of cancer pain in children and adolescents. J Pain. 2007;8(6):515–521. doi: 10.1016/j.jpain.2007.02.429. [DOI] [PubMed] [Google Scholar]
- 47.Ugur F, Gulcu N, Boyaci A. Oral ketamine for pain relief in a child with abdominal malignancy. Pain Med. 2009;10(1):120–121. doi: 10.1111/j.1526-4637.2008.00424.x. [DOI] [PubMed] [Google Scholar]
- 48.Brems H, Park C, Maertens O, et al. Glomus tumors in neurofibromatosis type 1: genetic, functional and clinical evidence of a novel association. Cancer Res. 2009;69(18):7393–7401. doi: 10.1158/0008-5472.CAN-09-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart DR, Sloan JL, Yao L, et al. Diagnosis, management, and complications of glomus tumors of the fingers in neurofibromatosis type 1. J Med Genet. 2010;47(8):525–532. doi: 10.1136/jmg.2009.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]