Abstract
Purpose
The cysteine cathepsins are a family of proteases that play important physiological roles in both normal cellular physiology and many human diseases. In cancer, the activity of many of the cysteine cathepsins is up-regulated and can be exploited for tumor imaging both in vivo and ex vivo. To characterize the behavior of a topically applied quenched fluorescent activity-based probe, GB119, ex vivo, we developed a basic immunohistochemistry technique to identify unquenched GB119 within tissue.
Procedures
Immunoblot assays were used to validate the utility of an anit-Cy5 antibody for the detection of unquenched GB119 generated by cathepsin-L. Following validation the anti-Cy5 antibody an immunohistochemical procedure was developed to detect the presence of unquenched GB119 in frozen sections of brain tumors derived from an orthotopic mouse model.
Results
These studies demonstrate that the anti-Cy5 antibody preferentially recognizes unquenched GB119 and that this differential can be used to identify the regions within the brain and the tumor that contained unquenched GB119. Using H&E staining and antibodies against other biochemical markers it was further determined that unquenched GB119 was localized to the peri-tumor space and co-localized with cathepsin-L expression.
Conclusion
Our data indicate that this methodology allows high-resolution detection of unquenched GB119 that can be correlated with other immunohistological stains.
Keywords: Molecular Optical Imaging, Quenched Activity Based Probe, Topical Application, Cathepsin-L, Brain cancer
INTRODUCTION
The cysteine cathepsins are a family of proteases that play an important role in both normal and diseased tissue physiology [1]. Although their physiological function had mainly been described as being confined to the endosomal pathway, evidence is accumulating that they may be major regulators of matrix degradation and cell motility, suggesting that they also function in an extracellular context during the disease process [2]. Members of the cysteine cathepsin family have also been elucidated to be major players in the development and progression of several types of cancer [3,4]. Recently, activity-based probes (ABPs) have been used as a tool for the direct assessment of the activity of these proteases in the context of a native tumor microenvironment [5–7]. These reagents form activity dependent covalent bonds with protease active site nucleophiles, thereby providing readout of the levels of active protease in a cell, tissue, or even whole organisms [8]. In particular, fluorescently quenched ABPs (qABPs) have proven to be powerful tools for the noninvasive optical imaging of cancer and subsequent characterization of the target cathepsins on a histological, cellular, and protein level [9,10]. Previously, we have presented an attractive and unique approach to utilize the ability of qABP to elucidate overexpression of tumor-associated cathepsin-L (CTS-L) ex vivo [11]. Utilizing one member of the qABP class of probes, GB119 [9,11], we have shown that topical application of GB119 can rapidly identify active proteases present in the peri-tumor spaces and margins when it was used in an animal model of brain cancer [11]. More specifically, such topical administration appears to be more effective than systemic administration for identification of small clumps of tumor cells that presumably have not develop a vasculature [11]. Additionally, since GB119 is labeled with a Cy5 dye that fluoresces in the near-infrared region of the spectrum, it has significant advantage potentially eliminating much of the auto-fluorescence background encountered with probes in the visible wavelength such as FITC.
However, in spite of the attractiveness and potential efficacy of this approach for rapid detection of cancerous tissue in surgical samples ex vivo, our understanding of probe’s behavior inside of the tumor and benign tissues is still illusive. Unfortunately, high-resolution microscopic analysis of treated brain tumor tissues is at best difficult because fixation and staining of tissues eliminates most of the fluorescence from unquenched probe. To further understand the unquenching of the probe and associate the activity with particular cell types within the specimen, we have developed an immune-staining technology to visualize the probe. This approach has allowed us to selectively identify unquenched GB119 using an anti-Cy5 antibody, Fig. 1, and has demonstrated that the major sites of unquenching of the probe resides in the peri-tumor spaces and is not isolated to only tumor cells.
Figure 1. Mechanism for immunorecognition of unquenched GB119.
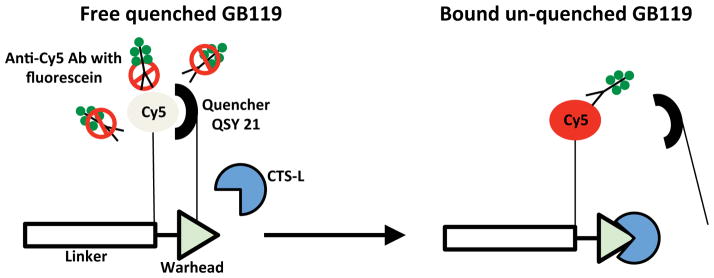
GB119 consists of four parts: i) a reactive element (warhead) that confers specificity towards cysteine proteases, ii) a linker that often contains recognition elements, iii) a fluorescent molecule (Cy5) for detection, and iv) a quencher moiety (QSY 21) that keeps Cy5 optically silent. When qABP is in its quenched form it is envisioned that QSY 21 may restrict the availability of Cy5 for anti-Cy5 antibody. Activation and unquenching of GB119 releases the QSY 21 and allows access of the anti-Cy5 antibody to bind Cy5. Each anti-Cy5 antibody carries from 3 to 6 molecules of fluorescein after conjugation serving to amplify the presence of Cy5 labeled probe, which only has one Cy5 per unquenched GB119 molecule.
MATERIALS and METHODS
Ethics Statement
All procedures were performed aseptically according to the Case Western Reserve University Institutional Animal Care and Use Committee (Case IACUC: Animal Experimental Protocol: 2012-0024).
Cell culture preparation for orthotopic implants and animal surgery
Human Gli36Δ5EGRF glioblastoma cell lines obtained as a gift from Dr. EA Chiocca were grown as described [11,12]. Athymic nude female mice (nu/nu, 6–8 weeks at time of surgery) were implanted with tumors as described previously [11].
Immunostaining, Imaging, and Histology
Staining of nitrocellulose membranes for Cy5
For immuno-detection of unquenched GB119 applied to nitrocellulose membranes (NCMs) a mouse anti-Cy5 antibody (clone CY5-15, Sigma) conjugated with fluorescein (AbD Serotec, LNK061F kit) was used. A 1:250 dilution of anti-Cy5 antibody in Protein-Free Blocking Buffer (Thermo Sci, IL) was added to the wells in 12-well plates and incubated with agitation for 1 hour at room temperature (RT) in the dark with agitation.
Imaging of nitrocellulose membranes
After washing twice in TBST for 5 min with agitation in the dark the NCMs were imaged. Fluorescence of GB119/cathepsin-dependent Cy5 fluorescence and anti-Cy5/fluorescein activity from the same spot on the surface of NCM was measured in parallel and was separated in time only by changing of filter sets to separate Cy5 GB119 (yellow filter set, Ex 595 nm, Em 635 nm) and fluorescein signals (blue filter set, Ex 455 nm, Em 490 nm) using a Maestro Imaging device (Maestro™ Multispectral In-Vivo Imaging System (CRi, Inc., Woburn, MA). Maestro stage positions were set to either position 1 or 3 depending on the intensity of the fluorescence signal.
Imaging of Tissue Sections
The brain was sectioned in 2 mm slices through the Gli36Δ5 tumor xenograft using an acrylic 1 mm frontal brain matrix (Kent Scientific). Baseline imaging of the tissue was acquired prior to probe application. Following initial imaging brain specimens were washed with saline, dried with KimWipes followed by topical application of 5 μL of GB119 (10 μM) in 100% DMSO [11]. At the indicated times the sections were imaged in the Maestro Imaging device with a filter set appropriate for Cy5. Prior to the final image at 30 minutes the treated areas were washed with 100 μL of saline twice to remove any unbound probe.
Probe Application
Freshly resected tissue was washed with phosphate buffered saline (PBS) and blotted dry with gauze. Probe application to tissue was performed after drying by placing a paper applicator on top of blotted dry tissue and immediately wetting it with GB119 in 100% DMSO. Complete contact of the paper applicator to the tissue was ensured by gentle patting of the applicator with a pipet tip. This ensured complete, even, and rapid probe distribution and application to the tissue.
Staining of tissue sections for Cy5, CTS-L, vimentin, and CD11b macrophages
Following imaging sections were snap-frozen in optimum cutting temperature compound (OCT) for cryosectioning (Leica CM3050S). Sections, 10 μm thick, were collected serially directly on to slides and stored at −80°C until processing. For immunohistochemical analysis the slides were warmed to RT for 10 minutes, fixed with 2% buffered formalin, and blocked in Background Sniper (Biocare Medical) for 10 min and incubated in primary antibody for 1–2 hours at RT followed by 3 five minute washes in TBST. For visualization of human tumor tissue, a rabbit anti-vimentin antibody (clone SP20, Spring Bioscience) was used at a 1:10 dilution. The tumor and brain expression of human CTS-L (Athens Res Tech Inc., Athens, GA) was evaluated by goat anti-human CTS-L antibody (RD Systems Cat#: AF952) at a 1:10 dilution. Presence of brain tumor associated macrophages and/or macroglia was evaluated by rat anti-CD11b antibody (Abcam, clone M1/70) using a 1:10 dilution. For detection and visualization of unquenched GB119, a mouse anti-Cy5 antibody conjugated with fluorescein was used at a 1:20 dilution. Manufacturer suggests standard labeling typically results in 3–6 fluorescein molecules attached per antibody, providing target amplification. IHC was performed by incubating with the indicated primary antibodies simultaneously. After washing the slides were treated with the appropriate combination of secondary antibody (donkey anti-rabbit, donkey anti-goat, and donkey anti-rat antibodies labeled with Alexa350, Alexa594, and Alexa594 respectively; Invitrogen Inc.) for 20 min at RT followed by triple washing with TBST for 5 minutes. Then slides were coverslipped with Crystal-Mount media (Electron Microscopy Sciences). Hematoxylin and eosin staining was carried out on adjacent sections by standard procedures.
In Vitro Detection of Unquenched/Activated Form of GB119
In vitro detection of cathepsin-L-induced unquenching of GB119 is detectable using anti-Cy5 antibodies
For detection of unquenched GB119, 2.5 μL of the probe (10 μM, 1% dimethylsulfoxide (DMSO) in sterile PBS) were spotted on to circles of NCM and placed on the bottom of the wells in a 12-well plate. After drying in the dark for 30 minutes, the NCMs were pre-washed in the dark for 2x5 min in sterile PBS. For detection of unquenched GB119, human liver CTS-L at a final concentration 1.35 μg/mL in sterile PBS was incubated with spots of GB119 on NCMs at RT for 20 min in the dark. As a control for specificity of unquenching of GB119, CTS-L was pre-incubated with 200 μM JPM-OEt, a protease inhibitor (PI) for 1 hr. and then applied onto pre-washed NCM with GB119. Negative controls included incubation in solvent alone (1% DMSO) in sterile PBS or solvent in combination with PI. Next, NCMs were blocked with Protein-Free Blocking Buffer for 30 min with constant agitation in the dark, followed by immunostaining with fluorescein-labeled anti-Cy5 antibodies and fluorescent imaging for either Cy5 or fluorescein. To study anti-Cy5 antibody recognition of the probe in a complex biological mileu similar experiments were conducted using cellular lysates derived from Gli36Δ5 in place of pure human CTS-L.
Competitive assay of availability of Cy5 in GB119 for anti-Cy5 antibody in vitro
To generate a source of nitrocellulose bound Cy5 2.5 μL of Cy-5-labelled donkey anti-chicken IgG polyclonal antibody (250 μg/mL, Chemicon, Temecula, CA), was applied to NCM, dried for 30 min in the dark, blocked with Protein-Free Blocking Buffer for 2 hr. with constant agitation in the dark, and then immunostained for 1 hr. with anti-Cy5 antibody conjugated with fluorescein as described in above. Prior to staining anti-Cy5 antibodies (6 ng) were pre-adsorbed in the dark for 1 hour with either Cy5-labelled donkey IgG (25 μg) in a final volume of 250 μL, quenched GB119 (2.5 nmoles in PBS), CTS-L unquenched GB119 (2.5 nmoles of GB119 were incubated with 0.337 μg CTS-L in a volume of 250 μL for 30 min at RT) or GB119 unquenched in the presence of CTS-L and an excess of a cathepsin PI, JMP-OEt. Protease inhibition was performed for 1 hour at RT by combing an excess of JMP-OEt (50 nmoles) with CTS-L (0.337 μg) in a total volume of 250 μL. Levels of auto-fluorescence for fluorescein from NCM blots of donkey antibody labeled with Cy5 were determined by not including anti-Cy antibodies in a set of samples (negative control) and this value subtracted from the levels of fluorescence in all experiments. Data are presented as a percentage of fluorescein fluorescence intensity remaining after blotting. To control for the amount of Cy-5 labeled donkey IgG adherent to the NCM, measurements of Cy5 fluorescence were also made with no difference in Cy5 fluorescence among all NCM blots (data not shown).
Microscopy
Fluorescent images were viewed with a Leica DM4000B upright microscope (for Alexa350 bandpass (BP) 360/470 (vimentin), fluorescein BP 480/527 (anti-Cy5 antibody conjugated with fluorescein), and Alexa594 BP 560/645 (CTS-L or CD11b). An Olympus VS120-S5 versatile microscope-based fluorescent scanner system was used to generate images larger than a single field of view. Commercially available software (QCapturePro, Version 5.1) was used to analyze and present the fluorescent images. Overlays between H&E and IHC images were done manually.
Imaging and Analysis
To compare signal intensities, regions of interest (ROI) were selected over the entire treated area and for control regions (for in vitro assays regions of nitrocellulose that did not contain human CAT-L or lysate; for tissue sections images derived prior to probe application to the tissue). Commercially available software (Maestro™ software 3.0.1.2, Cri, Woburn MA) was used for analysis of total signal in the ROI.
Statistics
The values are expressed as mean +SD. The significance of measured values between the groups was performed by one-way analysis of variance (ANOVA) with 95% confidence limits for multiplex analysis. Spearman rank test was used for correlation analysis. The p values less than 0.05 were taken as significant in experiments.
RESULTS
Identification of GB119 unquenching using an anti-Cy5 antibody
GB119 is a compound that has been engineered to covalently modify enzyme targets in an activity dependent manner. Rather than acting as a substrate that is processed by the protease, they act as suicide inhibitors of the protease. GB119, as a typical quenched ABP, contains a reactive functional group (warhead) linked to fluorophore tag (Cy5) and a quencher QSY 21 (Fig. 1) [5,9]. This “smart probe” only produces a fluorescent signal when catabolized by a particular protease. For GB119 the protease it interacts with most robustly is CTS-L.
Previous studies have demonstrated that fixation, sectioning, and staining of fluorescent tissue containing unquenched GB119 reduces its fluorescent signal so that it is not detectable using fluorescence microscopy, except in very thick sections where it is difficult to clearly elucidate the location of fluorescent signal [11]. Therefore, in order to identify the location of unquenched GB119 within tissues we developed an immunohistochemistry assay to detect unquenched probe. We noticed that anti-Cy5 antibody did not recognize intact GB119 very well, but that after activation by proteases the reaction with antibody was much stronger. We speculated that removal of the quencher made Cy5 more bioavailable and able to be recognized by an anti-Cy5 antibody (Fig. 1). We tested this idea by utilizing a blot assay to capture unquenched GB119 on nitrocellulose and then probe it with anti-Cy5 antibody.
First, we confirmed that unquenching of the probe results in bioavailable Cy5 for antibody recognition using a competitive binding assay, Fig. 2a–c. Donkey IgG labeled with Cy5 was adsorbed to nitrocellulose membranes to serve as a target for the anti-Cy5 antibodies. As Fig. 2a shows, anti-Cy5 antibodies recognized the substrate bound to the nitrocellulose and that binding was blocked by pre-adsorbtion of the antibodies with substrate. Next, either GB119 or GB119 that had been first unquenched with CTS-L was used to pre-absorb anti-Cy5 antibodies, which were then used to probe Cy5-IgG on a nitrocellulose filter. Unquenching of GB119 dramatically increased the ability of the probe to block antibody binding to the nitrocellulose fixed Cy5, Fig. 2b. Inclusion of a cathepsin inhibitor during the unquenching step prevented any blocking of antibody binding to Cy5, Fig. 2b. Fluorescent microscopy of anti-Cy5 antibody fluorescence from nitrocellulose blots in Fig. 2b showed a strong differential suggesting that this approach might be suitable for visualization of unquenched GB119 in tissue, Fig. 2c. Altogether these data suggest that both levels of unquenching of GB119 and availability of Cy5 to anti-Cy5 Ab are consequences of the same event.
Figure 2. Cathepsin-L-induced unquenching of GB119 increases availability of Cy5 in GB119 for anti-Cy5 antibody in vitro.
a) Assay Validation. Donkey IgG labeled with Cy5 (Dky IgG/Cy5) was adhered to nitrocellulose membrane (NCM) and then probed with Anti-Cy5 antibodies that were untreated (Anti-Cy5 Ab) or pre-adsorbed with donkey Dky IgG/Cy5 (Anti-Cy5 Ab, Dky IgG/Cy5). Data was normalized to average signal measured when the anti-Cy5 was not pre-adsorbed. Color inset represents typical signal measured from the nitrocellulose using the Maestro imaging device. b) Pre-adsorption of anti-Cy5 Ab with GB119. Dky IgG/Cy5 was adhered to NCM and then probed with i) anti-Cy5 antibody pre-incubated with quenched GB119 (Anti-Cy5, GB119); ii) anti-Cy5 antibody pre-incubated with GB119 that had first been unquenched with human cathepsin-L (CTS-L) (Anti-Cy5Ab, GB119, CTS-L); and iii) anti-Cy5 antibody pre-incubated with GB119 that had been unquenched with human CTS-L in the presence of a general papain family protein inhibitor, JMP-OEt (PI), i.e. negative control, (Anti-Cy5 Ab, GB119, CTS-L, PI). Color inset represents typical signal measured from the nitrocellulose using the Maestro imaging device. c) Pseudo green color images of anti-Cy5 antibody fluorescence from NCM blots of donkey antibody labeled with Cy5 as in (b) that were taken by Leica microscope (1.25x) indicating differential is suitable for visualization of unquenched GB119 in tissue. d) Pre-washed blots of GB119 on NCM were incubated with i) CTS-L alone, ii) PBS alone, or as controls a mixture of iii) CTS-L and an excess of a PI, or iv) a mixture of PBS and the PI. Fluorescent images indicate strong unquenching of GB119 by CTS-L only in the absence of inhibitor. PBS alone did not unquench GB119. e) Quantitative analysis of levels of Cy5 fluorescence. f) Nitrocellulose membranes from inset (2d) were probed with fluorescein-labeled anti-Cy5 antibodies and imaged for fluorescence. Results were similar to those determine in inset (d). g) Quantitative analysis of levels of anti-Cy5 Ab fluorescence.
For a) the difference in signal was significant, p<0.001; for b) the difference in signal was compared to anti-Cy5Ab, GB119 and was significant for both cases, p<0.001 and p<0.05, respectively. Statistical data (e) and (g) are presented as mean levels of fluorescence from which matched PBS only controls were subtracted. For d) p<0.001; for g) p<0.05. Cy5 fluorescence (emission filter = 645 nm); Fluorescence of anti-Cy5 Ab (emission filter = 515 nm). Error bars represent +SD.
Next, we analyzed whether fluorescence generated from unquenched GB119 correlated with presence of CTS-L. To assess this, quenched GB119 was blotted on to nitrocellulose filters, washed, and then treated with active human CTS-L or saline and imaged for Cy5 fluorescence resulting from probe unquenching, Fig. 2d & e. (Washing was performed as a first step to ensure that only tightly bound GB119 was assessed for these studies.) To control for specificity JPM-OEt, a broad-spectrum cathepsin inhibitor, was incubated with the CTS-L and then used to activate another set of samples. Cy5-fluorescence directly from unquenched GB119 was readily visible from samples treated with CTS-L but not those treated with saline alone or CTS-L plus JPM-OEt. Further, we analyzed whether fluorescence generated from unquenched GB119 correlated with anti-Cy5 antibody detection of activation-exposed Cy5, Fig. 2f & g. To assess this, the blots from Fig. 2d were probed with commercially available anti-Cy5 antibody labeled with fluorescein and re-imaged. Since the wavelength spectra of excitation and emission of these two fluorochromes minimally overlap we can compare the signal from each assay. Figure 2f indicates that unquenched GB119 is preferentially recognized by the anti-Cy5 antibody in samples that were incubated with CTS-L alone. Inclusion of the inhibitor also reduced the signal resulting from antibody probing. It is worth noting, that both washed and un-washed blots of GB119 show the same patterns of Cy5 and anti-Cy5 Ab-dependent fluorescein fluorescence (data not shown), indicating stable binding of GB119 to the NCMs in these experiments.
All together, these data indicate that binding and unquenching of GB119 by human CTS-L is specific and can be detected using an immunochemical assay. Further these data suggest that anti-Cy5 immunodetection of unquenched GB119 may be a tool to detect the unquenched form of GB119 and to potentially analyze its behavior in pathological sections of solid tissue.
Unquenching of GB119 as a function of tumor cell lysate
The next step in validation of this assay was to demonstrate that probe unquenching could be achieved by increasing amounts of human tumor cell extracts. GB119 (10 μM) was spotted on to filters and then treated (without washing) with different concentrations of cell lysates generated from Gli36Δ5, a human tumor cell line that expresses CTS-L. As a negative control GB119 was treated with similar concentrations of pre-immune rabbit IgG. After incubation the mixture was washed blocked, and immunoblotted with fluorescein–labeled anti-Cy5 antibodies. The blots were then assayed for fluorescence both of Cy5, Fig. 3a, and fluorescein, Fig. 3b. Both protease-dependent GB119 fluorescence (Fig. 3a) and detection of GB119-associated Cy5 by anti-Cy5 antibody (Fig. 3b) correlated with increased concentration of tumor lysate and were highly correlated to each other, r = 0.9, Fig. 3c.
Figure 3. Tumor lysate unquenching of GB119 is detectable using anti-Cy5 antibodies.

A constant amount of GB119 was spotted onto nitrocellulose filters, and then incubated with either rabbit polyclonal IgG (control) or increasing amounts of cell lysate derived from a human Gli36Δ5 cancer cell line to unquench GB119. Nitrocellulose membranes were imaged for: a) Cy5 fluorescence or b) anti-Cy5 antibody signal (fluorescein). Correlation between the signal from direct Cy5 fluorescence and anti-Cy5 detected GB119 unquenching was statistically correlated in inset (c). Vertical bars (a) and (b) represent mean fluorescence +SD for each point of lysate concentration. Spearman rank correlation test was used (r= 0.9; p< 0.01) to analyze relationship between levels of two types of fluorescence (c).
Identification of unquenched GB119 in brain tumor sections, ex vivo
We next assessed the ability of fluorescein–labeled anti-Cy5 antibody to detect unquenched probe in pathological sections derived from a mouse brain tumor model. For these studies an orthotopic brain tumor model was used [11]. Mice were unilaterally stereotactically implanted with Gli36Δ5 cells. When tumors were 9–10 days old, animals were sacrificed; the brains removed and sectioned into 2 mm thick sections. Only the brain sections containing visible tumor (black asterisk, Fig. 4a and 4b) were used in these studies. Age matched normal mouse brains or sham-implanted brains were used as controls for background unquenching of the probe (not shown). After obtaining base-line fluorescent image of the slices, we topically applied GB119 to both hemispheres of the brain slice and measured probe activation, i.e. fluorescence, using a Maestro imaging device. The tumor-containing area of brain consistently and rapidly unquenched the probe at the tumor-brain interface with no probe unquenching visible on normal brain tissues (Fig. 4c), as previously described [11]. The slices were washed after 25 min and re-imaged at 30 min to determine the stability of the signal. These manipulations demonstrated that the signal intensity was stable but that background could be further reduced by washing [11].
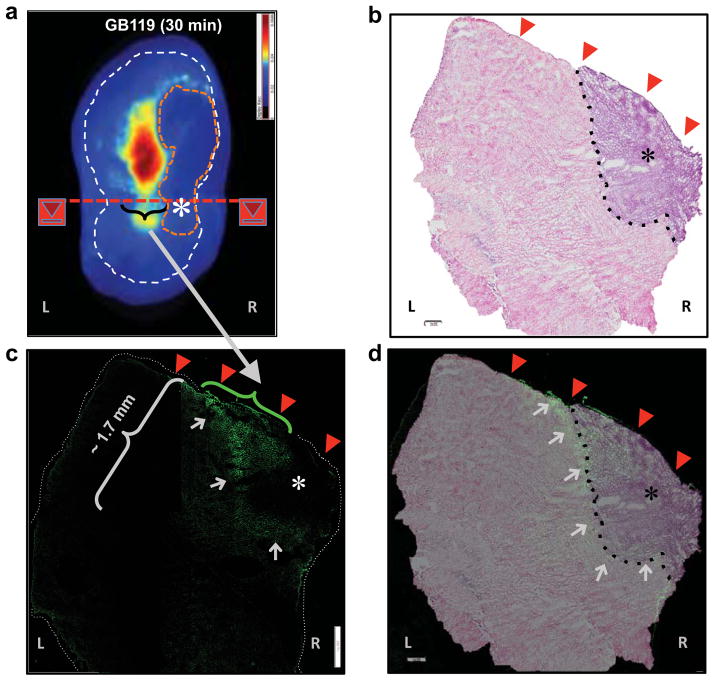
Figure 4. Topical application of GB119 to brain sections containing a tumor unquenches GB119.
Mice were stereotactically injected unilaterally with Gli36Δ5 tumor cells. After 9–10 days the animal was sacrificed and the brain sectioned as indicated in insets (a) & (b). Prior to GB119 application there was no auto-fluorescence, inset (c), left. Thirty minutes following application of GB119 significant unquenching of GB119 as demarked by fluorescence visible in the peri-tumor spaces, inset (c), right. Representative images from a single mouse are displayed (n = 3). (a) & (b): black asterisk – visible Gli356Δ5 tumor xenograft; (c): white contour – perimeter of the brain tissue surface used for probe application. White asterisk and pink dotted contour – location and approximate visible perimeter of the tumor xenograft, respectively.
In order to determine the location of unquenched GB119 we cut the 2 mm brain section perpendicular to its axis and created sections to reveal the tumor and surrounding normal brain, Fig. 5. Immunhistochemical staining of the microscopic sections with the anti-Cy5 antibody revealed unquenched probe most prevalently at the tumor-brain interface (green staining), with some lessor amounts present within the tumor mass itself. This is in agreement with our previous results [11] and those of others describing overexpression of cathepsins at the borders of human brain tumors [13]. Figure 5d is an overlay of the H&E and IHC stained sections revealing localization of the Cy5 to the tumor and peri-tumor spaces (white arrows).
Figure 5. Unquenching of GB119 can be detected by immunohistochemical analysis of tissue sections.
The brain section from Fig. 4 was cut perpendicular to the plane of the section and indicated in inset (a) and underwent IHC analysis. b) H&E staining of the sections derived from the cut described in inset (a). The tumor is clearly visible – black asterisk. c) Anti-Cy5 antibody staining for unquenched GB119 of an adjacent section of tissue. Gray arrows highlight anti-Cy5 staining, green. The dotted white line in inset (a) approximates the edges of the tissue slice, which are overlapped by the probe applicator. White asterisk – approximate the visible portion of the tumor xenograft (see Fig. 4); scale bar – 500 μm. d) Histology image (b) and image of IHC staining for Cy5 (c) overlay. Red arrowheads indicate location of topically applied GB119.
Staining with anti-Cy5 for unquenched GB119 is associated with the presence of CTS-L at the tumor-brain interface
Using our immunohistochemical assay for unquenched GB119 we next sought to determine the reason for the increased probe unquenching detected by Maestro imaging distal to the visible tumor in the brain section, black star Fig. 6a. As indicated in Fig. 6a we cut perpendicular to the 2 mm brain section revealing the z-axis of the tumor and brain tissues, Fig. 6b. This revealed tumor growth invisible from the surface of the brain slice that was replete with unquenched probe, black star Fig. 6b, corresponding to the fluorescent signals measured with the Maestro imaging device. Sections from this cut were then stained for tumor tissue (vimentin staining-blue), probe unquenching (anti-Cy5 staining-red), and CTS-L (anti-CTS-L-green) and are displayed in Fig. 6. The boxes on Fig. 6b correspond to the enlargements 1–8 in Fig. 6c.
Figure 6. Unquenching of GB119 localizes in the peri-tumor space and is associated with tumor cells, macrophages, and the expression of cathepsin-L.
The brain section from Fig. 4 was cut perpendicular to the plane of the section and indicated in inset (a) and underwent immunohistochemical analysis. b) H&E staining of the sections derived from the cut described in inset (a). Boxed regions correspond to the approximate area for the sections in inset (c). c) Frames 1–8: Immunological and H&E staining corresponding to regions 1–8 boxed in inset (b). Images are composites of three stains: Anti-Cy5 antibody staining (false red), cathepsin-L (CTS-L) (false green), and presence or absence of the cancerous tissue (vimentin – false blue). The yellow-orange color represents areas of Cy5/CTS-L co-registration along the tumor edge and on the surface of the sample. Purple indicates CTS-L and vimentin co-localization. Frame 9 (green box): H&E and IHC staining overlay derived from an adjacent section of the tumor area depicted as a green box in inset (b). Overlay of staining demonstrate that macrophages and unquenched GB119 co-localize, but that some macrophages are not localized with unquenched GB119. In non-tumor areas no macrophages were found (data not shown). Unquenched GB119 (false red), tumor tissue (vimentin staining – false blue) and macrophages (CD11b staining – false green). The yellow-orange color represents areas of Cy5/CD11b (macrophages) co-registration. Black dotted lines – approximate the edge of the tumor xenograft. Red arrowheads indicate surface where the probe was topically applied and direction of the probe penetration; scale bars =100 μm.
To confirm the specificity of IHC staining for Cy5 as a part of an unquenched GB119 molecule, we assessed co-localization of CTS-L and unquenched GB119 in our brain samples. As internal controls, tumor-free areas of mouse brain were assessed in comparison (Fig. 6c, frames 6 and 7). This staining revealed a significant reason both for lack of Cy5 fluorescence at a core of the tumor xenograft (Fig. 6a, white asterisk) and the intensive Cy5 fluorescence at tumor-brain interface (Fig. 6a, black star). Indeed, there was a lack of expression of CTS-L in the core of the tumor xenograft (Fig. 6c, frames 1–5, lack of green color). In contrast, at the tumor-brain interface expression of CTS-L was highest and co-localized with unquenching of the probe (Fig. 6c, frames 2–5). No expression of CTS-L was detected in normal brain tissue and no unquenching of GB119 was detected by the immunostain against Cy5 (Fig. 6c, frames 6 & 7). Strong co-registration of Cy5 and CTS-L expression was also detected deep inside of brain tissue along of tumor edge (Fig. 6c, frame 8). The regions of brain represented by frames 7 and 8 represent brain tissue that is at the same distance from the surface of the sample where probe was applied (Fig. 6c, red arrowheads) suggesting that lack of GB119 unquenching is not due to penetration issues with the topically applied probe.
Previously, we have shown for this animal model that a portion of tumor-associated fluorescent signals from unquenched GB119 might be related to presence of macrophages along the tumor edge [11]. In the current study we stained for macrophages using anti-CD11b antibodies and demonstrate a strong co-registration of CD11b marker and Cy5 (unquenched form of GB119) at peri-tumor space (Fig. 6c, green colored frame 9). For all IHC, staining in the absence of primary antibodies revealed no background or auto-fluorescence, data not shown.
DISCUSSION
The use of activity based probes (ABPs) for imaging applications has the major advantage of the formation of a permanent covalent bond with the enzyme, thus allowing direct biochemical identification of targets. However, the major limitation of these probes is their general fluorescence both when bound to an enzyme target and when free in solution. This limitation has been overcome when quenched probes (qABPs) became available [14]. The qABPs become fluorescent only after covalent modification by a protease target [14]. Because of their covalent modification of target proteases these probes allow an excellent opportunity to visualize the cell types that overexpress activated proteases. Unfortunately, the robust signal from unquenching of these probes after application to brain tissue, is reduced substantially by preparing tissues for histopathological analysis, impacting the ability to co-localize probe unquenching with cell type. This is likely due in part to secreted cathepsin-L that has unquenched GB119 but has been secreted from cells and is removed during the preparation of the sections. Here we have developed an alternative method to develop a tool to allow visualization of the probe in immunohistologically-studied tissues. We hypothesized that the change in conformation that occurs upon unquenching of qABPs might enable immunological detection of remaining activated probes, via their exposed Cy5 chromophore. We further reasoned that the 3–6 fluorescein molecules per anti-Cy5 antibody would serve to amplify unquenched GB119 that was not removed during the staining process. To test this hypothesis we studied levels of binding of anti-Cy5 Ab conjugated with fluorescein to Cy5 both in activated/unquenched and non-activated/quenched forms of GB119. Using either purified human CTS-L or extracts from tumor cells expressing active CTS-L we were able to generate unquenched GB119 and demonstrate a tight correlation between immunological detection of unquenched GB119 and optical imaging of unquenched GB119. Studies with sections derived from mouse brain xenografts demonstrated that the immunological detection performed in vitro with enzymes or cellular extracts was also possible using immunohistological probing of tissue sections generated from tissue treated ex vivo with GB119. Therefore, IHC detection of Cy5 may be useful to identify GB119 localization to specific regions within resected tissues.
In our study we used the validated immunodetection of unquenched GB119 to demonstrate the localization of unquenched GB119 within tissues. In every case the localization of the signal generated from imaging the tissue prior to processing for histology, was highly correlated with the location of immunohistochemical detection of unquenched GB119. Remarkably these data demonstrate that most of the probe unquenching occurs at the tumor brain interface. We further investigated the location of brain tumor unquenching of the GB119 and demonstrated the heterogeneity of the signal is replicated using the immunolocalization of the unquenched GB119, and co-localized with higher expression of CTS-L, the protease that is the most robust target for GB119. Interestingly there was much lower fluorescence from the interior of the tumor for both the fresh tissue imaging and immunological detection of GB119 and again this was associated with little to no expression of CTS-L at these locations.
Topical application of imaging probes for detection of cancer in near real-time is a fairly new technology [11,15]. Kobyashi and co-workers have developed a promising approach for rapid detection of ovarian cancer with a γ-glutamyl hydroxymethyl rhodamine green quenched probe, which is activated and internalized after interaction with γ-glutamyltranspeptidase [15]. Simply spraying this probe onto the surface of internal organs of the mouse abdominal cavity was enough to visualize presence of ovarian cancer tissue among loops of the intestine. However, it is not clear from these studies how deep the probe penetrated into target tissues.
In contrast to water-based solvents, dimethylsulfoxide (DMSO, 100%) was used to dissolve the probe in this study. DMSO has some advantages both in preservation and storage of chemical compounds. It is also well known as a universal carrier of chemicals through the tissue [16]. We have used 100% DMSO to apply our probes exploiting these characteristics of DMSO and have detected probe unquenching to approximately 1.7 mm from the site of topical application (Fig. 5c). The penetration of the probe is relatively rapid; there was no significant difference between fluorescent images of tumor xenografts that were incubated with the probe for 5 or 25 minutes (data not shown).
In the current study we found that the core of tumor xenograft lacked significant GB119-cathepsin-dependent fluorescence. There are at least two possible explanations for such fluorescent pattern in our animal tumor model: 1) the probe is unable to reach active CTS-L in the tumor tissue, or 2) that there are little to no active CTS-L proteases expressed within the core of the tumor. The first case is unlikely, as we have clearly shown that probe can penetrate deeply in to the brain tissue and become unquenched in peri-tumor regions deep within the brain. While it is possible that GB119 penetrates brain tissue differently than through tumor tissue, we have performed experiments that indicate good penetration of GB119 through tumor tissues (data not shown) making this an unlikely possibility. The second scenario likely prevails here and is related to the absence of CTS-L expression within the core of the tumor. Our immunohistological studies suggest that this is the case, as no staining for CTS-L is visible in the core of the tumor tissue. Since this antibody recognizes both inactive (30 kDa) single chain of CTS-L and heavy chains (25 kDa) of the active mature enzyme in different cancer cell line lysates [17] as well as in Gli36Δ5 cell lysate (our unpublished data), this suggests that the tumor core does not express the active form of CTS-L, although precursor CTS-L may be present. In all examples examined it appears that the expression of CTS-L and unquenching of GB119 occurs at the tumor margins where tissue remodeling is abundant. Thus, it seems likely that existence of this remodeled tissue is both crucial and compulsory to “ignite” GB119 and convert the probe from inactive/quenched to active/unquenched form.
Previously, we have shown that immunostaining of these sections for a microglial and macrophage marker, CD11b, suggested that some but not all of the tumor-associated signals resulted from microglial unquenching of the probe [11]. More recently, another qABP, BMV109, demonstrated that the margins of syngenic orthotopic mouse breast 4T1 tumor xenograft in BALB/c mice were replete with macrophages in the invasive edge of the tumor that had activated the probe [18]. It is difficult to extrapolate these data to our immune compromised animal model, however, some level of co-registration of CD11b+ macrophages and Cy5 at cancer-brain interface rather then inside of the tumor tissue, may suggest that the macrophages are partially responsible for Cy5 fluorescent tumor-associated signals in our system.
CONCLUSION
We have developed, validated and utilized an immunological assay to microscopically assess unquenching of topically applied qABPs in brain tissues, which loose unquenched probe upon fixation. This new approach may bring some advantages for further characterization of the qABPs labeled with Cy5 such as: i) tissue may be snap-frozen, collected, stored, and analyzed later at any time; ii) combination of routine histology and IHC analysis may be useful to analyze behavior of qABPs at the site of unquenching of the probe; and iii) our data may suggest that IHC staining for Cy5 is an attractive, promising, and useful tool to assess the distribution of active GB119 inside a tissue of interest.
Acknowledgments
Special thanks to Dr. Wilson for support of these studies by training and loan of his slide scanner and to Joe Meyers for his help in Fig. generation. Supported by a grant from the Coulter Foundation and the NFCR to J.P.B.
Footnotes
Conflict of Interest. JPB and MB are both board members and co-founders of Akrotome Imaging Inc. Akrotome is a company developing optical contrast agents and has licensed technology described in this manuscript from both Case Western Reserve and Stanford Universities. This does not alter the authors’ adherence to all the MIB policies on sharing data and materials.
References
- 1.Reiser J, Adair B, Reinheckel TJ. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120:3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bromme D, Wilson S. Role of Cysteine Cathepsins in Extracellular Proteolysis. In: Parks WC, Mecham RP, editors. Biology of Extracellular Matrix. Vol. 2. Berlin: Springer; 2011. pp. 23–51. [Google Scholar]
- 3.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 4.Palermo C, Joyce JA. Cysteine cathepsin proteases as pharmacological targets in cancer. Trends Pharmacol Sci. 2008;29:22–28. doi: 10.1016/j.tips.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Blum G, Weimer RM, Edgington LE, Adams W, Bogyo M. Comparative assessment of substrates and activity based probes as tools for non-invasive optical imaging of cysteine protease activity. PLoS One. 2009;4:e6374. doi: 10.1371/journal.pone.0006374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothberg JM, Bailey KM, Wojtkowiak JW, et al. Acid-mediated tumor proteolysis: contribution of cysteine cathepsins. Neoplasia. 2013;15:1125–1137. doi: 10.1593/neo.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edgington LE, Verdoes M, Ortega A, et al. Functional imaging of legumain in cancer using a new quenched activity-based probe. J Am Chem Soc. 2013;135:174–182. doi: 10.1021/ja307083b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serim S, Haedke U, Verhelst SH. Activity-based probes for the study of proteases: recent advances and developments. Chem Med Chem. 2012;7:1146–1159. doi: 10.1002/cmdc.201200057. [DOI] [PubMed] [Google Scholar]
- 9.Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat Chem Biol. 2007;3:668–677. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- 10.Verdoes M, Edgington LE, Scheeren FA. A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. Chem Biol. 2012;19:619–628. doi: 10.1016/j.chembiol.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutter JL, Cohen NT, Wang J, et al. Topical application of activity-based probes for visualization of brain tumor tissue. PLoS One. 2012;7:e33060. doi: 10.1371/journal.pone.0033060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe T, Wakimoto H, Bookstein R, Maneval DC, Chiocca EA, Basilion JP. Intra-arterial delivery of p53-containing adenoviral vector into experimental brain tumors. Cancer Gene Ther. 2002;9:228–235. doi: 10.1038/sj.cgt.7700437. [DOI] [PubMed] [Google Scholar]
- 13.Rempel SA, Rosenblum ML, Mikkelsen T. Cathepsin B expression and localization in glioma progression and invasion. Cancer Res. 1994;54:6027–6031. [PubMed] [Google Scholar]
- 14.Blum G, Mullins SR, Keren K. Dynamic imaging of protease activity with fluorescently quenched activity-based probes. Nat Chem Biol. 2005;1:203–209. doi: 10.1038/nchembio728. [DOI] [PubMed] [Google Scholar]
- 15.Urano Y, Sakabe M, Kosaka N. Rapid cancer detection by topically spraying a γ-glutamyltranspeptidase-activated fluorescent probe. Sci Transl Med. 2011;3:110ra119. doi: 10.1126/scitranslmed.3002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto H, Tokunaka S, Sasaki M, Nishihara M, Yachiku S. Dimethylsulfoxide enhances the absorption of chemotherapeutic drug instilled into the bladder. Urol Res. 1992;20:233–236. doi: 10.1007/BF00299723. [DOI] [PubMed] [Google Scholar]
- 17.Haugen MH, Johansen HT, Pettersen SJ, et al. Nuclear Legumain Activity in Colorectal Cancer. PLoS One. 2013;8:e52980. doi: 10.1371/journal.pone.0052980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdoes M, Oresic Bender K. Improved Quenched Fluorescent Probe for Imaging of Cysteine Cathepsin Activity. J Am Chem Soc. 2013;135:14726–1423. doi: 10.1021/ja4056068. [DOI] [PMC free article] [PubMed] [Google Scholar]