Abstract
The vertebrate olfactory epithelium (OE) is known for its ability to renew itself throughout life as well as to reconstitute after injury. Although this remarkable capacity demonstrates the persistence of stem cells and multipotent progenitor cells, their nature in the OE remains undefined and controversial, as both horizontal basal cells (HBCs) and globose basal cells (GBCs) have features in common with each other and with stem cells in other tissues. Here, we investigate whether some among the population of GBCs satisfy a key feature of stem cells, i.e., mitotic quiescence with retention of thymidine analogue label and activation by injury. Accordingly, we demonstrate that some GBCs express p27Kip1, a member of the Kip/Cip family of cyclin-dependent kinase inhibitors. In addition, some GBCs retain bromodeoxyuridine or ethynyldeoxyuridine for an extended period when the pulse is administered in neonates followed by a 1-month chase. Their identity as GBCs was confirmed by electron microscopy. All spared GBCs express Ki-67 in the methyl bromide (MeBr)-lesioned OE initially after lesion, indicating that the label-retaining (LR) GBCs are activated in response to injury. LR-GBCs reappear during the acute recovery period following MeBr exposure, as demonstrated with 2- or 4-week chase periods after labeling. Taken together, our data demonstrate the existence of LR-GBCs that are seemingly activated in response to epithelial injury and then re-established after the initial phase of recovery is completed. In this regard, some among the GBCs satisfy a common criterion for functioning like stem cells.
Indexing Terms: label-retaining cells, mitotic quiescence, tissue stem cells, neuronal progenitors
The vertebrate olfactory epithelium (OE) sustains neurogenesis throughout life. Proliferation, differentiation, and death of olfactory sensory neurons (OSNs) occur continuously, even in adult animals. In addition, the OE retains the capacity for robust anatomic and functional recovery after experimental or natural injury (Graziadei and Monti Graziadei, 1979; Monti Graziadei and Graziadei, 1979; Schwartz Levey et al., 1991; Schwob et al., 1995). By analogy to other regenerating tissues, such as the hematopoietic system (Heimfeld and Weissman, 1991) and the epidermis (Morris and Potten, 1994), it is generally accepted that neurocompetent stem cells persist in the OE that proliferate and differentiate into OSNs and the other epithelial cell types after injury. Several lines of evidence from study of rodent epithelium have demonstrated that the globose basal cell (GBC) population contains neuronal precursors that are capable of giving rise to OSNs during piecemeal neuronal turnover in the undamaged OE or wholesale neuronal replacement in response to injury to the olfactory bulb or nerve (Caggiano et al., 1994; Monti Graziadei and Graziadei, 1979; Schwartz Levey et al., 1991; Schwob et al., 1994). However, the potency of GBCs in the adult OE extends beyond the neuronal lineage. GBCs give rise to both neurons and all varieties of non-neuronal cells during the recovery of the OE following injury caused by exposure to the olfactotoxin methyl bromide (MeBr), as shown by lineage studies (Huard et al., 1998), immunohistochemical analysis in the OE (Goldstein and Schwob, 1996; Jang et al., 2007), and the outcome following transplantation of the various cell types of the neurogenic epithelium (Goldstein et al., 1998; Chen et al., 2004).
Numerous in vivo and in vitro studies imply that GBCs in the adult OE are a heterogeneous population that encompasses GBCs that make neurons (immediate neuronal progenitors), GBCs that are ostensibly committed to making neurons (transit-amplifying precursors), and still others that are almost certainly multipotent (multipotent precursors). The identification and classification of these multiple types is based on the pattern of expression of members of the basic helix–loop–helix (bHLH) and other transcription factor families in epithelial development and during epithelial regeneration (DeHamer et al., 1994; Gordon et al., 1995a; Cau et al., 1997, 2002; Manglapus et al., 2004, Guo et al., 2010; Packard et al., 2011a), gene knockout and epistasis experiments during development (Guillemot et al., 1993; Cau et al., 1997, 2002), lineage tracing experiments (Huard et al., 1998, Leung et al., 2007, Packard et al., 2011a), labeling with monoclonal antibodies that mark GBCs (GBC-1, GBC-2, and GBC-3) in neurogenic and regenerating epithelium (Goldstein and Schwob, 1996; Goldstein et al., 1997; Jang et al., 2007), and the dependence of epithelial regeneration on the persistence/generation of GBCs (Jang et al., 2003). As a result of these analyses, one can present a tentative hierarchy of functional types within the population of GBCs defined by gene expression as follows: Sox2/Pax6 → Ascl1(Mash1) → Neurog1→ NeuroD1 proceeding from multipotent to transit-amplifying to early immediate neuronal progenitor to late neuronal progenitor types of GBC, respectively.
However, the number and identity of the tissue stem cell types in the OE remain controversial. In addition to the evidence of GBC multipotency, recent fate-mapping studies using transgenic mice suggest that HBCs also act as multipotent progenitors limited, for the most part, to OE that is recovering from direct, severe injury (Iwai et al., 2008; Leung et al., 2007). In the absence of injury, HBCs tend to be quiescent, which is an item, in addition to their multipotency in the face of injury, cited in favor of the HBC-as-stem-cell hypothesis (Leung et al., 2007; Duggan and Ngai, 2007; Fletcher et al., 2011). However, some aspects of HBC biology indicate that it is too simplistic to conclude that HBCs are “the” stem cells of the OE. First, HBCs are late to emerge during the development of the OE (becoming fully established as a population around the end of the first postnatal week in mice and rats) and develop from cells that share some characteristics of GBCs, including the expression of GBC-typical bHLH transcription factors (Holbrook et al., 1995; Packard et al., 2011b). Second, aborting the differentiation of HBCs by eliminating the expression of the transcription factor ΔNp63 does not prevent the normal or near-normal development of the other cell types of the OE (Packard et al., 2011b). Third, HBCs require activation in situ in order to function as neuropotent progenitors following transplantation into the MeBr-lesioned OE (manuscript in preparation, Schnittke, Herrick, Packard, and Schwob, 2013). Fourth, HBCs apparently remain quiescent and do not function as neurocompetent stem cells in the OE of mouse and human in which neurogenesis has become exhausted (Largent et al., 1993; Holbrook et al., 2005, 2011). Fifth, HBCs contribute to the metaplastic respiratory epithelium that replaces olfactory epithelium lining the dorsal recess of the nose following injection of dichlobenil without making neurons (Xie et al., 2013). The convergence of these data suggests that HBCs may serve as “a” reserve stem cell population within the adult OE that is activated in response to direct injury to the epithelium and directed to regenerate olfactory epithelium only in response to particular signals.
In that regard, the OE resembles several other types of tissue in which multiple putative stem cell candidates have been identified. Among these tissues are the gut, the respiratory epithelium, the skin, the cornea, and even the subventricular zone of the adult central nervous system (CNS) (Cotsarelis et al., 1989; Johansson et al., 1999; Doetsch et al., 2002a; Rawlins et al., 2009; Blanplain and Fuchs, 2009; Rock et al., 2010; Tian et al., 2011). In these other settings, one or more categories of putative stem cells are distinguished by slow mitotic cycling and its operational counterpart—the retention of thymidine analogue label for an extended period. Despite their tremendous potential for cell division, such reserve stem cells normally remain quiescent or cycle slowly, presumably to preserve their proliferative potential and minimize DNA errors occurring during replication (Lajtha, 1983). Indeed, the feature of thymidine label retention has been used to identify candidate stem cells in multiple tissues, several of which have subsequently proven to be true tissue stem cells (Cotsarelis et al, 1990; Tian et al., 2011; Terskikh et al., 2012).
Accordingly, we have undertaken a further examination of the cell cycle characteristics of the basal cells of the OE, with particular reference to the GBCs. We have focused on the expression of a cyclin-dependent kinase inhibitor (CDKI), p27Kip1, within the normal and MeBr-lesioned/recovering OE and compared it with Ki-67, a marker that is characteristic of cells that are progressing through the mitotic cycle and that more completely identifies the population that is progressing within the cell cycle (Gerdes et al., 1984). Among the CDKIs, p27Kip1 appears to be a central mediator of many anti-mitogenic signals and to be closely involved in the imposition and maintenance of the quiescent state in a variety of tissues and of conditions (Sherr, 2000; Slingerland and Pagano, 2000). The levels and stability of p27Kip1 are high in quiescent cells (G0) and, in most eukaryotic cells, fall during G1 to a nadir in S-phase in response to mitotic stimulation (Pagano et al., 1995; Rivard et al., 1996). In addition, elevated levels of p27Kip1 enforce the maintenance of the quiescent state, whereas downregulation of p27Kip1 allows cells to recommence proliferation (Ladha et al., 1998; Rivard et al., 1996). In normal fibroblasts, increased expression of p27Kip1 mediates G1 arrest by contact inhibition, and it is necessary for the maintenance of cell quiescence (Rivard et al., 1996; Sherr and Roberts, 1995). In addition, we have searched for GBCs that retain thymidine-analogue label following multiple injections in the neonate or in the OE during the initial stages of recovery following MeBr lesion. Both approaches have indicated that some among the GBCs are quiescent and label-retaining, thus satisfying a common criterion for identification of tissue stem cells (Morris and Potten, 1994).
Materials and Methods
Animals
Outbred male Sprague-Dawley rats were obtained from a barrier isolated facility (Taconic Farm, Germantown, NY) and housed in a temperature and humidity controlled environment. All animals were maintained on a 12-hour light/dark cycle with ad libitum access to food and water. Rats exposed to MeBr weighed between 225 and 275 g. Postnatal 3-day-old rats were used for long-term bromodeoxyuridine (BrdU) (or ethynyldeoxyuridine [EdU]) labeling. All procedures using vertebrate animals were approved by the Institutional Animal Care and Use Committee at Tufts University School of Medicine.
MeBr lesion
Conscious rats were placed in a wire enclosure centered in a plexiglass box and exposed to MeBr gas (Matheson Gas Products, East Rutherford, NJ) for 6 hours at concentration of 330 ppm in a purified air at a low rate of 10 L/min. After lesion, the animals were kept on the same feeding schedule as before and perfused after 2 days.
Tissue processing
Rats were injected intraperitoneally with a triple anesthetic cocktail consisting of ketamine/xylazine/acepromazine (25 μg/g, 5 μg/g, 0.7 μg/g, respectively) and, after induction of deep anesthesia, were euthanized by intracardiac flush with phosphate-buffered saline (PBS; pH 7.2) followed by perfusion with Zamboni's fixative (for MeBr-lesioned rats), Carnoy's fixative (for BrdU-injected rats), or 4% paraformaldehyde (for EdU-injected rats). The OE was then decalcified with a saturated solution of ethylenediaminetetraacetic acid (EDTA; Sigma [St. Louis, MO] for Zamboni's-fixed tissue) or formic citrate (for Carnoy's fixed tissue) and cryoprotected with 30% sucrose before cryosectioning in the coronal plane at 5–8 μm. For whole-mount analysis, only the septum was stained.
Antibody characterization
Primary antibodies that were used in this study are shown in Table 1. Information on the antibodies is derived from the manufacturers' descriptions and our own data.
Table 1. Antibodies Used in This Study.
| Primary antibody | Immunogen and preparation | Source, species, clonality, and cat. no. | Dilution |
|---|---|---|---|
| BrdU | Bromodeoxyuridine (BrdU) | Harlan Sera-Lab, rat monoclonal, cat. # BU1/75 (ICR1) | 1:200 |
| Ki-67 | Recombinant protein corresponding to a 1,086-bp Ki67 motif-containing cDNA fragment | Novocastra, rabbit polyclonal, cat. # NCL-Ki67p | 1:200 |
| Ki-67 | 22-amino acid Ki-67 repeat motif (APKEKAQPLEDLASFQELSQ) | BD-Biosciences, mouse monoclonal, cat. # 556003 (clone B56) | 1:150 |
| p27Kip1 | Recombinant-full length mouse p27KIP1 (amino acids 1–197) | BD-Biosciences, mouse monoclonal, cat. # 610241 (clone 57) | 1:10,000 (TSA); 1:300 (FITC enhancement) |
| CK5/6 | Purified cytokeratin 5 | Millipore, mouse monoclonal, cat. # MAB1620 (clone D5/16B4) | 1:20 |
| CK14 | Synthetic peptide of the extreme C-terminal of human keratin 14 (last 15 amino acids) conjugated to thyroglobulin | Novocastra, mouse monoclonal, cat. # NCL-LL002 | 1:5 |
| CK14 | A synthetic peptide of 15 amino acid residues from the C-terminus of human keratin 14 | LabVision, rabbit polyclonal, cat. # RB-9020-P1 | 1:50 (AMCA) |
| CK18 | A synthetic peptide corresponding to residues on the C-terminus of human cytokeratin 18 | Abcam, rabbit polyclonal, cat. # 52948 | 1:200 |
| FITC | Fluorescein isothiocyanate (FITC)-conjugated keyhole limpet hemocyanin (KLH) | Jackson ImmunoResearch, mouse monoclonal, cat. # 200-002-037 | 1:100 |
| PGP9.5 | Human PGP9.5 protein purified from pathogen-free human brain | Cedarlane/Ultraclone, rabbit poly-clonal, cat. # 31A3 | 1:200 (AMCA) |
| Tuj-1 | Microtubules derived from rat brain | Covance, mouse monoclonal, cat. # MMS-435P (TuJ1 | 1:100 |
| NCAM | Affinity-purified neural cell adhesion molecule (NCAM) from embryonic chicken brain | Dr. J. Covault (Department of Psychiatry, University of Connecticut Health Center), rabbit polyclonal | 1:250 |
The Ki67 antiserum reacts with a 345/395-kDa protein doublet on western blots. In addition, binding to cells is blocked by the canonical anti-Ki67 monoclonal antibody MIB 1 (BD Biosciences [San Diego, CA] datasheet, 556003). The staining pattern of the Ki67 antibody in the OE has been described previously by our laboratory (Guo et al., 2010).
The p27KIP1 monoclonal antibody detects a band of 27 kDa on western blot (BD Biosciences datasheet, 610241). The staining pattern of the anti-p27KIP1 antibody in the OE has been described previously by our laboratory (Guo et al., 2010).
The fluorescein isothiocyanate (FITC) monoclonal antibody was shown to produce a dose-dependent quenching of FITC in a soluble assay as well as specific reactivity to FITC and dichlorotriazinylamino fluorescein (DTAF) but not tetramethylrhodamine isothiocyanate (TRITC; Jackson ImmunoResearch, West Grove, PA, personal communication). Its use for enhancing the FITC signal including immunohistochemical protocols has been shown by our laboratory (Guo et al., 2010).
Both monoclonal antibodies against CK5/6 and CK14 have been shown to stain the horizontal basal cells (Holbrook et al., 1995), and recognize multiple isoelectric variants of 54.5 kDa (anti-CK5/6) and a single spot of 53 kDa (anti-CK14) on western blots of two-dimensional electrophoresis (Holbrook et al., 1995).
The CK18 rabbit antiserum recognizes a 48-kDa band on western blots (Abcam [Cambridge, MA] datasheet, ab52948), and its staining on sustentacular cells and Bowman's gland and duct cells is identical to that of mouse CK18 monoclonal antibody (RGE53), which has been previously shown by our laboratory (Schwob et al., 1995).
The protein gene product (PGP)9.5 antiserum recognizes a single 27-kDa band on western blots of mouse brain. The subcellular labeling for PGP9.5 is cytoplasmic and restricted to neuronal populations in mice, including within the vomeronasal organ and olfactory epithelium (Guo et al., 2010).
The neural cell adhesion molecule (NCAM) antiserum recognizes all three modified forms of the protein, derived from rat brain, corresponding to their 120, 140, and 180 kDa molecular weights by western blots. NCAM antiserum has been shown previously to stain the entire neuronal population (both immature and mature receptor neurons) (Jang et al., 2007).
The Tuj1 monoclonal antibody is well characterized and has been shown to be highly reactive to neuron-specific class III β-tubulin (βIII) and not the β-tubulin found in glial cells. Its staining on the immature olfactory neurons has been described previously (Schwob et al., 1995).
Immunohistochemistry
Immunostaining was performed according to published procedures (Schwob et al., 1995; Guo et al., 2010; Packard et al., 2011a,b). Briefly, sections were blocked in PBS containing 10% normal serum, 4% bovine serum albumin (BSA; Sigma), 0.1% Triton X-100 (Sigma), and 5% nonfat dry milk for 30 minutes at room temperature. Sections were subsequently incubated with the primary antibody diluted in blocking solution for 1 hour at room temperature or overnight at 4° C. After washing, secondary antibody conjugated to fluorescein (FITC), Texas Red, 7-amino-4-methyl-coumarin-3-acetic acid (AMCA; Jackson ImmunoResearch), or Alexa 488 or Alexa 597 (Invitrogen, Carlsbad, CA) was applied for 1 hour at room temperature. Sections to be stained with anti-Ki67, anti-p27Kip1, or anti-BrdU were puddled with 0.01 M citric acid (pH 6.0) before being heated in a commercial food steamer for 10 minutes. Visualization of the anti-p27Kip1 staining was performed by using a tyramide signal amplification (TSA) kit (NEN Life Science Products, Boston, MA) or anti-FITC antibody (Jackson ImmunoResearch) for amplifying the signal (Guo et al., 2010). Visualization of incorporated EdU was performed according to the manufacturer's protocol (Invitrogen) using Alexa 594 azide (Invitrogen).
Processing of tissue whole mounts differed in detail from the staining of sections. After fixation and pretreatment (with 6 N HCl and dehydration–rehydration in alcohols in the case of BrdU, or only the latter in the case of Ki-67), the mucosa was stripped from the nasal septum and subsequent steps were carried out on the free-floating tissue. The duration of the incubations was lengthened considerably to accommodate the need for tissue penetration: 1 day in block, 7 days in primary, 1 day each in secondary antibody and the avidin-biotin-horseradish peroxidase complex, with extended washes between steps.
Sections were imaged with a Nikon 800E microscope equipped with B-2E, G-1A, UV-1A (Nikon), and dual FITC/Texas Red filters (Omega Optical, Brattleboro, VT) and photographed by using a digital SPOT camera (Diagnostic Instruments, Sterling Heights, MI). Photographic images were adjusted for intensity, contrast, and evenness of illumination in the Photoshop component of Adobe (San Jose, Ca) Creative Suite. Figures 5 and 6 were also filtered by using the “unsharp mask” command to render them crisper and more aesthetically pleasing. High-resolution images of the diaminobenzidine (DAB)-stained whole mounts were acquired as individual contiguous tiles by using a computer-controlled stage, and the tiles were then stitched into a mosaic. Individual stained cells and groups of cells were highlighted by density segmentation of the resulting mosaic image using IPLab Spectrum image processing software. The segmented objects were expanded and pseudo-colored to render individual cells visible at the lower resolution afforded by The Journal of Comparative Neurology.
Figure 5.
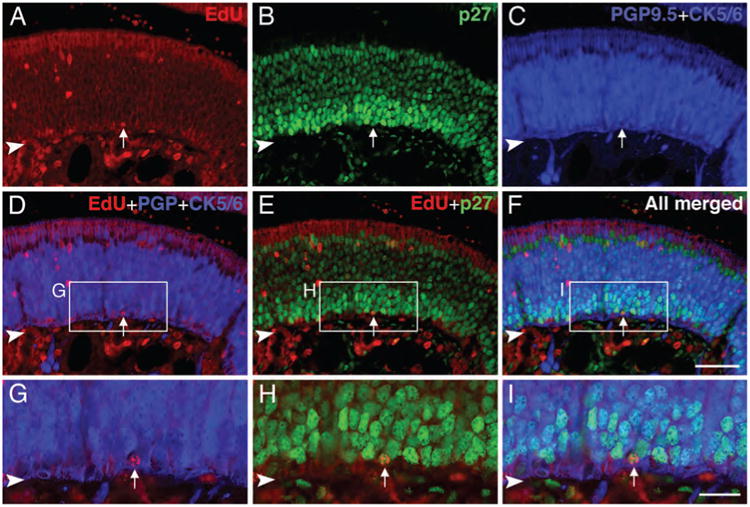
Some label-retaining cells in the normal OE are p27Kip1 (+) GBCs and presumably quiescent. Sections of the OE from rats that were injected with EdU daily for 3 consecutive days beginning on postnatal day 3 and euthanized 4 weeks after the last dose were reacted with Alexa Fluor 594-azide (red) and stained with anti-p27Kip1 (green), anti-PGP 9.5 (blue), and anti-CK5/6 (blue). A–F: EdU [+], p27Kip1 [+] GBCs (PGP9.5 [−]/CK5/6 [−]) (arrows) are found in the OE, and these label-retaining GBCs are presumed to be quiescent. G–I: Higher magnification of boxed area in D–F. Arrowhead indicates basal lamina. Magenta–green–blue versions of the photomicrographs are available online as Supplemental Figure S5. Scale bar = 50 μm in F (also applies to A–E); 20 μm in I (also applies to G–H).
Figure 6.
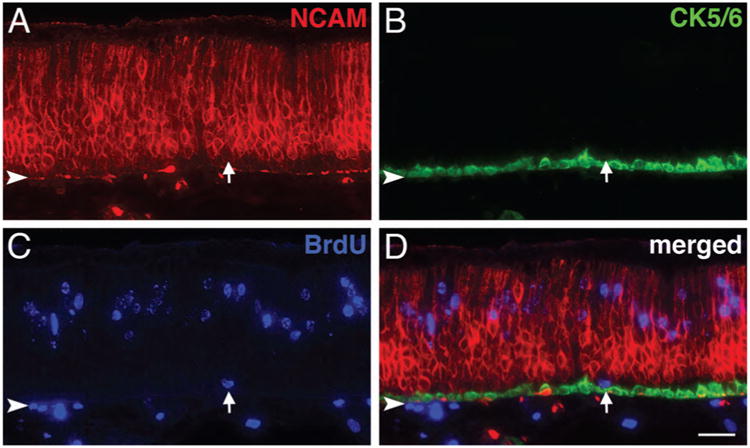
Label-retaining GBCs re-emerge after MeBr lesion. A–D: Sections of the OE from MeBr-lesioned rats that were injected with BrdU daily for 3 consecutive days beginning 3 days after exposure and euthanized 2 weeks after the last dose of BrdU were stained with anti-NCAM (red in A), anti-CK5/6 (green in B), and anti-BrdU (blue in C). The merged image (in D) clearly shows a label-retaining GBC that is positioned between the NCAM (+) neurons and the CK5/6 (+) HBCs (arrow); label-retention indicates onset of quiescence or slow cycling after a few days of recovery from damage and subsequent to the universal activation shown by general Ki-67 labeling in the immediate postlesion period (see Fig. 3). Arrowhead indicates basal lamina. Magenta–green–blue versions of the photomicrographs are available online as Supplemental Figure S6. Scale bar = 25 μm in D (also applies to A–C).
Detection of acutely proliferating cells
Rats intended for the detection of proliferating cells in the OE were injected intravenously with BrdU (Sigma-Aldrich, St. Louis, MO) or EdU (Invitrogen) at a single dose of 100 mg/kg, and euthanized 1 hour later by perfusion with fixative.
Counts of quiescent versus dividing GBCs
Cells near the base of the epithelium labeled with p27Kip1 but lacking CK5/6, Tuj-1, and Ki-67 labeling were considered quiescent GBCs, cells with Ki-67 labeling but lacking CK5/6, Tuj-1, and p27Kip1 labeling were considered dividing GBCs, and GBCs labeled with both Ki-67 and p27 were identified. Profiles of the nuclei of quiescent, dividing, and Ki-67/p27 double-labeled GBCs were counted in OE harvested from normal, 2 days, and 7 days post MeBr-lesioned, 4 days and 10 days post bulbectomized animals (n = 3 for each of the five groups). Two sections taken at each of three levels— anterior, middle, and posterior—of the OE from each animal were used for analysis. On each section, profiles of the nuclei of quiescent and dividing GBCs were manually counted in two adjacent areas (total 570 μm) from dorsal, middle, and ventral parts along the septum. Raw data were expressed as the number of positive profiles/mm of OE.
Measurements of the greatest diameter of the labeled nuclei for each category of cell for each condition (normal, 4 or 10 days post OB ablation, 2 or 7 days post MeBr exposure) were made on 60× micrographs of a single field from four to seven sections. Nuclear profiles were measured and counted only where the outlines of the nucleus and of the cell soma were also clearly visible, which had the practical effect of eliminating debris or fragmented cells measuring less than 2 μm. Each of the mean values for greatest diameter (for each cell type and condition) fell within 1 standard deviation of all the others (with means ranging from 5.5 μm to 7.2 μm and standard deviations averaging 1.25 μm), indicating that there was no substantial difference in size across the groups, and accordingly the number of profiles was not subject to any correction.
Detection of label-retaining cells
To label slow-cycling cells, neonatal rats were injected subcutaneously with BrdU (5 μg/g body weight) or EdU (10 μg/g body weight) daily for 4 days beginning on postnatal day 3. Rats then survived for 4 weeks after the last BrdU/EdU injection. After perfusion and removal of the cranium and the bones overlying the nose, nasal tissue was decalcified by using formic acid/sodium citrate solution (5.4 M and 0.4 M, respectively), cyroprotected, frozen in liquid nitrogen, and sectioned. Sections from BrdU-injected rats were stained with anti-BrdU as described above. Sections from EdU-injected rats were stained according to the manufacturer's instructions (Invitrogen), by using a fluorophore–azide conjugate to mark the labeled cells. Cells retaining the thymidine-analogue label for 4 weeks were classified as label-retaining cells. We also investigated the reappearance of label-retaining cells in the OE following MeBr lesion. In this case, lesioned rats were administered 20 mg/kg of BrdU daily by subcutaneous injection for a variety of time periods (postlesion day [PLD]1–3, 3–5, 3–6, or 4–7) and euthanized either 2 weeks (PLD1–3 and 3–5), or 4 weeks (PLD3–6 and 4–7) after the last injection. For those harvested at 4 weeks, sections were stained with antibodies to BrdU, CK5/6, and NCAM, as outlined below, and the BrdU-labeled profiles were classified on the basis of labeling profile and morphology and counted from three sections at each of seven levels (total 21 sections) along the anteroposterior axis of the OE for each animal.
Electron microscopic examination of label-retaining cells
Rats that received multiple subcutaneous injections of EdU in the postnatal period were euthanized 1 month later (see “Detection of label-retaining cells” above) by perfusion with 2% glutaraldehyde/0.6% paraformaldehyde in 0.06 M Na cacodylate buffer (pH 7.2) and decalcified with EDTA. Other 1-month-old rats that received a single injection of EdU intravenously were euthanized by fixative perfusion 1 hour later. Olfactory mucosa from these rats was dissected and embedded in 4% agarose for sectioning with a Vibratome (Leica VT1000S) at a thickness of 100 μm. To detect incorporated EdU, sections from septum were washed in 3% BSA in PBS for 5 minutes, quenched for endogenous peroxidase in 0.3% H2O2, and washed again in 3% BSA in PBS for 5 minutes. Sections were permeabilized with 0.5% Triton-X in PBS for 30 minutes followed by washing in 3% BSA in PBS for 5 minutes. The EdU detection protocol followed the manufacturer's instructions (Invitrogen), with the exception that the concentration of biotin azide in the detection cocktail was half what is recommended for fluorophore azide. After 30 minutes in the detection cocktail, sections were washed three times for 5 minutes each in 3% BSA in PBS, and the coupling of EdU and biotin azide was visualized by a peroxidase-based ABC kit (Vector, Burlingame, CA) and DAB. Subsequently, tissues were fixed with osmium tetroxide and embedded in araldite before being ultrathin-sectioned and examined with a Philips CM-10 transmission electron microscope at Tufts University School of Medicine Electron Microscopy Facility.
Results
Proliferating cells in the unlesioned OE
Actively proliferating cells were identified in the OE in two ways, which can be used to rule out mitotic quiescence: first, rats received an intravenous injection of BrdU or EdU 1 hour before euthanasia in order to detect cells in S-phase at the time of injection (Huard and Schwob, 1995) (Fig. 1B); second, sections of the OE were immunolabeled with anti-Ki-67 antibodies, which recognize a nuclear antigen present exclusively in proliferating cells that are in the G1-, S-, G2-, or M-phases of the mitotic cycle, but do not label quiescent or postmitotic cells (G0-phase) (Gerdes et al., 1983; 1984). As expected, staining with anti-Ki-67 labels a larger number of basal cells than those that are labeled by a single injection of BrdU, and does encompass all of the BrdU (+) cells (Fig. 1). The majority of the Ki-67 (+)/BrdU (+) cells in the OE were observed in the basal cell layer, but at a remove from the basal lamina, placing them primarily within the lamina occupied by GBCs (Fig. 1C). However, in contrast to the scant distribution of BrdU (+) non-basal cells, Ki67 (+) cells were relatively more numerous within the upper zones of the OE than expected by the relative proportions of Ki67-and BrdU-labeled basal cells. These cells appear to be slowly dividing sustentacular cells and duct cells (Graziadei and Monti Graziadei, 1979; Monti Graziadei and Graziadei, 1979; Weiler and Farbman, 1998), and their identity was confirmed by staining for CK18, the keratin marker characteristic of sustentacular and duct cells (Fig. 1D).
Figure 1.
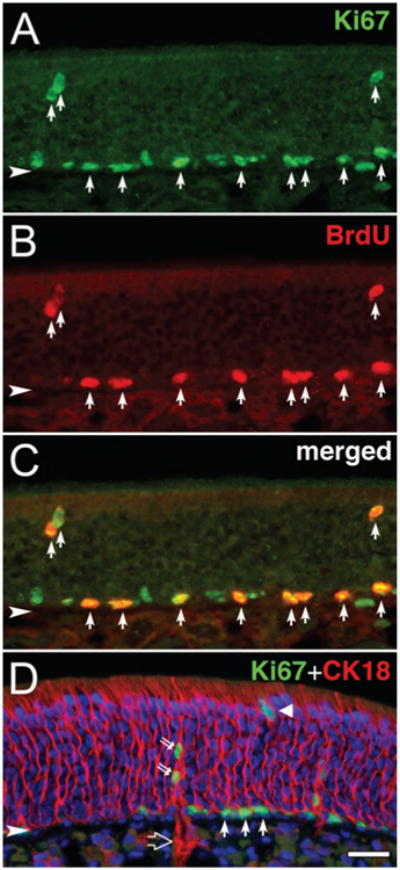
Proliferating cells in the OE are identified by anti-Ki-67 and anti-BrdU. A–C: Immunostaining with anti-Ki-67 and anti-BrdU: anti-Ki67 (A; green), anti-BrdU (B; red), and merged image (C). Note that all BrdU-labeled cells are Ki67 (+) (arrows in A–C). D: Double immunostaining with anti-Ki-67 (green) and anti-CK18 (red) and counterstaining with Hoechst dye. CK18 (+) cells are sustentacular cells (triangle) and duct and gland cells (large open arrow). Ki-67 (+) duct cells (double arrows) are found along with Ki67 (+) basal cells (arrows). Arrowhead indicates basal lamina. Magenta–green-blue versions of the photomicrographs are available online as Supplemental Figure S1. Scale bar = 25 μm in D (applies to A–D).
That Ki67 marks more cells than BrdU, and thus other phases of the cell cycle than S, was further confirmed by the whole-mount staining (Fig. 2). Close examination of the whole mounts revealed two contributors to the enhanced Ki67 numbers: 1) additional Ki67 (+) basal cells, which presumably are caught in the G1-and G2-phases (rather than S-phase); because 97.6% of the cells in the basal compartment of the OE in S-phase are GBCs (Huard and Schwob, 1995) and because most of the cells labeled by anti-Ki-67 are situated between the HBCs and neurons, the vast majority of the Ki-67 (+) basal cells are presumably GBCs; and 2) additional Ki67 (+) duct/gland cells and sustentacular cells that BrdU fails to mark. The latter can be identified by focusing through the depth of the epithelium in the whole mount since the Ki67 (+) cells that are found in the Bowman's duct and gland span the depth of the OE from the basal lamina to its apical surface. Our results clearly demonstrate that anti-Ki-67 is an excellent operational marker for proliferating cells in the OE, and, by exclusion, the absence of Ki-67 can mark quiescence.
Figure 2.
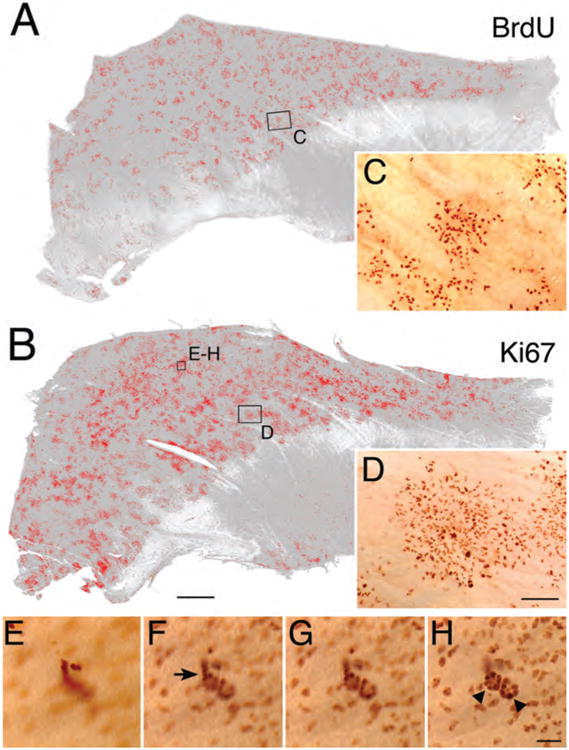
The distribution of proliferating cells as marked by Ki-67 expression is broader and more numerous than acute labeling with BrdU both within the basal cell population and within duct-gland units. A,B: Whole mounts of normal septal mucosae were stained with anti-BrdU (A) and anti-Ki-67 (B), and stained cells were highlighted on mosaics of both whole mounts using IPLab Spectrum image analysis software; anterior is to the right. C,D: Actual photographs at higher magnification of boxed area in A and B, respectively. E–H: Through-focus micrographs of the boxed area in B (E is most apical and H is most basal) illustrate Ki-67 labeling of duct (arrows in F) and gland (triangles in H) cells, whose identification is based on their morphology. All the cells in the duct-gland units are Ki-67(+), even though the BrdU labeling index is disproportionately low compared with the GBC population. Scale bar = 1 mm in B (also applies to A); 100 μm in D (also applies to C); 25 μm in H (also applies to E–G).
Identification of quiescent cells in the OE (normal vs lesioned)
The foregoing demonstrates that many of the GBCs (defined as the cells situated between the HBCs and neurons) are Ki-67 (+) and that the Ki-67 (+) cells are clustered and distributed in a discontinuous pattern across the epithelium. However, close examination demonstrates that some GBCs, defined as above, are not Ki-67 (+), raising the possibility that they are quiescent (Fig. 3). To determine whether quiescent GBCs exist and to compare their distribution with actively dividing, Ki67 (+) GBCs, we stained the epithelium with antibodies against p27Kip1, a cyclin-dependent kinase inhibitor (CDKI), which can be used as a marker for quiescent cells (Rivard et al., 1996; Kato et al., 1997), in addition to Ki-67. Many p27Kip1 (+) cells are superficial to the layer formed by the Ki-67 (+) cells, suggesting that they are differentiating neurons, which is confirmed when p27Kip1 staining is combined with the neuronal marker Tuj-1. Among the OSNs, the highest level of p27Kip1 staining is observed in the cells near the basal cell layer and then decreases gradually from the basal to the apical levels of the OE (Fig. 3C,G). This decline in p27Kip1 staining suggests that its expression in olfactory neurons peaks as they exit the cell cycle and then falls off as the neurons mature.
Figure 3.
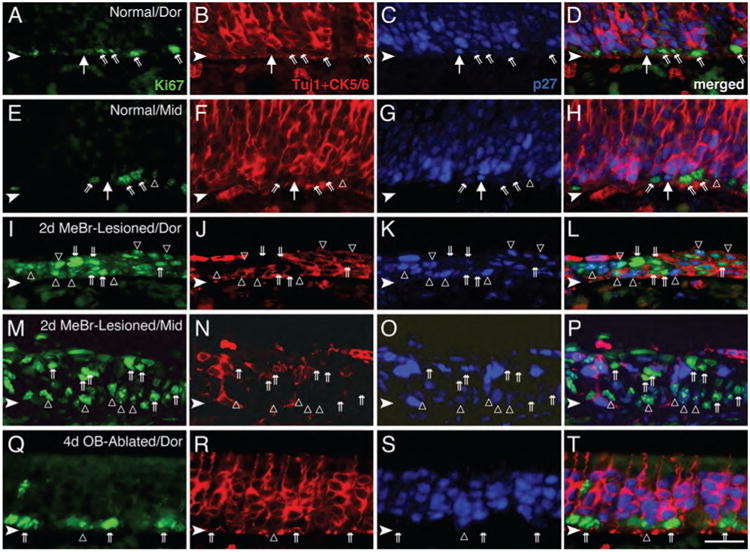
Quiescent GBCs are present in the normal OE and disappear after MeBr lesion. A–H: The normal OE from dorsal (A–D) and mid-ventral (middle, E–H) septum was stained with anti-Ki-67 (green), anti-CK5/6 and Tuj-1 (red), and anti-p27Kip1 (blue). The GBC population is identified as those cells that label with neither Tuj-1 nor anti-CK5/6 and are located in the space between the CK5/6 (+) HBCs (deep to them) and Tuj-1 (+) neurons (just superficial to them). A large majority of the GBCs are Ki-67 (+) and p27Kip1 (−) (angled double arrows). However, some of the GBCs are p27Kip1 (+)/Ki-67 (−) (large arrows) and are presumed to be quiescent. Occasionally, cells that are p27Kip1 (+)/Ki-67 (+) are found (open triangles in E–H). I–P: At 2 days after MeBr lesion, the septal OE from dorsal (I–L) and ventral (M–P) regions show increased numbers of proliferating cells that are Ki-67 (+) (double arrows) compared with the normal OE (A–H), whereas p27Kip1 (+)-only cells are absent. Note that cells that are both p27Kip1 (+) and Ki-67 (+) (open triangles) are numerous after lesion. As described previously (Schwob et al., 1995), HBCs that are marked with anti-CK5/6 almost disappear in the ventral OE immediately after MeBr lesion (as in N). Q–T: At 4 days after olfactory bulb ablation, Ki-67 (+) dividing cells (double arrows) are more numerous than in normal epithelium, and cells labeled with p27Kip1 are less numerous and frequently also labeled with Ki-67 (open triangles). Abbreviations: Dor, dorsal septum; Mid, mid-point of septum. Arrowheads indicate the basal lamina. Magenta–green–blue versions of the photomicrographs are available online as Supplemental Figure S3. Scale bar = 25 μm in T (also applies to A–S).
In addition, a small number of p27Kip1 (+)/Ki67 (−) cells (or p27Kip1-only cells) are scattered near the basal lamina, within what is the basal zone of the epithelium, and intermingled with Ki-67 (+) cells. Their identity as GBCs is confirmed by their lack of staining with Tuj-1 or anti-CK5/6 (Fig. 3A–H). The p27Kip1 (+) GBCs are relatively sparse by comparison with the Ki67 (+) ones; along the septum, there are 25.4 p27Kip1 (1) GBCs/mm length as opposed to 90.1 Ki67 (+) cells/mm (Table 2). Cells double-labeled for both p27Kip1 and Ki-67 are also observed in the OE; their significance is considered in detail in the Discussion (Table 2). Consistent with the previous results using [3H]-thymidine or BrdU incorporation (Huard and Schwob, 1995; Schwartz Levey et al., 1991), HBCs are seldom marked by Ki-67, being no more than 2% of the Ki-67 (+) cells in the basal compartment of normal OE (for example, in Fig. 3D,H, none of the CK5/6 (+) HBCs are Ki-67 [1]), nor do HBCs label strongly with anti-p27Kip1 (Fig. 3D,H; also see Fig. 5H,I).
Table 2. Prevalence of Dividing and Quiescent GBCs as a Function of Epithelial Status1.
| Status of OE | Ki-67(+) GBCs per mm length (%) | Ki-67(+)/p27Kip1 (+) GBCs per mm length (%) | p27Kip1 (+) GBCs |
|---|---|---|---|
| Normal | 90.1 ± 0.5 (66.0) | 20.1 ± 4.0 (15.3) | 25.4 ± 4.2 (18.7) |
| 2 days post MeBr lesion | 217.2 ± 10.4 (76.4) | 67.0 ± 3.1 (23.6) | 0 (0) |
| 7 days post MeBr lesion | 203.2 ± 1.8 (94.3) | 11.3 ± 2.7 (5.3) | 1.0 ± 0.4 (0.4) |
| 4 days post OB ablation | 178.7 ± 16.6 (87.9) | 12.5 ± 0.5 (6.2) | 12.0 ± 2.0 (5.9) |
| 10 days post OB ablation | 144.4 ± 8.0 (86.2) | 10.4 ± 1.2 (6.2) | 12.6 ± 0.8 (7.6) |
n = 3 for each treatment condition. See Materials and Methods for details of the profile counts.
Abbreviations: GBCs, globose basal cells; OE, olfactory epithelium; MeBr, methyl bromide; OB, olfactory bulb.
The number and distribution of p27Kip1 (+) cells were also assessed during the reconstitution of the OE that follows MeBr lesion. At 2 days after MeBr lesion (PLD2), neurons have yet to reappear in the OE (Fig. 3J,N), and HBCs have also vanished from all but the lining of the epithelium on either side of the dorsal recess (i.e., the middle, ventral, and lateral regions of the OE; Fig. 3N). The CK5 (+) HBCs persist in the dorsal OE, proliferate, and extend to the apical surface (Fig. 3J) (Schwob et al., 1995). Some p27Kip1 (+)/Tuj-1 (+) cells were seen loosely associated with the apical surface, and presumably are dead neurons that have not been shed or phagocytosed (Fig. 3L,P). The population of Ki-67 (+) proliferating cells is strikingly expanded compared with normal controls (Fig. 3I,M). At this time, the proliferating cells are distributed throughout the apicobasal width of the epithelium, which is only a few cells thick. The count of Ki-67 (+) proliferative GBCs reaches 217.2 cells/mm length of the epithelium (Table 2). Although some p27Kip1 (+) cells are evident even at this short survival time after lesion, all of them are also marked by the expression of Ki-67 (Fig. 3L,P), such that the number of p27Kip1 (+)/Ki-67 (+) GBCs reaches 22.5 cells/mm length of OE. In other words, none of the GBCs (CK5 [−] basal cells) express p27Kip1 by itself, and thus, none fit our criterion for a quiescent GBC (Table 2). It is important to re-emphasize that this time-point is in advance of the return of neurons (which are not seen in significant numbers for another 2 days) (Schwob et al., 1995; Manglapus et al., 2004). Small numbers of p27Kip1 (+)-only, i.e., quiescent, GBCs reappear by PLD7 (Fig. 3M–P, Table 2).
In contrast to the findings with MeBr lesion, in which case putatively quiescent, p27Kip1 (+) GBCs are absent early after lesion, some p27Kip1 (+)/Ki-67 (−) GBCs are evident both at early and intermediate times after olfactory bulb ablation. However, the number of these putatively quiescent GBCs is reduced to roughly 50% of that in normal OE during the first 10 days following olfactory bulb ablation (Fig. 3Q–T; Table 2).
Taking these data together, we have identified at least two subpopulations of GBCs in the normal rat OE—proliferating and quiescent. Quiescent GBCs rapidly disappear after MeBr lesion and are reduced substantially in absolute and relative number following ablation of the olfactory bulb, both of which increase proliferation markedly, suggesting that these GBCs are recruited and directly contribute to the pool of proliferating GBCs and subsequent OE reconstitution.
Identification of label-retaining GBCs in the unlesioned and lesioned-recovering OE
As indicated in the Introduction, many tissues have been shown to contain a population of slow-cycling, potentially reserve stem cells, which can be identified on the basis of their retention of thymidine-analogue label (Quesenberry and Levitt, 1979; Cotsarelis et al., 1989; Morris and Potten, 1994). The results described above suggest that quiescent GBCs are present in the normal OE and can be recruited to the proliferating pool in response to damage, but they did not provide detailed information on the dynamics of quiescent GBCs in the normal OE. In particular, the expression of p27Kip1 might be indicative of either a transient quiescence, i.e., a brief pause in mitotic cycle progression, or a longer term withdrawal (and potential reserve status). Indeed, previous analysis of cell cycle kinetics in the unlesioned OE suggests that about one-third of the population of mitotically active GBCs has come into the cell cycle after a period (undefined) of quiescence, because the percentage of daughter cells that re-enters the cycle without delay (roughly 30% of daughter cells, which corresponds to 60% of the original cohort of thymidine-labeled cells) is insufficient to maintain the number of dividing GBCs seen at any one time (Huard and Schwob, 1995). Moreover, the demonstration that p27Kip1-only GBCs are absent during the initial stages in the recovery of the post-MeBr lesion suggest that all spared quiescent cells are activated in response to damage, allowing us to assay for their re-establishment as epithelial reconstitution proceeds. Thus, we undertook analysis of thymidine label retention as a measure of long-term quiescence in order to satisfy a criterion for putative stem cell status in both unlesioned and MeBr lesioned/recovering OE.
In the setting of the normal epithelium, we looked for the establishment of label-retaining cells during the postnatal period, under the rationale that stem cells were more likely to be set aside at that time when the OE is expanding with respect to area (each part presumably requiring its own stem cell populations) and becoming adult-like in cellular composition rather than when the epithelium has stabilized after reaching maturity. To that end, neonatal pups were injected with BrdU daily for 3 consecutive days beginning on postnatal day 3 and examined 4 weeks after the last injection. By using anti-NCAM and anti-CK5/6 antibodies to label neurons and HBCs, respectively, GBCs were easily identified as the unstained cells sandwiched between the neurons and HBCs. Among the NCAM (−)/CK5/6 (−) basal cells, there is a small population of BrdU-labeled cells (Fig. 4A–E), indicating that label-retaining GBCs are indeed established during the early postnatal period and then persist for at least 1 month. The identity of some label-retaining cells as GBCs necessarily depends on location and shape in addition to molecular phenotype (Fig. 4F–H). The label-retaining basal cells identified as GBCs are clearly not duct cells from Bowman's glands, as ruled out on the basis of two morphological criteria; duct cells are more elongated in shape and oriented along the apicobasal axis and generally arranged as a continuous chain of cells extending from base to apex of the OE. We did observe a few label-retaining Bowman's gland/duct cells, identified as such by the morphological criteria specified here (see below). In addition, a small number of BrdU-labeled HBCs are also observed, scattered within the CK5/6 (+) HBC layer. Other BrdU-retaining cells within the epithelium are marker-confirmed olfactory sensory neurons (Fig. 4A,B). Although there are also label-retaining cells within the lamina propria that appear to be duct/gland cells and fibroblasts, these were not marker-identified as to type and are not considered further here.
Figure 4.
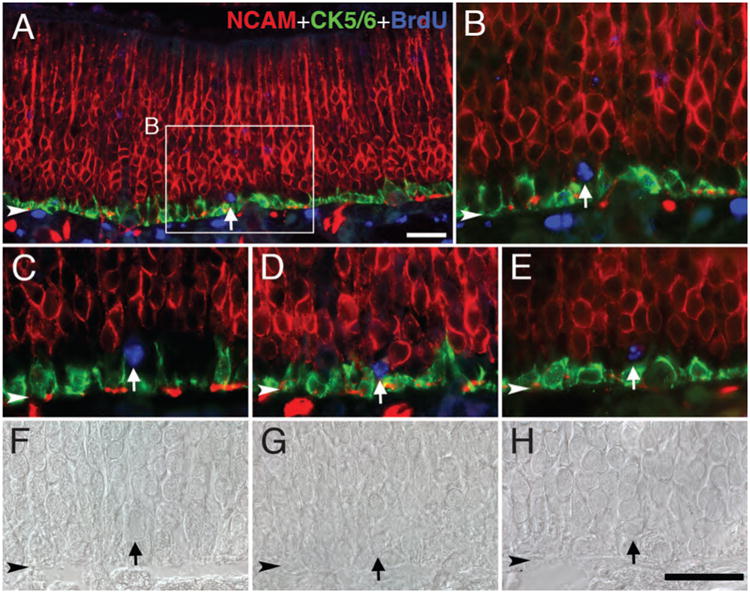
Label-retaining GBCs are present in the normal OE. Sections of the OE from rats that were injected with BrdU daily for 3 consecutive days beginning on postnatal day 3 and euthanized 4 weeks later were stained with anti-CK5/6 (green), anti-NCAM (red), and anti-BrdU (blue). A: Some of the BrdU-labeled cells (blue) are situated between neurons and HBCs and are, accordingly, classified as GBCs (arrow). B: Higher magnification showing boxed area in A. C–E: Other examples of label-retaining GBCs (arrows). F–H: Differential interference contrast images of C–E, respectively, showing that the morphology of the label-retaining GBCs is that of GBCs and distinct from HBCs or Bowman's duct cells. Arrowhead indicates basal lamina. Magenta–green–blue versions of the photomicrographs are available online as Supplemental Figure S4. Scale bar = 25 μm in A and H (also applies to B–G).
Identification of label-retaining GBCs as long-term quiescent was further supported by comparing thymidine label retention and staining with p27Kip1. For reasons of technical compatibility, we substituted EdU as the thymidine analogue (Salic and Mitchison, 2008), and “Click-iT” chemistry as the means of visualizing EdU-labeled cells (Breinbauer and Kohn, 2003). In the OE, EdU labeling is coextensive with that of BrdU when both are injected simultaneously (data not shown). When EdU was sequentially injected on postnatal days 3–5 following the same protocol as BrdU and the tissue harvested after a chase period of 1 month, a cohort of EdU-labeled, i.e., label-retaining, CK5/6 (−)/Tuj-1 (−) GBCs was evident, some of which stain with p27Kip1 (Fig. 5). The pattern of EdU labeling in differentiated cell types—neurons, duct/gland cells, and HBCs—is similar to what we described for BrdU label retention above (cf. Figs. 4, 5).
In addition, we analyzed the reappearance of label-retaining GBCs during the reconstitution of the epithelium that follows MeBr lesion. The data on p27Kip1 and Ki-67 expression post-MeBr lesion suggest that all cells are mitotically active in the immediate postlesion period (exemplified by the analysis of tissue harvested 2 days after MeBr exposure shown in Fig. 3), but that p27Kip1 (+)/Ki-67 (−), putatively quiescent GBCs emerge later in the first week of epithelial reconstitution. We expected and indeed found that label-retaining GBCs emerge in parallel with the reappearance of marker-defined, putatively quiescent ones.
To that end we administered BrdU via sequential injections on PLD1–3, PLD3–5, PLD3–6, or PLD4–7 and then harvested the tissue 2 or 4 weeks following the last injection. Label-retaining GBCs, defined as such by the criteria listed above, were not observed in the animals in the PLD1–3 group. However, they were observed at both 2 and 4 weeks after injection in the other groups of animals in which the series of sequential injections began on either PLD3 or PLD4 (Fig. 6). Some label-retaining HBCs were observed with each of the four injection protocols, and the relative distributions of each cell type has been determined in the PLD3–6 and PLD4–7 groups that were analyzed 4 weeks after the last injection of BrdU (Table 3).
Table 3. BrdU-Labeled Cell Types in MeBr-Lesioned/Recovering OE Found 28 Days After Last Pulse of Nucleotide1.
| Animal identification | OSNs | Sus | BD/G | GBC | HBC | Total | |
|---|---|---|---|---|---|---|---|
| Injection at PLD3–6 | MBM5 | 431 | 247 | 26 | 62 | 68 | 834 |
| MBM6 | 342 | 187 | 26 | 35 | 72 | 662 | |
| Injection at PLD4–7 | MBM3 | 512 | 234 | 19 | 53 | 47 | 865 |
| MBM4 | 374 | 162 | 16 | 79 | 75 | 706 |
The counts were obtained from three sections at each of seven levels (total 21 sections) along the anteroposterior axis of the OE for each animal.
Abbreviations: PLD, post lesion day; OSNs, olfactory sensory neurons; Sus, sustentacular cells; BD/G, Bowman's duct and gland; GBC, globose basal cell; HBC, horizontal basal cell; MeBr, methyl bromide.
Electron microscopic confirmation of label-retaining GBCs
EdU labeling is compatible with EM fixation and examination of the label-retaining cells as described in Materials and Methods to provide ultrastructural confirmation that they are GBCs. To verify the appearance of GBCs at the EM level in material prepared in this way, we examined cells in the OE of 1-month-old rats labeled acutely by the incorporation of EdU that was injected 1 hour before fixation and tissue harvest (Fig. 7). As seen in semithin sections (Fig. 7A) and confirmed with EM (Fig. 7B,C), the vast majority of EdU-labeled cells are located near the basal lamina but not on it, and are indeed GBCs that are situated immediately superficial to HBCs; the latter are easily recognized by lying flat against the basal lamina and being tightly attached to it. These EM data are consistent with the light microscopic (LM) immunohistochemical observations of BrdU labeling (cf. Figs. (1 and 7)).
Figure 7.
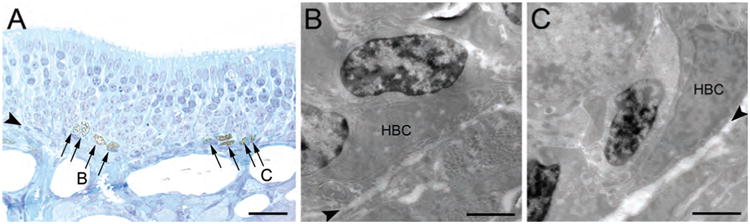
Electron microscopic (EM) examination confirms that most of the EdU (+) cells observed in the OE acutely after pulse label are GBCs. A: In semithin plastic sections counterstained with Toluidine blue, the EdU (+) cells are mostly found superficial to the layer of horizontal basal cells (HBCs) and are classified as GBCs (arrows). Electron micrographs of the areas labeled B, C are shown in the corresponding panels. B,C: EM examination demonstrates that the EdU (+) cells (those with the darkened nuclei) have the ultrastructural characteristics described for GBCs and are found superficial to the HBCs (HBC), which rest on the basal lamina (arrowheads). Arrowheads in A–C indicate basal lamina. Scale bar = 25 μm in A; 2 μm in B,C.
Likewise, when EdU was injected sequentially on postnatal days 3–5, and the tissue fixed and harvested 1 month later, some EdU-labeled GBCs can be identified (Fig. 8). As seen in semithin sections (Fig. 8A,C,E) and confirmed by EM examination (Fig. 8B,D,F), the labeled GBCs are round with scant cytoplasm and are situated at a remove from the basal lamina, superficial to HBCs (which are usually unlabeled), (e.g., Fig. 8A–D); on occasion the labeled GBCs nestle between the HBCs and touch the basal lamina in a single spot (Fig. 8C,D). Cells of the latter type have been observed with conventional EM in both mice and rats (Graziadei and Monti Graziadei, 1979; Holbrook et al., 1995, respectively), and are best classified as a kind of GBC, as they can be labeled acutely as well by thymidine analogues (viz. Fig. 5B of Holbrook et al., 1995). Label-retaining HBCs are also observed at the EM level and can be recognized by the usual features—tight apposition to the basal lamina and the arching of their somata over bundles of olfactory axons (Fig. 8E,F). Other labeled cells also reflect the distribution noted at the LM level (Fig. 5); in addition to the GBCs, there are labeled olfactory sensory neurons, sustentacular cells, and Bowman's duct/gland cells (Fig. 8A,C,E).
Figure 8.
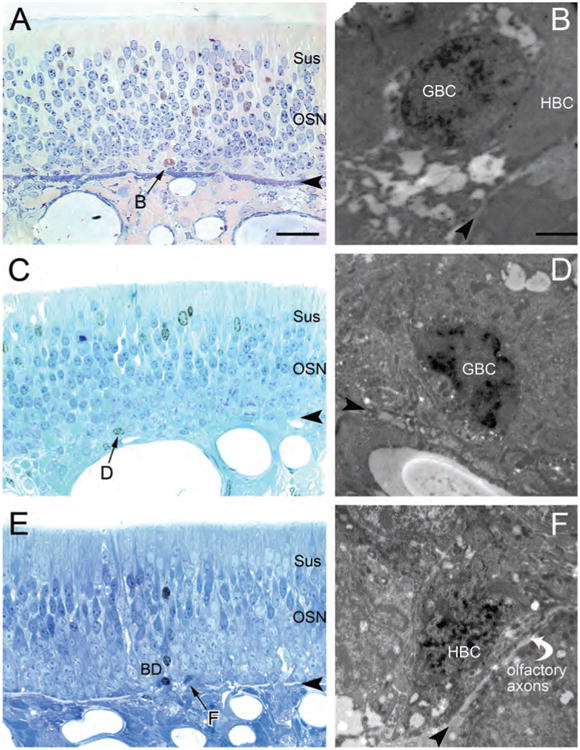
Electron microscopic (EM) examination confirms that some of the label-retaining cells within the basal compartment are globose basal cells (GBCs), as shown by their ultrastructure and cellular relationships. A,C,E: Three examples of the label-retaining basal cells, generated by three sequential injections in the postnatal period, were identified in Toluidine blue-counterstained semithin plastic sections (arrows) and examined in adjacent ultrathin sections with the EM (B,D,F). Other label-retaining cells are found to be more differentiated cell types—neurons (i.e., in the middle lamina of the OE in A; olfactory sensory neurons [OSNs]), sustentacular cells (i.e., the most superficial nuclei in C; Sus), and Bowman's duct/gland cells (the line of three labeled duct cells in E; BD). B,D,F: EdU-labeled basal cells are both GBCs (B,D) and horizontal basal cells (HBCs; F). The GBCs in B and D are separated from the basal lamina by the interposed HBCs either completely (B) or with a minimal focal contact with the basal lamina (D). GBCs of the latter type have been previously illustrated and analyzed in the rat OE (Holbrook et al., 1995) as well as in the mouse OE (Graziadei and Monti Graziadei, 1979). F: The labeled basal cell is most likely an HBC, based on the arch it forms over a small bundle of olfactory axons sitting at the basal lamina (Holbrook et al., 1995). Arrowheads indicate basal lamina. Scale bar = 25 μm in A (also applies to C,E); 2 μm in B (also applies to D,F).
Discussion
The current work examines the kinetic behavior of GBCs within the rat olfactory epithelium, and focuses on identifying cells that are actively cycling, as shown by labeling for Ki-67, which is expressed in all phases of the mitotic cycle (with the exception of G0) (Gerdes et al., 1984; Scholzen and Gerdes, 2000), and those that are quiescent and have withdrawn from the cycle, as marked by the retention of thymidine analogue label and by staining for the cyclin-dependent kinase inhibitor p27Kip1. The motivation for the work comes from the need to understand the stem cell population(s) of the OE in greater depth, in order to begin to dissect the molecular regulation of stem/progenitor cell function in more detail. To date, the putative identification of the neurocompetent stem cells of the OE has depended largely on the demonstration that a particular class of cell has the capacity to generate all of the various epithelial cell types, which has been shown for both HBCs and GBCs (Goldstein et al., 1997; Huard et al., 1998; Chen et al., 2004; Leung et al., 2007).
The other canonical criterion for stemness, the capacity for self-renewal, is a difficult criterion to satisfy and has not been fully met for either of the two candidates cell types, in part because a stringent assay has not yet been devised. Both HBCs and GBCs will regenerate themselves and each other (Goldstein et al., 1997; Huard et al., 1998; Chen et al., 2004; Leung et al., 2007), but this outcome represents a more limited degree of renewal than has typically been demonstrated for other stem cells. An indirect indicator of self-renewal capacity has been the identification of cells that are normally quiescent, but can be activated to produce the various cell lineages in the tissue, before returning to dormancy (however, it is important to note that some cell types that satisfy functional criteria for stemness are robustly mitotic; Simons and Clevers, 2011). The mitotic quiescence of HBCs, which has been recognized for years, and the more recent demonstration that HBCs undergo a cycle of quiescence–activation–return to quiescence in response to epithelial damage have been cited as evidence that HBCs are “the” olfactory stem cells (Leung et al., 2007; Duggan and Ngai, 2007; Fletcher et al., 2011).
Our interpretation that some among the broadly defined population of GBCs are also quiescent for an extended period, called upon to divide by injury, and then return again to quiescence, is based on two kinds of data: thymidine label-retention and CDKI expression. First, with respect to the former, some among the GBCs in the unlesioned OE retain thymidine analogue for a prolonged period, are recruited into the cell cycle by lesion, and then they or others are then restored to label retention beginning several days into the process of recovery of the OE after lesion. That all label-retaining cells are activated by injury is suggested strongly by our observation that each and every one of the spared cells in the lesioned OE is labeled by the expression of Ki-67 2 days after MeBr exposure and that, simultaneously, the index of labeled cells is increased many-fold compared with normal (data presented here; Schwob et al., 1995). That some of the label-retaining cells in the normal and lesioned–recovered OE are GBCs is confirmed by their immunohistochemical profile (i.e., that they do not label with neuronal or HBC markers) and by their morphology at the ultrastructural level (small, round cells, with sparse cytoplasm, at a remove from the basal lamina, and not a component of a Bowman's duct). We are not the first to demonstrate label-retaining basal cells in the fully formed olfactory epithelium (Mackay-Sim and Kittel, 1991), and HBCs, by virtue of their mitotic quiescence in the absence of lesion, were suspected before and shown here to be label retaining. However, the previous report did not distinguish between HBC and GBC populations, nor was there any demonstration that such cells were activated and then restored after epithelial lesion (Mackay-Sim and Kittel, 1991). Nonetheless, the label-retaining basal cells identified in the earlier study were considered to be putative stem cells of the OE.
The significance of label retention emerges from the study of cells with this phenotype in other tissues. Although the mechanism responsible for DNA label retention—slow cycling versus immortal strand retention—has been controversial (Lansdorp, 2007; Rando, 2007; Waghmare et al., 2008), stem cells and label-retaining cells have been linked together. From their earliest identification in gut, cornea, and epidermis, label-retaining cells have been identified as strong stem cell candidates (Cotsarelis et al., 1990; Morris and Potten, 1994; Taylor et al., 2000; Morris et al., 2004; Claudinot et al., 2005). Perhaps the most convincing evidence derives from the small intestine, where the +4 cell (referring to its position relative to the crypt base) retains thymidine label (Cheng and LeBlond, 1974). By expressing Cre-recombinase from the Bmi-1 locus (a +4 cell marker) for purposes of lineage tracing, the +4 cell has been shown to give rise to all of the various cell types of the crypt–villus unit, including the Lgr-5 (+) cells, which are a continually proliferating stem cell population at the crypt base (Tian et al., 2005).
Second, some among the GBCs express the CDK1 p27Kip1, a finding that was suggested although not definitively demonstrated previously (Legrier et al., 2001). p27Kip1 is often responsible, at least in part, for governing the withdrawal from the mitotic cycle (Coats et al., 1996; Rivard et al., 1996; Chen and Segil, 1999; Levine et al, 2000) and keeping cells quiescent (Koff and Polyak, 1995; Millard et al., 1997; Doetsch et al., 2002b; Besson et al., 2006; Liu et al., 2012; Cheung and Rando, 2013). It is important to acknowledge that the expression of p27Kip1 is complex and does not always imply an exit from active progression in the cell cycle (Harper and Elledge, 1996). For example, in some GBCs, p27Kip1 is expressed by itself, whereas other p27Kip1 (+) GBCs are also marked by expression of Ki-67. In human bone marrow, a small number of mega-karyocytes are positive for both Ki-67 and p27Kip1 during cell cycle exit (Taniguchi et al., 1999). Furthermore, in primitive human CD34 (+) hematopoietic stem cells, reduction of p27Kip1 is coincident with the elevation of Ki-67 and cell cycle re-entry (Dao et al., 1998). In addition, low levels of p27Kip1 help to stabilize cyclin D– cyclin-dependent kinase interactions and permit G1 progression to S-phase (Sherr and Roberts, 1999). As a consequence, it is not surprising that some GBCs are labeled by both Ki-67 and p27Kip1, and the double-labeled GBCs may mark some cells that are in G1 and actively cycling or, alternatively ones that pause in G1 for a period of time. The existence of a pause in G1 for some GBCs is suggested by the demonstration that too few GBCs return immediately to the cell cycle to account for the numbers of cells in S-phase at any one time (Huard and Schwob, 1995). A disjunction between the expression of Ki-67 and progression into S-phase is also apparently true of duct cells as well, because the number of Ki-67 (+) duct cells is far more than twice the number of ones labeled by the incorporation of BrdU, suggesting that in the case of the duct cells a substantial number are pausing for a period of time, most likely in G1 based on analogy to other cell types (Shirane et al., 1999). Nonetheless, that some GBCs are labeled by p27Kip1 and not Ki-67 as shown here (and also by Guo et al., 2010) and that expression of p27Kip1 overlaps with thymidine label retention by GBCs suggest strongly that p27Kip1 expression without concurrent Ki-67 labeling is a useful means of identifying quiescent GBCs.
It is also important to emphasize that p27Kip1 expression is not limited to GBCs. Immature neurons also express p27Kip1, as shown here, along with other CDKIs, as shown elsewhere (Legrier et al., 2001). Interestingly, the intensity of p27Kip1 expression, as well as other CDKIs (Legrier et al., 2001) declines as cells make the transition to maturity, suggesting that establishment of the postmitotic state requires higher levels of this and the other CDKIs than its maintenance in mature olfactory neurons.
As a final point, it is worth considering further the re-emergence of label-retaining, quiescent GBCs during the process of tissue regeneration following MeBr lesion. As indicated in the introduction, the population of GBCs is heterogeneous when analyzed by the expression of various transcription factors, among which are Sox2, Pax6, Ascl1 (previously called Mash1), Neurog1, and NeuroD1 (Cau et al., 1997, 2002, Manglapus et al., 2004; Guo et al., 2010). The data presented here demonstrate the re-establishment of label-retaining GBCs beginning 4 days after lesion (because injections of BrdU from 1 to 3 days post MeBr lesion do not result in label-retaining GBCs, although label-retaining HBCs are produced within that time-frame). By comparison with the time course of renewed expression of these transcription factors during the post-MeBr recovery period (Manglapus et al., 2004), the resumption of neurogenesis coincides with a return to quiescence of some among the GBCs. These data suggest a complex regulation of the transition between mitotic activity and quiescence, and raise the possibility that cells go “backward” from activity into quiescence rather than the usual model of stem cell asymmetric division, in which one of the daughters exits the cell cycle immediately. Taken in total, published data and the current results support a model of stem cell–progenitor cell relationships that recognize two distinct categories of stem cells—both a defined subset of GBCs and also HBCs—along with defined GBC stages downstream of the stem cell populations (Fig. 9). Much remains to be discovered regarding the regulation of the transitions among the various types of GBCs and the population of HBCs held in reserve, but the model provides a heuristic framework for further investigating cell renewal in the OE.
Figure 9.
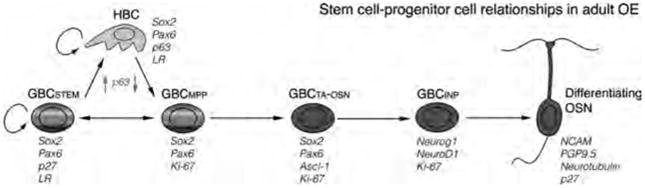
Model of stem cell and progenitor cell relationships in the olfactory epithelium (OE), based on data presented here and previous published findings regarding globose basal cell (GBC) molecular heterogeneity (Goldstein and Schwob, 1996; Cau et al., 1997, 2002; Manglapus et al., 2004; Jang et al., 2007; Leung et al., 2007; Guo et al., 2010; Packard et al., 2011a,b; Fletcher et al., 2011), results following transplantation of GBCs (Goldstein et al., 1998; Chen et al., 2004), and data obtained via retroviral lineage tracing in the MeBr-lesioned epithelium (Huard et al., 1998). Key points to emphasize include: 1) the subdivision of the population of GBCs on the basis of transcription factor expression and kinetic status into putative stem (STEM), multipotent progenitor (MPP), transit amplifying (TA), and immediate neuronal precursor (INP) stages; the rainbow of colors at stem and multipotent progenitor stages represents the demonstrated capacity of these GBC types to generate all the cell types of the OE; 2) the restoration of label-retaining (LR) and p27-expressing GBCs (GBCSTEM) following the universal proliferation (Ki-67 expression) induced by MeBr lesion of the OE (double-headed arrow between GBCSTEM and GBCMPP); and 3) the role of upregulation and downregulation of p63 (up arrow and down arrow, respectively) in the conversion of horizontal basal cells (HBCs), a putative reserve stem cell population, to and from dormancy, respectively. OSN, olfactory sensory neuron. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In summary, the data presented here demonstrate that quiescent, label-retaining GBCs exist in the normal OE and can be activated to proliferate and contribute to OE reconstitution after MeBr lesion. The cycle of GBC quiescence-activation after injury–return to quiescence during recovery satisfies one of the key characteristics of stem cells, and strongly suggests that a subset of GBCs constitutes a population of stem cells critical to the health and maintenance of the OE (Naessen, 1970; Holbrook et al., 2005, 2011).
Supplementary Material
Acknowledgments
The authors thank Po Kwok-Tse and Meghan Bliss-Moreau for their excellent technical contributions and Cathy Linsenmayer for her outstanding assistance with electron microscopy.
Grant sponsor: National Institutes of Health; Grant number: R01 DC002167.
Footnotes
Additional Supporting information may be found in the online version of this article.
Conflict of Interest Statement: Authors claim no conflicts of interest.
Role of Authors: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: WJ, XC, and JES. Acquisition of data: WJ, XC, DF, and MH. Analysis and interpretation of data: WJ, XC, DF,MH, and JES. Drafting of the manuscript: WJ, XC, and JES. Critical revision of the manuscript for important intellectual content: WJ and JES. Obtained funding: JES.
Literature Cited
- Besson A, Gurian-West M, Chen X, Kelly-Spratt KS, Kemp CJ, Roberts JM. A pathway in quiescent cells that controls p27Kip1 stability, subcellular localization, and tumor suppression. Genes Dev. 2006;20:47–64. doi: 10.1101/gad.1384406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breinbauer R, Kohn M. Azide-alkyne coupling: a powerful reaction for bioconjugate chemistry. Chembiochem. 2003;4:1147–1149. doi: 10.1002/cbic.200300705. [DOI] [PubMed] [Google Scholar]
- Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. doi: 10.1016/0896-6273(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Fode C, Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–1880. doi: 10.1242/dev.129.8.1871. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;128:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457–474. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci U S A. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Dao MA, Taylor N, Nolta JA. Reduction in levels of the cyclin-dependent kinase inhibitor p27(kip-1) coupled with transforming growth factor beta neutralization induces cell-cycle entry and increases retroviral transduction of primitive human hematopoietic cells. Proc Natl Acad Sci U S A. 1998;95:13006–13011. doi: 10.1073/pnas.95.22.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHamer MK, Guevara JL, Hannon K, Olwin BB, Calof AL. Genesis of olfactory receptor neurons in vitro: regulation of progenitor cell divisions by fibroblast growth factors. Neuron. 1994;13:1083–1097. doi: 10.1016/0896-6273(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002a;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Verdugo J, Gaille I, Alvarez-Buylla A, Chao M, Casaccia-Bonnefil P. Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesis. J Neurosci. 2002b;22:2255–2264. doi: 10.1523/JNEUROSCI.22-06-02255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan CD, Ngai J. Scent of a stem cell. Nat Neurosci. 2007;10:673–674. doi: 10.1038/nn0607-673. [DOI] [PubMed] [Google Scholar]
- du Manoir S, Guillaud P, Camus E, Seigneurin D, Brugal G. Ki-67 labeling in postmitotic cells defines different Ki-67 pathways within the 2c compartment. Cytometry. 1991;12:455–463. doi: 10.1002/cyto.990120511. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Prasol MS, Estrada J, Baudhuin A, Vranizan K, Choi YG, Ngai J. p63 regulates olfactory stem cell self-renewal and differentiation. Neuron. 2011;72:748–759. doi: 10.1016/j.neuron.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker H, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Goldstein BJ, Schwob JE. Analysis of the globose basal cell compartment in rat olfactory epithelium using GBC-1, a new monoclonal antibody against globose basal cells. J Neurosci. 1996;16:4005–4016. doi: 10.1523/JNEUROSCI.16-12-04005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BJ, Wolozin BL, Schwob JE. FGF2 suppresses neuronogenesis of a cell line derived from rat olfactory epithelium. J Neurobiol. 1997;33:411–428. [PubMed] [Google Scholar]
- Gordon MK, Mumm JS, Davis RA, Holcomb JD, Calof AL. Dynamics of MASH1 expression in vitro and in vivo suggest a non-stem cell site of MASH1 action in the olfactory receptor neuron lineage. Mol Cell Neurosci. 1995a;6:363–379. doi: 10.1006/mcne.1995.1028. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Mumm JS, Davis RA, Holcomb JD, Calof AL. Dynamics of MASH1 expression in vitro and in vivo suggest a non-stem cell site of MASH1 action in the olfactory receptor neuron lineage. Mol Cell Neurosci. 1995b;6:363–379. doi: 10.1006/mcne.1995.1028. [DOI] [PubMed] [Google Scholar]
- Graziadei PP, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Guo Z, Packard A, Krolewski RC, Manglapus GL, Harris M, Schwob JE. Expression of Pax6 and Sox2 in adult olfactory epithelium. J Comp Neurol. 2010;518:4395–4418. doi: 10.1002/cne.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Elledge SJ. Cdk inhibitors in development and cancer. Curr Opin Genet Dev. 1996;6:56–64. doi: 10.1016/s0959-437x(96)90011-8. [DOI] [PubMed] [Google Scholar]
- Heimfeld S, Weissman IL. Development of mouse hematopoietic lineages. Curr Top Dev Biol. 1991;25:155–175. doi: 10.1016/s0070-2153(08)60415-9. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Szumowski KE, Schwob JE. An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol. 1995;363:129–146. doi: 10.1002/cne.903630111. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Leopold DA, Schwob JE. Abnormalities of axon growth in human olfactory mucosa. Laryngoscope. 2005;115:2144–2154. doi: 10.1097/01.MLG.0000181493.83661.CE. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121:1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard JM, Schwob JE. Cell cycle of globose basal cells in rat olfactory epithelium. Dev Dyn. 1995;203:17–26. doi: 10.1002/aja.1002030103. [DOI] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–486. [PubMed] [Google Scholar]
- Iwai N, Zhou Z, Roop DR, Behringer RR. Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium. Stem Cells. 2008;26:1298–1306. doi: 10.1634/stemcells.2007-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Youngentob SL, Schwob JE. Globose basal cells are required for reconstitution of olfactory epithelium after methyl bromide lesion. J Comp Neurol. 2003;460:123–140. doi: 10.1002/cne.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Kim KP, Schwob JE. Nonintegrin laminin receptor precursor protein is expressed on olfactory stem and progenitor cells. J Comp Neurol. 2007;502:367–381. doi: 10.1002/cne.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kato A, Takahashi H, Takahashi Y, Matsushime H. Inactivation of the cyclin D-dependent kinase in the rat fibroblast cell line, 3Y1, induced by contact inhibition. J Biol Chem. 1997;272:8065–8070. doi: 10.1074/jbc.272.12.8065. [DOI] [PubMed] [Google Scholar]
- Koff A, Polyak K. p27 KIP1, an inhibitor of cycline-dependent kinases. Prog Cell Cycle Res. 1995;1:141–147. doi: 10.1007/978-1-4615-1809-9_11. [DOI] [PubMed] [Google Scholar]
- Ladha MH, Lee KY, Upton TM, Reed MF, Ewen ME. Regulation of exit from quiescence by p27 and cyclin D1-CDK4. Mol Cell Biol. 1998;18:6605–6615. doi: 10.1128/mcb.18.11.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajtha LJ. Stem cell concepts. In: Potten CS, editor. Stem cells: their identification and characterization. New York: Churchill Livingstone; 1983. pp. 1–11. [Google Scholar]
- Lansdorp PM. Immortal strands? Give me a break. Cell. 2007;129:1244–1247. doi: 10.1016/j.cell.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Largent BL, Sosnowski RG, Reed RR. Directed expression of an oncogene to the olfactory lineage in transgenic mice. J Neurosci. 1993;13:300–12. doi: 10.1523/JNEUROSCI.13-01-00300.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrier ME, Ducray A, Propper A, Chao M, Kastner A. Cell cycle regulation during mouse olfactory neurogenesis. Cell Growth Differ. 2001;12:591–601. [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Levine EM, Close J, Fero M, Ostrovsky A, Reh TA. p27(Kip1) regulates cell cycle withdrawal of late multipotent progenitor cells in the mammalian retina. Dev Biol. 2000;219:299–314. doi: 10.1006/dbio.2000.9622. [DOI] [PubMed] [Google Scholar]
- Liu Z, Walters BJ, Owen T, Brimble MA, Steigelman KA, Zhang L, Mellado Lagarde MM, Valentine MB, Yu Y, Cox BC, Zuo J. Regulation of p27Kip1 by Sox2 maintains quiescence of inner pillar cells in the murine auditory sensory epithelium. J Neurosci. 2012;32:10530–10540. doi: 10.1523/JNEUROSCI.0686-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A, Kittel P. Cell dynamics in the adult mouse olfactory epithelium: a quantitative autoradiographic study. J Neurosci. 1991;11:979–984. doi: 10.1523/JNEUROSCI.11-04-00979.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglapus GL, Youngentob SL, Schwob JE. Expression patterns of basic helix-loop-helix transcription factors define subsets of olfactory progenitor cells. J Comp Neurol. 2004;479:216–233. doi: 10.1002/cne.20316. [DOI] [PubMed] [Google Scholar]
- Millard SS, Yan JS, Nguyen H, Pagano M, Kiyokawa H, Koff A. Enhanced ribosomal association of p27(Kip1) mRNA is a mechanism contributing to accumulation during growth arrest. J Biol Chem. 1997;272:7093–7098. doi: 10.1074/jbc.272.11.7093. [DOI] [PubMed] [Google Scholar]
- Monti Graziadei GA, Graziadei PP. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979;8:197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Potten CS. Slowly cycling (label-retaining) epidermal cells behave like clonogenic stem cells in vitro. Cell Prolif. 1994;27:279–289. doi: 10.1111/j.1365-2184.1994.tb01425.x. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Naessen R. The identification and topographical localisation of the olfactory epithelium in man and other mammals. Acta Otolaryngol. 1970;70:51–57. doi: 10.3109/00016487009181858. [DOI] [PubMed] [Google Scholar]
- Packard A, Giel-Moloney M, Leiter A, Schwob JE. Progenitor cell capacity of NeuroD1-expressing globose basal cells in the mouse olfactory epithelium. J Comp Neurol. 2011a;519:3580–3596. doi: 10.1002/cne.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard A, Schnittke N, Romano RA, Sinha S, Schwob JE. DNp63 regulates stem cell dynamics in the mammalian olfactory epithelium. J Neurosci. 2011b;31:8748–8759. doi: 10.1523/JNEUROSCI.0681-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Quesenberry P, Levitt L. Hematopoietic stem cells. N Engl J Med. 1979;301:755–761. doi: 10.1056/NEJM197910043011404. [DOI] [PubMed] [Google Scholar]
- Rando TA. The immortal strand hypothesis: segregation and reconstruction. Cell. 2007;129:1239–1243. doi: 10.1016/j.cell.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivard N, L'Allemain G, Bartek J, Pouyssegur J. Abrogation of p27KIP1 by cDNA antisense suppress quiescence (G0 state) in fibroblast. J Biol Chem. 1996;271:18337–18341. doi: 10.1074/jbc.271.31.18337. [DOI] [PubMed] [Google Scholar]
- Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schwartz Levey M, Chikaraishi DM, Kauer JS. Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J Neurosci. 1991;11:3556–3564. doi: 10.1523/JNEUROSCI.11-11-03556.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Meiri KF. On the formation of neuromata in the primary olfactory projection. J Comp Neurol. 1994;340:361–380. doi: 10.1002/cne.903400307. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J Comp Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Shibui S, Hoshino T, Vanderlaan M, Gray JW. Double labeling with iodo- and bromodeoxyuridine for cell kinetics studies. J Histochem Cytochem. 1989;37:1007–1011. doi: 10.1177/37.7.2659659. [DOI] [PubMed] [Google Scholar]
- Shirane M, Harumiya Y, Ishida N, Hirai A, Miyamoto C, Hatakeyama S, Nakayama K, Kitagawa M. Down-regulation of p27(Kip1) by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J Biol Chem. 1999;274:13886–13893. doi: 10.1074/jbc.274.20.13886. [DOI] [PubMed] [Google Scholar]
- Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Endo H, Chikatsu N, Uchimaru K, Asano S, Fujita T, Nakahata T, Motokura T. Expression of p21(Cip1/Waf1/Sdi1) and p27(Kip1) cyclin-dependent kinase inhibitors during human hematopoiesis. Blood. 1999;93:4167–4178. [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Terskikh VV, Vasiliev AV, Vorotelyak EA. Label retaining cells and cutaneous stem cells. Stem Cell Rev. 2012;8:414–425. doi: 10.1007/s12015-011-9299-6. [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, He XC, Hood L, Li L. Bridging the BMP and Wnt pathways by PI3 kinase/Akt and 14-3-3zeta. Cell Cycle. 2005;4:215–216. [PubMed] [Google Scholar]
- Waghmare SK, Bansal R, Lee J, Zhang YV, McDermitt DJ, Tumbar T. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 2008;27:1309–1320. doi: 10.1038/emboj.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler E, Farbman AI. Proliferation decrease in the olfactory epithelium during postnatal development. Ann N Y Acad Sci. 1998;855:230–234. doi: 10.1111/j.1749-6632.1998.tb10572.x. [DOI] [PubMed] [Google Scholar]
- Xie F, Fang C, Schnittke N, Schwob JE, Ding X. Mechanisms of permanent loss of olfactory receptor neurons induced by the herbicide 2,6-dichlorobenzonitrile: effects on stem cells and noninvolvement of the inflammatory cytokine IL-6. Toxicol Appl Pharmacol. 2013;272:598–607. doi: 10.1016/j.taap.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


