Abstract
In spite of its well-documented anticancer chemopreventive and therapeutic activity, the clinical development of curcumin has been limited by its poor oral bioavailability. Curcumin has low aqueous solubility and undergoes extensive first pass metabolism following oral dosing. We hypothesized that oral bioavailability of curcumin can be enhanced by increasing its absorption and decreasing its metabolic clearance simultaneously. To test this hypothesis, we formulated curcumin with naturally occurring UGT inhibitors (piperine, quercetin, tangeretin, and silibinin) in a self-microemulsifying drug delivery system (SMEDDS). Mouse liver microsome studies showed that silibinin and quercetin inhibited curcumin glucuronidation effectively. When dosed orally in mice, the SMEDDS containing curcumin alone increased curcumin glucuronide concentrations in plasma without significantly affecting parent drug concentration. Of the four inhibitors examined in vivo, silibinin significantly improved the Cmax (0.15 μM vs. 0.03 μM for curcumin SMEDDS) and the overall bioavailability (3.5-fold vs. curcumin SMEDDS) of curcumin. Previous studies have shown that silibinin has anticancer activity as well. Thus, co-delivery of silibinin with curcumin in SMEDDS represents a novel and promising approach to improve curcumin bioavailability.
Keywords: Metabolism, absorption, oral drug delivery, microemulsion, cancer chemoprevention
Introduction
Curcumin, a dietary polyphenol derived from turmeric, is a potent antioxidant and anti-inflammatory and has shown significant anti-cancer effects [1–4]. The clinical potential of curcumin, however, has been limited due to its low bioavailability (<2%) [5–7]. Low aqueous solubility and extensive hepatic and intestinal metabolism contributes to its poor absorption [8–10]. Curcumin undergoes extensive phase one and phase two hepatic metabolism. Primarily, UDP-glucuronosyltransferase (UGT) and sulfotransferase (SULT) convert curcumin to its glucuronide and sulfate metabolites, respectively. Glucuronidation by UGT1A1, 1A8, and 1A10 accounts for the majority of curcumin metabolism [9, 11]. Thus, to improve the oral bioavailability of curcumin, both low solubility and high metabolic clearance must be overcome.
Several alternative drug delivery approaches have been explored to improve the bioavailability of curcumin [12]. Nanoparticle systems containing curcumin have the benefit of shielding curcumin from metabolizing enzymes, and can improve curcumin availability in tumors through passive and active targeting approaches. Implantable polymeric devices capable of sustaining curcumin plasma levels for at least 90 days have also been reported [13, 14]. We have previously developed an injectable polymeric microparticle formulation of curcumin, which sustained curcumin plasma concentrations for at least one month and resulted in significant inhibition of tumor growth in an orthotopic mouse model [15, 16]. While these approaches are highly promising, there are concerns regarding the acceptability of frequent injections for chronic disease prevention and the cost associated with clinic visits required for such administration [17]. Formulations that can be administered orally can circumvent these problems and are thus highly suitable for chronic chemoprevention.
Previous studies have shown that self-microemulsifying drug delivery systems (SMEDDS) can be used to increase the solubility and oral absorption of hydrophobic compounds [18–20]. SMEDDS are comprised of one or more surfactants dispersed in an oil phase. When added to an aqueous phase (either prior to dosing or in situ in the gut), a thermodynamically stable nanoemulsion is formed. Previous studies have shown that SMEDDSs can be used to dissolve high concentrations of curcumin (up to 50 mg/ml) [18, 21], making them a promising formulation for the oral delivery of curcumin.
There have been very few previous attempts to decrease curcumin metabolism through adjuvant therapies. A study by Shoba et al showed that administering piperine, an alkaloid found in black pepper, with curcumin improved curcumin bioavailability 2000% compared to curcumin alone [22]. However, this study did not firmly establish the mechanism of improved curcumin bioavailability with piperine. Several other natural compounds, particularly flavonoids such as silibinin, quercetin, and tangeretin, have been shown to inhibit UGT activity [23, 24], and therefore would be beneficial adjuvant compounds. Additionally, these natural compounds have shown chemopreventive and chemotherapeutic activity against a variety of cancers [25, 26] providing further benefit from co-administration. Thus, flavonoids offer great potential as an adjuvant therapy to increase curcumin bioavailability and anticancer activity.
The goal of the current study was to evaluate the effect of co-delivering a natural inhibitor of curcumin metabolism using a SMEDDS formulation on the oral bioavailability of curcumin. In vitro hepatic microsomal assays were used to identify promising UGT inhibitors. The ability of these inhibitors to improve the oral bioavailability of curcumin was then investigated in vivo.
Materials and methods
Materials
Curcumin (>98% curcuminoid content), piperine, silibinin, quercetin, epigallocatechin gallate (EGCG), uridine 5′-diphospho-glucuronic acid (UDPGA), alamethicin, Carbitol™ and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO). Tangeretin was generously provided by the Florida Department of Citrus. Cremophor® EL was from BASF (Florham Park, NJ). Captex® 355 was from Abitec Corp (Columbus, OH). Acetonitrile was purchased from Fisher Scientific (Pittsburg, PA). CD-1 female pooled mouse liver microsomes were purchased from BD Biosciences (San Jose, CA).
Microsomal studies
Curcumin and a selected inhibitor (piperine, silibinin, quercetin, EGCG, tangeretin or salicylic acid) were first dissolved in DMSO. This solution was then added to Tris buffer (10 mM, pH 7.1) containing 5 mM magnesium chloride, 5 μg alamethicin, and 100 μg/mL of microsomal protein. Final DMSO concentration was 2% for all samples. Final volume of this reaction mix was 0.2 mL. Concentration of curcumin in the assay buffer was 1μM, and was chosen based on reported Km values for UGT1A1 metabolism [27]. Samples were first incubated at 37 °C for two minutes, followed by the addition of UDPGA (3 mM) to start the reaction. The reaction was stopped after 5 minutes by addition of ice-cold acetonitrile (3:1). Samples were refrigerated for 15 minutes and then centrifuged for 10 minutes at 14,000 rpm. Curcumin concentration in the supernatant was determined by HPLC.
SMEDDS
The SMEDDS used for all the studies consisted of Cremophor® EL, an FDA approved excipient, and Carbitol™ (2:1 w/w), mixed with Captex® 355 (10:1 w/w, surfactants to oil). Equilibrium solubility of flavonoids in this formulation was determined by adding excess compound to the formulation and incubating at room temperature overnight with stirring. The saturated formulation was centrifuged at 14000 rpm for 10 min. Supernatant was then diluted with methanol and analyzed by HPLC (see below). Particle size distribution was determined using a Beckman-Coulter Delsa Nano C Particle Analyzer (Brea, CA). SMEDDSs were diluted with distilled water (1:200 v/v) prior to analysis.
Pharmacokinetic studies
All the animal studies were approved by the Institutional Animal Care and Use Committee of University of Minnesota. Animals were housed in specific pathogen-free environment, with access to food and water ad libitum. Female BALB/c or CD-1 mice, 8–12 weeks of age, were dosed by oral gavage with curcumin suspension prepared in 1% carboxymethylcellulose, curcumin solution (DMSO solubilized), curcumin SMEDDS, curcumin with piperine SMEDDS, curcumin with tangeretin SMEDDS, curcumin with quercetin SMEDDS, or curcumin with silibinin SMEDDS. All SMEDDS formulations were dosed in the oil phase, without prior addition of water. Curcumin, quercetin, and silibinin were dosed at 100 mg/kg for all the studies, while piperine was dosed at 125 mg/kg and tangeretin was dosed at 33 mg/kg. Mice were sacrificed at 0.5, 1, 2, 4, or 6 hours post-dose. Blood was collected from the facial vein and/or by cardiac puncture into a heparinized tube (Terumo Capiject, Somerset, NJ) and then centrifuged at 20000 × g for 5 minutes to obtain plasma. Other tissues were collected in pre-weighed glass test tubes. Tissues and plasma were stored at −20° C until analysis. Non-compartmental analysis of the plasma concentrations was performed using Phoenix WinNonLin software version 1.2 (Pharsight, St. Louis, MO) to estimate Area Under the Curve (AUC).
Curcumin extraction from plasma and tissue
Acetonitrile was added to thawed plasma (1:4 v/v, plasma to acetonitrile) to precipitate proteins. Plasma samples were centrifuged at 20000 × g for 10 minutes, and the supernatant was used directly for HPLC analysis. Other tissues were weighed and homogenized in 4 mL distilled water using a hand-held homogenizer (Omni International, Kennesaw, GA) and then lyophilized for 48 hours (Labconco, Kansas City, MO). Curcumin was extracted from dried tissues using acetonitrile for ~18 hrs at room temperature on a rotary extractor. Tissues were centrifuged twice, first at 3200 × g and then at 20000 × g to rid samples of tissue debris. Final supernatant was used directly for HPLC analysis. Stability of curcumin during the extraction and the efficiency of extraction were determined by spiking tissues from untreated animals with 50 μg of curcumin dissolved in DMSO prior to lyophilizing, followed by extraction and analysis of curcumin as described above.
HPLC analysis
All HPLC analyses were performed using a Beckman Coulter HPLC system attached to UV-PDA and fluorescence (Jasco, Easton, MD) detectors. Sample injection volume was 50 μL for all analytes.
Curcumin and curcumin glucuronide
The mobile phase consisted of acetonitrile and 10 mM ammonium acetate (65:35 v/v) with a flow rate of 1 mL/min. Compounds were separated on a Scherzo SM C-18 (150 × 4.6 mm, 5 μm) column (Imtakt, Philadelphia, PA). Curcumin (retention time, 3.9 min) and curcumin glucuronide (retention time 6.6 min) were detected using a fluorescence detector (excitation 420 nm, emission 470 nm for both compounds). Total run time was 9 minutes.
Curcumin, tangeretin and piperine
The three compounds were separated on an Agilent Zorbax SB-C18 column (250 × 4.6 mm; 5 μm, Santa Clara, CA), fitted with an Agilent Zorbax cartridge guard column (C-18, 12.5 × 4.6 mm). Mobile phase consisted of acetonitrile and 10 mM ammonium acetate buffer adjusted to pH 4 with glacial acetic acid (65:35), running at a flow rate of 1 ml/min. Curcumin (λmax = 430 nm) eluted at 4.9 min. Piperine (λmax = 340 nm) eluted at 5.6 min. Tangeretin (λmax = 280 nm) eluted at 6.7 min. Total run time was 9 min. Curcumin glucuronide co-eluted with the solvent front (1.6 min), making it difficult to accurately determine curcumin glucuronide concentrations in this assay. Incubation of plasma diluted with 50 mM phosphate buffer and 5000 U β-glucuronidase (Sigma) confirmed the identity of this peak (data not shown). Linear standard plots were obtained over the range of 0.015–0.5 μg/mL with R2 > 0.99.
Curcumin and quercetin
A gradient method was used to separate curcumin and quercetin (λmax = 370 nm) using an Agilent Eclipse XBD-C18 (150 × 4.6 mm, 5 μm) column with an Agilent Zorbax cartridge guard column (C-18, 12.5 × 4.6 mm). The mobile phase consisted of A: 10 mM ammonium acetate, B: acetonitrile. The gradient conditions were – 0–2 min B 35%, 2–4 min B 35–65%, 4–9 min B 65%, 9–10 min B 65–35%. Quercetin eluted at 3.9 minutes. The retention time for curcumin was 7.9 minutes. Curcumin and quercetin produced linear standard plots over the range of 1–25 μg/mL with R2 > 0.99.
Curcumin and silibinin
Silibinin (λmax = 288 nm) and curcumin were separated on the Agilent XBD Eclipse C-18 column with an Agilent Zorbax cartridge guard column (C-18, 12.5 × 4.6 mm). Mobile phase consisted of A: 10 mM ammonium acetate, B: acetonitrile. The gradient conditions were – B 35%. 0–2 min B 20%, 2–4 min B 20–70%, 4–8 min B 70%, 8–9 min B 70–20%. Retention time of curcumin and silibinin were 7.6 and 5.6 minutes, respectively. Curcumin and silibinin produced linear standard plots over the range of 0.015–0.5 μg/mL with R2 > 0.99.
Statistical analysis
SigmaPlot software was used to test for the significance of differences using ANOVA followed by post-hoc Newman Keuls testing, with p < 0.05 being considered statistically significant.
Results
Microsomal Studies
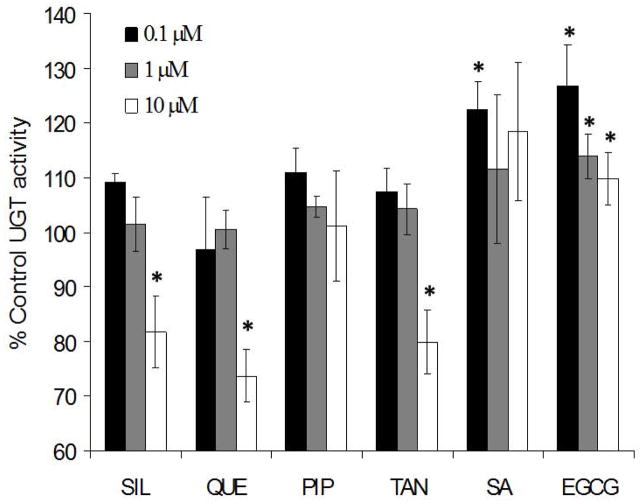
Flavonoids share chemical structure attributes with curcumin (Figure 1), making them attractive candidates for competitively inhibiting curcumin metabolism. Mouse liver microsomal assays were carried out with a panel of naturally occurring compounds to identify molecules that have inhibitory effects on curcumin glucuronidation. Members of the panel were selected based on previously reported inhibitory activity on UGTs as well as anticancer activity. For example, piperine has been shown to increase curcumin bioavailability [22] and to modulate a variety of metabolic enzymes and cancer pathways [28–30]. Silibinin (derived from milk thistle) and quercetin (from fruit peels and onions) [23] have been shown to modulate UGT enzymatic activity and have anticancer effects [31, 32]. In our studies, tangeretin, quercetin and silibinin showed significant inhibitory effects on curcumin metabolism (Figure 2). Inhibitor concentrations were limited to 10 μM to mimic concentrations achievable in vivo. Quercetin, silibinin and tangeretin showed 20–30% inhibition at the maximum concentration tested compared to blank controls. The other compounds used showed either no inhibitory effect (piperine) or slightly increased UGT activity (EGCG and salicylic acid).
Figure 1.
Structure of curcumin and the natural inhibitors of UGT metabolism used in this study.
Figure 2.
Inhibition of curcumin glucuronidation. Curcumin (1 μM) was incubated with piperine (PIP), quercetin (QUE), silibinin (SIL), tangeretin (TAN), epigallocatechin gallate (EGCG) or salicylic acid (SA) at various concentrations with mouse liver microsomes (n = 3). Data are presented as % of control curcumin glucuronidation activity ± SD. *p<0.05 as determined by a Students t-test versus incubations with no inhibitor.
SMEDDS
Previous studies have shown that SMEDDS containing Cremophor® EL solubilized curcumin to a greater extent than those formulated with some other surfactants [18, 33, 34]. The SMEDDS used in our studies showed high curcumin and inhibitor (piperine, silibinin, or quercetin) solubility (Table 1). A concentration of 30 mg/ml was suitable for 100 mg/kg dosing (equivalent to 1 g dose in a human [35]), making this formulation suitable for human dosing in the future. Dynamic light scattering was used to characterize the nanoemulsion formed by the addition of SMEDDS to water. No significant difference in size was observed between curcumin-loaded and curcumin and inhibitor-loaded formulations.
Table 1.
Solubility of compounds in SMEDDS formulation with corresponding particle size
| Compound | Solubility (mg/g) | Particle size (nm) |
|---|---|---|
| Curcumin | 51.5 | 17.6 ± 4.7 |
| Piperine | 45.2 | 21.5 ± 8.8 |
| Quercetin | 34.4 | 15.1 ± 3.0 |
| Silibinin | 32.8 | 16.8 ± 2.4 |
Effect of formulation on curcumin plasma levels
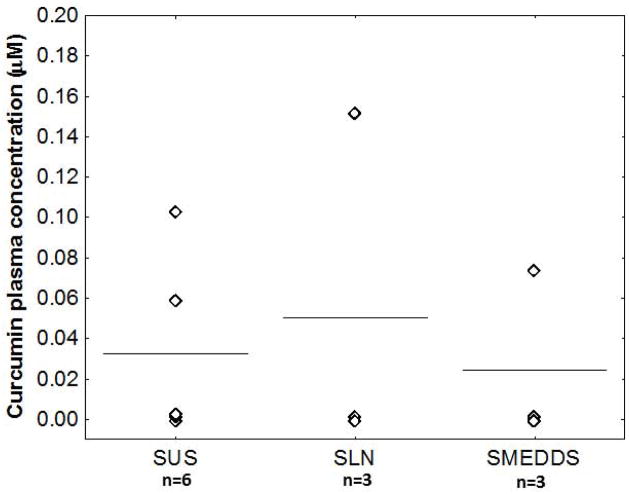
Several different formulations were investigated for oral delivery of curcumin. Plasma concentrations observed one hour post-dose [suggested tmax of curcumin [34]] are shown in Figure 3. The SMEDDS containing curcumin alone did not show a significant increase in plasma concentration compared to curcumin suspension or curcumin solubilized in DMSO. All formulations showed significant variability, with some animals having no detectable curcumin in the plasma. The observed variability in plasma levels is likely the result of differences in metabolic enzyme levels between animals, as well as in food consumption and gastric emptying time. Overall, the SMEDDS resulted in much higher plasma concentrations of curcumin glucuronide, which was essentially absent in animals treated with either the suspension or solution formulations (data not shown).
Figure 3.
SMEDDS formulation does not increase plasma levels of curcumin. Mice (n = 3–6) were dosed orally with curcumin (100 mg/kg) either as a suspension (SUS), solubilized in DMSO (SLN) or in the SMEDDS formulation (SMEDDS). Mice were sacrificed after one hour and curcumin plasma concentrations were determined by HPLC. Data points represent curcumin plasma concentration in individual animals, with lines signifying group mean.
Effect of metabolic inhibitors on curcumin pharmacokinetics
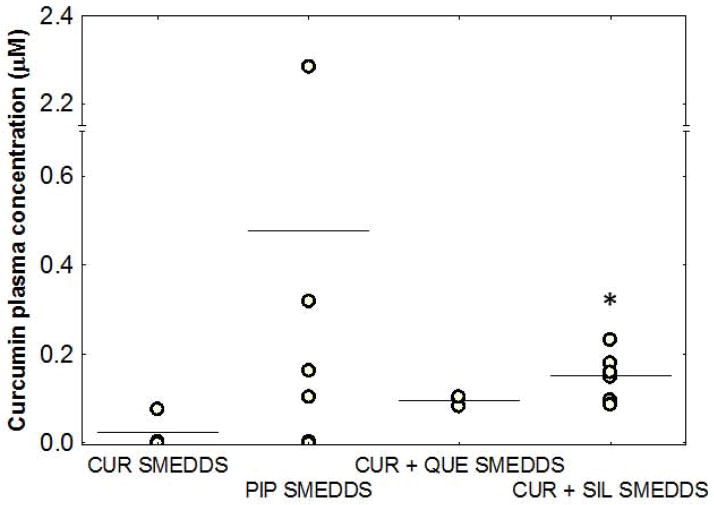
Administration of tangeretin with curcumin in the SMEDDS formulation produced no detectable levels of curcumin in the plasma one hour after administration (data not shown). However, co-administration of piperine with curcumin using the SMEDDS formulation resulted in higher average Cmax values than administration of curcumin alone, but was also associated with high variability (Figure 4). Piperine was well absorbed from this formulation and resulted in high (~30 μM) plasma concentrations one hour after dosing. However, piperine treatment appeared to stress the mice, as evidenced by decreased mobility and squinting eyes, almost immediately after dosing. Although piperine co-delivery resulted in higher curcumin levels in this study, the effect was highly variable (0–2.4 μM curcumin plasma levels), which was deemed unacceptable.
Figure 4.
Addition of quercetin (QUE) or silibinin (SIL) reduces variability and improves curcumin plasma concentrations. Mice were administered orally with SMEDDS containing curcumin (100 mg/kg, n=3) and either piperine (125 mg/kg, n=6), QUE (100 mg/kg, n=3), or SIL (100 mg/kg, n=6). Mice were sacrificed after one hour and blood was collected and processed to obtain plasma. Curcumin plasma concentrations were analyzed by HPLC. Data points represent curcumin plasma concentration in individual animals, with lines signifying group mean. *p<0.05 as determined by ANOVA followed by post hoc Newman Keuls testing compared to CUR SMEDDS.
Co-administration of either quercetin or silibinin reduced the variability in plasma concentrations of curcumin and increased the average Cmax of curcumin after oral dosing (Figure 4) compared to that without the inhibitor. Silibinin showed significantly higher plasma levels one hour after dosing (p<0.05) and therefore the highest potential to improve curcumin bioavailability. Based on this, a full time-course study was conducted for the curcumin-silibinin combination formulation. As can be seen from Figure 5A, plasma levels of curcumin were much higher and were still detectable four hours after dosing of the combination compared to that when curcumin was dosed alone. Silibinin co-administration improved the overall bioavailability of curcumin by ~3.5-fold [AUC(0–6 hrs) = 0.2613 ± 0.0368 vs. 0.0808 ± 0.0469 hr*μmol/L for curcumin SMEDDS]. Silibinin plasma concentrations peaked at 30 minutes and remained steady throughout the study (Figure 5B). Mice tolerated the curcumin-silibinin combination well, and no overt signs of toxicity were observed in this short-term study.
Figure 5.
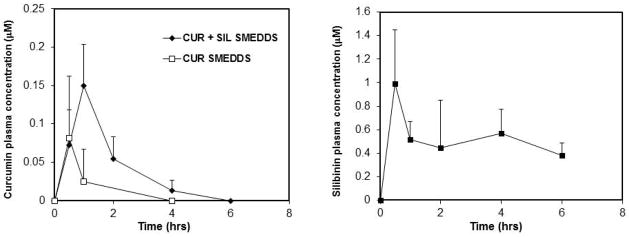
Addition of silibinin improves curcumin oral bioavailability. Female mice (n = 3–6) were dosed orally with curcumin SMEDDS (CUR SMEDDS) or curcumin and silibinin combination SMEDDS (CUR + SIL SMEDDS). Mice were sacrificed at various time points, and plasma was analyzed for curcumin (A) and silibinin (B) concentrations. Data are presented as mean plasma concentration. Error bars represent SD.
Discussion
The chemotherapeutic potential of curcumin is well documented. In various cancer cell lines, curcumin has been shown to inhibit the activation of NF-κB [36], a pathway critical for tumorigenesis and inflammation [37]. However, many of these effects have been tested at curcumin concentrations ranging from 20–100 μM. These concentrations are likely unrealistic and not physiologically relevant, given the poor bioavailability of curcumin. Interestingly, the IC50 of curcumin has been shown to decrease with the duration of exposure in vitro [38, 39]. A previous study investigating the anti-inflammatory effects of curcumin showed that oral administration of 150 mg of curcumin daily over 8 weeks resulted in a significant decrease in inflammatory cytokine levels in humans [40]. These in vitro and in vivo results suggest that a long-term dosing regimen, resulting in low, steady concentrations of curcumin, could be an effective chemopreventive approach. The overall goal of the current study was to examine an oral dosing strategy to improve curcumin bioavailability.
The low solubility of curcumin (~2 ng/mL in water) combined with its poor absorption through the small intestine [41] results in a significant portion of an oral dose being excreted in the feces [42]. To overcome this problem, we used a SMEDDS capable of solubilizing a large dose of curcumin (Table 1). SMEDDS formulations have also been shown to improve the absorption of hydrophobic drugs and increase the shelf-life of otherwise unstable compounds [20]. SMEDDSs have been used previously for oral dosing of curcumin. For example, Setthacheewakul et al [34] formulated a SMEDDS, consisting of Cremophor EL, Labrasol, Labrafac PG and Capryol 90. This formulation showed average plasma concentrations as high as 5 μM in rats, ~8–9 times higher than the suspension control. Compared to this previous study, the lower Cmax observed in our study could be due to the differences in UGT enzyme levels between rodent species [43]. The effectiveness of SMEDDS in other studies was less clear. Cui et al reported the curcumin concentrations remaining in the gastrointestinal tract, rather than the plasma concentrations [18]. Zhongfa et al [21] used a curcumin dose of 1.8 g/kg corresponding to a 126 g average human dose. It is not clear whether such high doses can be used in humans. Recently, Zhang et al successfully fabricated a folate-modified SMEDDS for targeting colon cancer [44]. Since this formulation is intended for colon cancer treatment, no systemic levels of curcumin were reported. Our laboratory recently showed that folate modulated PLGA nanoparticles increase gut absorption in vitro [45], and thus it is possible that folate-modified SMEDDS could be useful for systemic delivery.
In our studies, solubilizing curcumin in the SMEDDS formulation appeared to improve the absorption of curcumin, as evidenced by higher plasma concentrations of the glucuronide. The lack of significantly higher plasma concentrations of parent drug suggested that inhibition of curcumin metabolism in the gut and during first pass through the liver was probably necessary to improve its oral bioavailability. UGT enzymes account for the majority of curcumin metabolism in the gut and liver. UGT1A1 and 1A3 (liver) as well as UGT1A8 and UGT1A10 (gut) show high activity for curcumin compared to other UGT enzymes [27], with UGT1A1 showing the highest activity for curcumin of all UGT enzymes tested. Inhibition of UGT activity offered a possible avenue to decrease curcumin clearance, and thereby increase the overall systemic exposure of curcumin.
Previous studies have examined the effects of naturally occurring compounds on UGT activity [23, 24, 46]. As an additional benefit, some of these natural compounds also have complementary anticancer activity [26, 31, 32, 47, 48]. This is especially true for piperine which, when combined with curcumin, affects cellular pathways important for cancer stem cells such as Wnt [28]. In our studies, silibinin, quercetin and tangeretin decreased curcumin glucuronidation in mouse liver microsomes, while piperine, EGCG and salicylic acid did not. This was an unexpected finding for piperine, which was previously shown to increase curcumin bioavailability. Because prior reports suggested that piperine is highly effective in improving curcumin bioavailability [22], we included piperine in our in vivo studies. Addition of piperine to SMEDDS increased curcumin plasma levels but the results were highly variable. Shoba et al showed a 2000% increase in curcumin bioavailability in humans, with a lesser effect in rats [22]. A closer look at the analytical technique used in the study could partly explain the dramatic increase in oral bioavailability observed with piperine. Prior to analysis, plasma samples were heated at 80 C for 30 min, which likely converted curcumin glucuronide back to the parent compound. The paper did not report on the stability of curcumin metabolites during the sample processing and extraction procedure used. Also, piperine has been shown to increase intestinal brush border membrane fluidity, leading to increased absorption of co-delivered molecules [49]. Thus, in addition to potential effects on metabolism, the apparent effects of piperine on curcumin bioavailability could be due to increased gut absorption. Plasma from animals treated with curcumin-piperine combination had high concentrations of curcumin glucuronide, providing further evidence that piperine improved the absorption of curcumin. Although some animals showed high plasma levels of curcumin, piperine was not investigated further because of the high variability and the observed side effects.
Both quercetin and silibinin increased curcumin bioavailability and were associated with reduced variability. Extended pharmacokinetic studies showed a 3.5-fold increase in area under the curve (AUC) for curcumin and silibinin SMEDDS compared to SMEDDS with curcumin alone. Silibinin was previously shown to inhibit UGT1A1-mediated glucuronidation of β-estradiol [23] but is not a commonly used natural product for metabolic inhibition. To our knowledge, this is the first study showing an increase in the bioavailability of curcumin using silibinin as an inhibitor. Similar to piperine, silibinin also modulates cellular pathways important for cancer proliferation and invasion. Previous studies demonstrated a decrease in epidermal growth factor receptor (EGFR) activation [50, 51] by silibinin, leading to apoptosis and decreased MAPK activity [52], a pathway important for tumor cell proliferation. Silibinin has been shown to decrease vascular endothelial growth factor (VEGF) production [53], leading to decreased angiogenesis in vivo [54]. Additionally, silibinin has been shown to inhibit tumor invasion through up-regulation of E-cadherin [55] and decreased MDA-MB-231 migration in vitro [56]. Silibinin has been combined previously with curcumin to effectively induce apoptosis in adult myeloid leukemia cells in vitro through activation of caspase pathways [57]. Pharmacokinetic studies show that the combination SMEDDS improves plasma concentrations of both curcumin and silibinin, and this formulation can, therefore, be expected to have enhanced chemopreventive activity.
Conclusions
Our studies showed that oral bioavailability of curcumin can be increased by formulating curcumin in a soluble form using the SMEDDS and by decreasing its metabolic clearance. Co-delivery of silibinin was used as a novel approach to decrease UGT-mediated clearance of curcumin and to improve curcumin bioavailability in vivo. Both curcumin and silibinin have complementary antioxidant, anti-inflammatory and anticancer activities. Therefore, there is a great potential for this combination formulation to be utilized as an oral dosage form for chemoprevention. Future studies will examine the safety and chemopreventive efficacy of this formulation following chronic dosing.
Abbreviations
- SUS
suspension
- SLN
solution
- SMEDDS
self-microemulsifying drug delivery system
- SIL
silibinin
- QUE
quercetin
- PIP
piperine
- TAN
tangeretin
- UGT
UDP-glucuronosyltransferase
- EGCG
epigallocatechin gallate
- VEGF
vascular endothelial growth factor
- EGFR
Epidermal growth factor receptor
Footnotes
Conflict of Interest
None
References
- 1.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 2.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Singh U, Barik A, Singh BG, Priyadarsini KI. Reactions of reactive oxygen species (ROS) with curcumin analogues: Structure-activity relationship. Free Radic Res. 2011;45:317–325. doi: 10.3109/10715762.2010.532493. [DOI] [PubMed] [Google Scholar]
- 5.Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853:183–189. doi: 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 7.Liu A, Lou H, Zhao L, Fan P. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. 2006;40:720–727. doi: 10.1016/j.jpba.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- 9.Hoehle SI, Pfeiffer E, Solyom AM, Metzler M. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J Agric Food Chem. 2006;54:756–764. doi: 10.1021/jf058146a. [DOI] [PubMed] [Google Scholar]
- 10.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- 11.Pfeiffer E, Hoehle SI, Walch SG, Riess A, Solyom AM, Metzler M. Curcuminoids form reactive glucuronides in vitro. J Agric Food Chem. 2007;55:538–544. doi: 10.1021/jf0623283. [DOI] [PubMed] [Google Scholar]
- 12.Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced Drug Delivery Systems of Curcumin for Cancer Chemoprevention. Cancer Prevention Research. 2011;4:1158–1171. doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bansal S, Vadhanam M, Gupta R. Development and In Vitro-In Vivo Evaluation of Polymeric Implants for Continuous Systemic Delivery of Curcumin. Pharm Res. 2011;28:1121–1130. doi: 10.1007/s11095-011-0375-z. [DOI] [PubMed] [Google Scholar]
- 14.Bansal SS, Kausar H, Vadhanam MV, Ravoori S, Gupta RC. Controlled systemic delivery by polymeric implants enhances tissue and plasma curcumin levels compared with oral administration. European Journal of Pharmaceutics and Biopharmaceutics. 2012;80:571–577. doi: 10.1016/j.ejpb.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahani K, Panyam J. Highly loaded, sustained-release microparticles of curcumin for chemoprevention. J Pharm Sci. 2011;100:2599–2609. doi: 10.1002/jps.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahani K, Swaminathan SK, Freeman D, Blum A, Ma L, Panyam J. Injectable sustained release microparticles of curcumin: a new concept for cancer chemoprevention. Cancer Res. 2010;70:4443–4452. doi: 10.1158/0008-5472.CAN-09-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganta S, Devalapally H, Amiji M. Curcumin enhances oral bioavailability and anti-tumor therapeutic efficacy of paclitaxel upon administration in nanoemulsion formulation. J Pharm Sci. 2010;99:4630–4641. doi: 10.1002/jps.22157. [DOI] [PubMed] [Google Scholar]
- 18.Cui J, Yu B, Zhao Y, Zhu W, Li H, Lou H, Zhai G. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009;371:148–155. doi: 10.1016/j.ijpharm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Spernath A, Aserin A. Microemulsions as carriers for drugs and nutraceuticals. Adv Colloid Interface Sci. 2006;128–130:47–64. doi: 10.1016/j.cis.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhongfa L, Chiu M, Wang J, Chen W, Yen W, Fan-Havard P, Yee LD, Chan KK. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother Pharmacol. 2012;69:679–689. doi: 10.1007/s00280-011-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 23.Williams JA, Ring BJ, Cantrell VE, Campanale K, Jones DR, Hall SD, Wrighton SA. Differential modulation of UDP-glucuronosyltransferase 1A1 (UGT1A1)-catalyzed estradiol-3-glucuronidation by the addition of UGT1A1 substrates and other compounds to human liver microsomes. Drug Metab Dispos. 2002;30:1266–1273. doi: 10.1124/dmd.30.11.1266. [DOI] [PubMed] [Google Scholar]
- 24.Grancharov K, Naydenova Z, Lozeva S, Golovinsky E. Natural and synthetic inhibitors of UDP-glucuronosyltransferase. Pharmacol Ther. 2001;89:171–186. doi: 10.1016/s0163-7258(00)00109-1. [DOI] [PubMed] [Google Scholar]
- 25.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 26.Weng CJ, Yen GC. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012 doi: 10.1007/s10555-012-9347-y. [DOI] [PubMed] [Google Scholar]
- 27.Hoehle SI, Pfeiffer E, Metzler M. Glucuronidation of curcuminoids by human microsomal and recombinant UDP-glucuronosyltransferases. Mol Nutr Food Res. 2007;51:932–938. doi: 10.1002/mnfr.200600283. [DOI] [PubMed] [Google Scholar]
- 28.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reen RK, Jamwal DS, Taneja SC, Koul JL, Dubey RK, Wiebel FJ, Singh J. Impairment of UDP-glucose dehydrogenase and glucuronidation activities in liver and small intestine of rat and guinea pig in vitro by piperine. Biochem Pharmacol. 1993;46:229–238. doi: 10.1016/0006-2952(93)90408-o. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr. 2007;47:735–748. doi: 10.1080/10408390601062054. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y. Silibinin inhibits cell growth and induces apoptosis by caspase activation, down-regulating survivin and blocking EGFR-ERK activation in renal cell carcinoma. Cancer Lett. 2008;272:61–69. doi: 10.1016/j.canlet.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 2008;11:733–740. doi: 10.1097/MCO.0b013e32831394b8. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Xu J, Huang X, Wen C. Self-microemulsifying drug delivery system improves curcumin dissolution and bioavailability. Drug Dev Ind Pharm. 2011;37:15–23. doi: 10.3109/03639045.2010.489560. [DOI] [PubMed] [Google Scholar]
- 34.Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur J Pharm Biopharm. 2010;76:475–485. doi: 10.1016/j.ejpb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- 36.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- 37.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 38.Hong RL, Spohn WH, Hung MC. Curcumin inhibits tyrosine kinase activity of p185neu and also depletes p185neu. Clin Cancer Res. 1999;5:1884–1891. [PubMed] [Google Scholar]
- 39.Moiseeva EP, Almeida GM, Jones GD, Manson MM. Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol Cancer Ther. 2007;6:3071–3079. doi: 10.1158/1535-7163.MCT-07-0117. [DOI] [PubMed] [Google Scholar]
- 40.Usharani P, Mateen AA, Naidu MU, Raju YS, Chandra N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D. 2008;9:243–250. doi: 10.2165/00126839-200809040-00004. [DOI] [PubMed] [Google Scholar]
- 41.Wahlang B, Pawar YB, Bansal AK. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur J Pharm Biopharm. 2011;77:275–282. doi: 10.1016/j.ejpb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, Williams ML, Steward WP, Gescher AJ. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002;11:535–540. [PubMed] [Google Scholar]
- 43.Shiratani H, Katoh M, Nakajima M, Yokoi T. Species differences in UDP-glucuronosyltransferase activities in mice and rats. Drug Metab Dispos. 2008;36:1745–1752. doi: 10.1124/dmd.108.021469. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Zhu W, Yang C, Guo H, Yu A, Ji J, Gao Y, Sun M, Zhai G. A novel folate-modified self-microemulsifying drug delivery system of curcumin for colon targeting. Int J Nanomedicine. 2012;7:151–162. doi: 10.2147/IJN.S27639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roger E, Kalscheuer S, Kirtane A, Guru BR, Grill AE, Whittum-Hudson J, Panyam J. Folic Acid Functionalized Nanoparticles for Enhanced Oral Drug Delivery. Mol Pharm. 2012 doi: 10.1021/mp2005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mano Y, Usui T, Kamimura H. In vitro inhibitory effects of non-steroidal antiinflammatory drugs on UDP-glucuronosyltransferase 1A1-catalysed estradiol 3beta-glucuronidation in human liver microsomes. Biopharm Drug Dispos. 2005;26:35–39. doi: 10.1002/bdd.430. [DOI] [PubMed] [Google Scholar]
- 47.Chen D, Dou QP. Tea polyphenols and their roles in cancer prevention and chemotherapy. Int J Mol Sci. 2008;9:1196–1206. doi: 10.3390/ijms9071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardiner PS, Gilmer JF. The medicinal chemistry implications of the anticancer effects of aspirin and other NSAIDs. Mini Rev Med Chem. 2003;3:461–470. doi: 10.2174/1389557033488033. [DOI] [PubMed] [Google Scholar]
- 49.Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine. 2002;9:224–231. doi: 10.1078/0944-7113-00114. [DOI] [PubMed] [Google Scholar]
- 50.Qi L, Singh RP, Lu Y, Agarwal R, Harrison GS, Franzusoff A, Glode LM. Epidermal growth factor receptor mediates silibinin-induced cytotoxicity in a rat glioma cell line. Cancer Biol Ther. 2003;2:526–531. doi: 10.4161/cbt.2.5.452. [DOI] [PubMed] [Google Scholar]
- 51.Kim S, Han J, Kim JS, Kim JH, Choe JH, Yang JH, Nam SJ, Lee JE. Silibinin suppresses EGFR ligand-induced CD44 expression through inhibition of EGFR activity in breast cancer cells. Anticancer Res. 2011;31:3767–3773. [PubMed] [Google Scholar]
- 52.Ascencio M, Estevez JP, Delemer M, Farine MO, Collinet P, Mordon S. Comparison of continuous and fractionated illumination during hexaminolaevulinate-photodynamic therapy. Photodiagnosis Photodyn Ther. 2008;5:210–216. doi: 10.1016/j.pdpdt.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Kim JS, Kim JH, Choe JH, Yang JH, Nam SJ, Lee JE. Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine. 2009;16:573–580. doi: 10.1016/j.phymed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Deep G, Gangar SC, Rajamanickam S, Raina K, Gu M, Agarwal C, Oberlies NH, Agarwal R. Angiopreventive efficacy of pure flavonolignans from milk thistle extract against prostate cancer: targeting VEGF-VEGFR signaling. PLoS One. 2012;7:e34630. doi: 10.1371/journal.pone.0034630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deep G, Gangar SC, Agarwal C, Agarwal R. Role of E-cadherin in antimigratory and antiinvasive efficacy of silibinin in prostate cancer cells. Cancer Prev Res (Phila) 2011;4:1222–1232. doi: 10.1158/1940-6207.CAPR-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dastpeyman M, Motamed N, Azadmanesh K, Mostafavi E, Kia V, Jahanian-Najafabadi A, Shokrgozar MA. Inhibition of silibinin on migration and adhesion capacity of human highly metastatic breast cancer cell line, MDA-MB-231, by evaluation of beta1-integrin and downstream molecules, Cdc42, Raf-1 and D4GDI. Med Oncol. 2012;29:2512–2518. doi: 10.1007/s12032-011-0113-8. [DOI] [PubMed] [Google Scholar]
- 57.Pesakhov S, Khanin M, Studzinski GP, Danilenko M. Distinct combinatorial effects of the plant polyphenols curcumin, carnosic acid, and silibinin on proliferation and apoptosis in acute myeloid leukemia cells. Nutr Cancer. 2010;62:811–824. doi: 10.1080/01635581003693082. [DOI] [PMC free article] [PubMed] [Google Scholar]