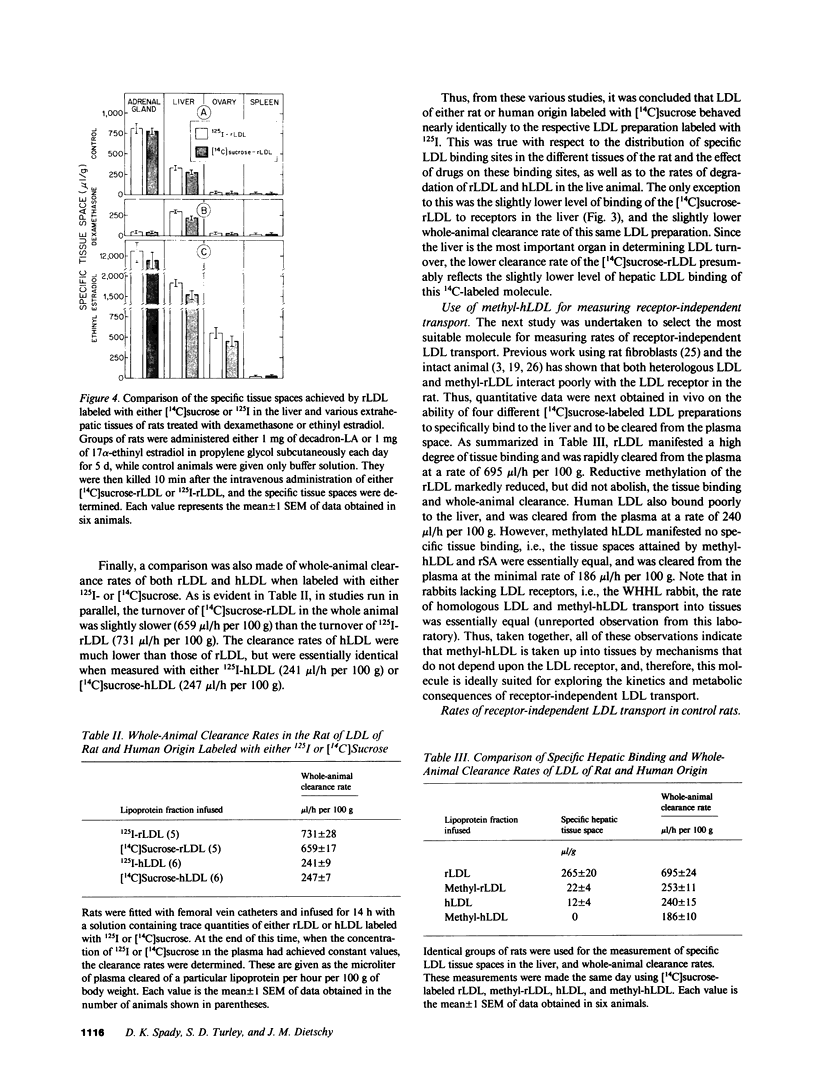
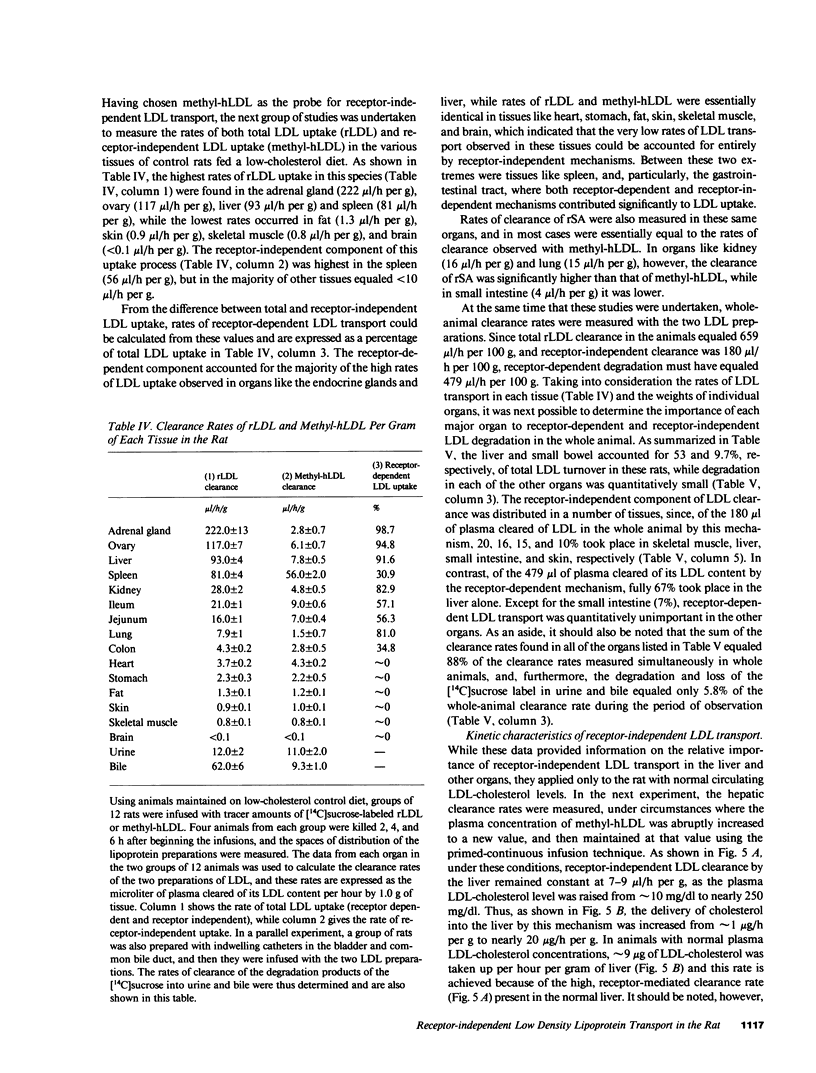
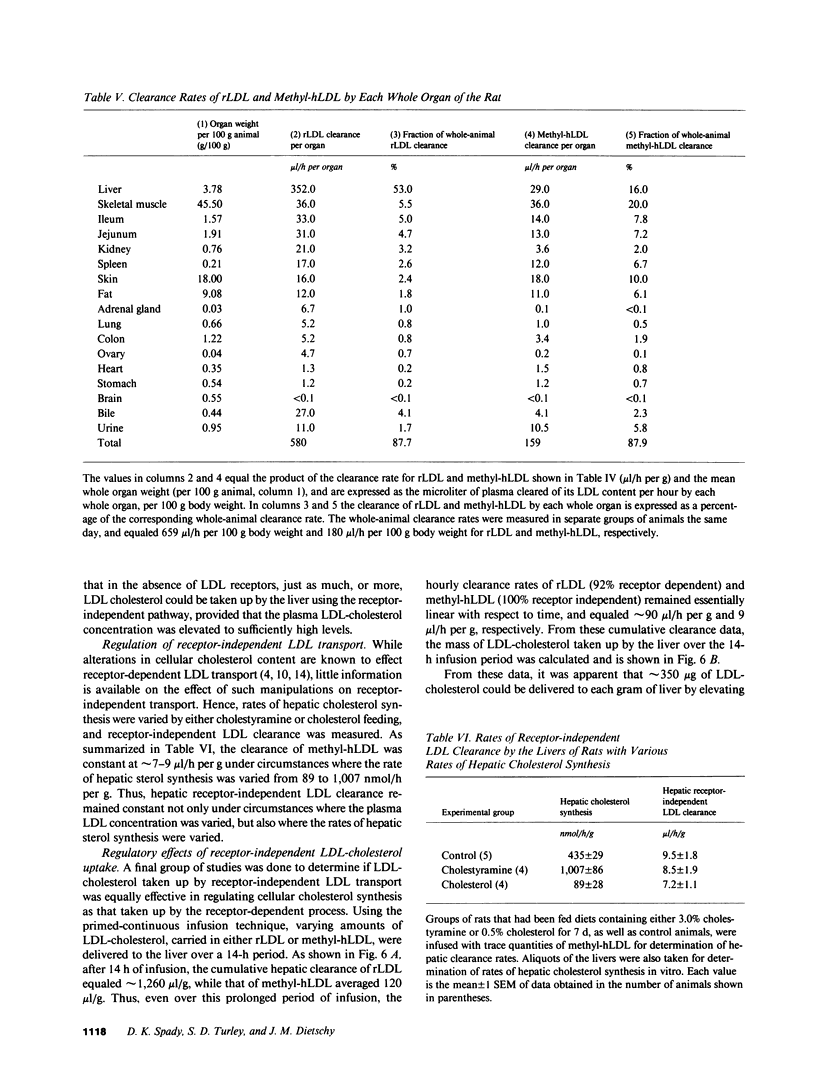
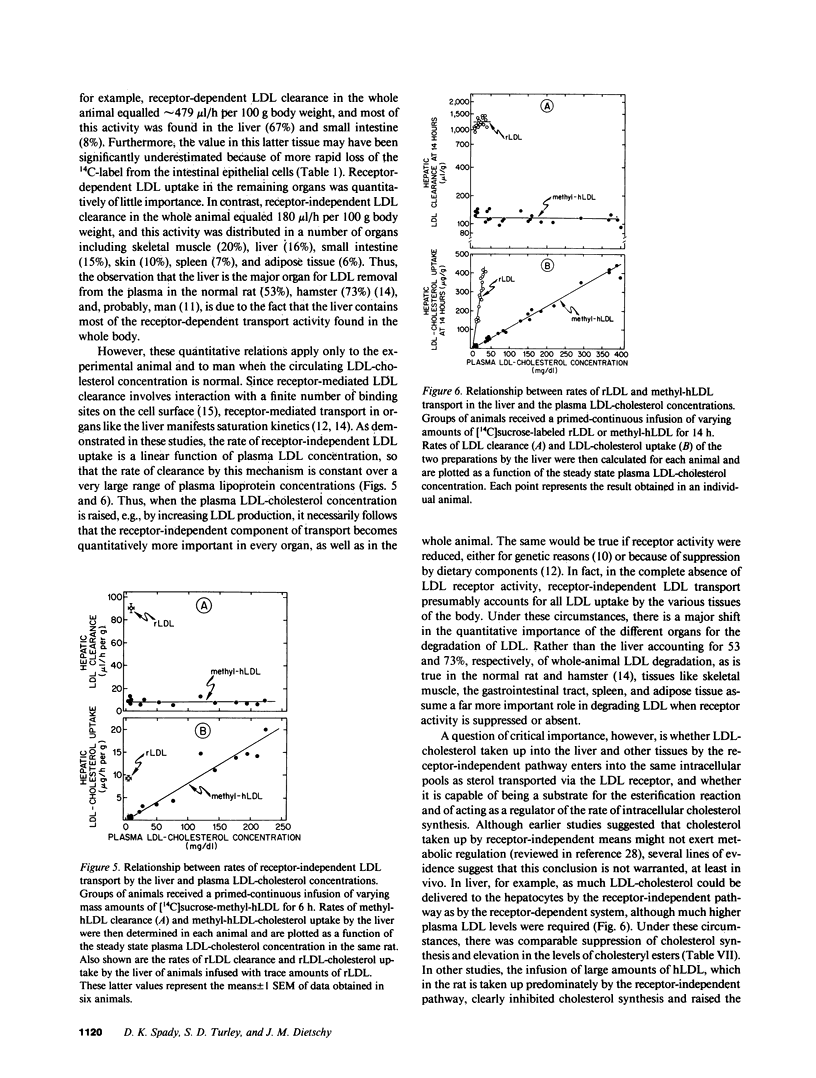
Abstract
Receptor-independent low density lipoprotein (LDL) transport plays a critical role in the regulation of plasma cholesterol levels; hence, these studies were done to characterize this process in the tissues of the rat. High rates of receptor-independent clearance were found in the spleen, but other organs, like liver, gastrointestinal tract, and endocrine glands manifested lower clearance rates that varied from 3 to 9 microliter/h per g, while the rates in nervous tissue, muscle, and adipose tissue were less than 1 microliter/h per g. Receptor-dependent uptake was much higher in liver (85 microliter/h per g) and adrenal gland (219 microliter/h per g), but was also low in most other tissues. At normal plasma LDL concentrations, 67% of the receptor-dependent transport in the whole animal was accounted for by LDL uptake in the liver. In contrast, the receptor-independent uptake found in the whole animal took place in many organs, including skeletal muscle (20%), liver (16%), small bowel (15%), skin (10%), and spleen (7%). Furthermore, in liver, the rate of cholesterol synthesis could be varied 11-fold, yet the rate of receptor-independent LDL clearance remained constant at approximately 8 microliter/h per g. When the circulating levels of LDL were systematically increased, receptor-independent LDL clearance also remained constant, so that hepatic LDL-cholesterol uptake by this mechanism increased linearly, from 1 to 20 micrograms/h per g, as the plasma LDL-cholesterol level was increased from 10 to 250 mg/dl. Finally, when equal amounts of LDL-cholesterol were delivered into the liver by either the receptor-dependent or receptor-independent mechanism, there was significant suppression of cholesterol synthesis and an increase in cholesteryl esters. Thus, in any situation in which receptor-dependent LDL degradation is lost, cholesterol balance in the whole animal and across individual organs is maintained by receptor-independent mechanisms, although when the new steady state is achieved, circulating levels of LDL must necessarily be very much increased.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Absolute rates of cholesterol synthesis in extrahepatic tissues measured with 3H-labeled water and 14C-labeled substrates. J Lipid Res. 1979 Aug;20(6):740–752. [PubMed] [Google Scholar]
- Andersen J. M., Dietschy J. M. Regulation of sterol synthesis in 15 tissues of rat. II. Role of rat and human high and low density plasma lipoproteins and of rat chylomicron remnants. J Biol Chem. 1977 Jun 10;252(11):3652–3659. [PubMed] [Google Scholar]
- Andersen J. M., Turley S. D., Dietschy J. M. Low and high density lipoproteins and chylomicrons as regulators of rate of cholesterol synthesis in rat liver in vivo. Proc Natl Acad Sci U S A. 1979 Jan;76(1):165–169. doi: 10.1073/pnas.76.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attie A. D., Pittman R. C., Steinberg D. Hepatic catabolism of low density lipoprotein: mechanisms and metabolic consequences. Hepatology. 1982 Mar-Apr;2(2):269–281. doi: 10.1002/hep.1840020215. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Goldstein J. L., Grundy S. M., Starzl T. E., Brown M. S. Liver transplantation to provide low-density-lipoprotein receptors and lower plasma cholesterol in a child with homozygous familial hypercholesterolemia. N Engl J Med. 1984 Dec 27;311(26):1658–1664. doi: 10.1056/NEJM198412273112603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Stone N. J., Grundy S. M. Metabolic studies in familial hypercholesterolemia. Evidence for a gene-dosage effect in vivo. J Clin Invest. 1979 Aug;64(2):524–533. doi: 10.1172/JCI109490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Watanabe Y., Kita T. Impaired receptor-mediated catabolism of low density lipoprotein in the WHHL rabbit, an animal model of familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1982 May;79(10):3305–3309. doi: 10.1073/pnas.79.10.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic. J Clin Invest. 1983 Sep;72(3):743–747. doi: 10.1172/JCI111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
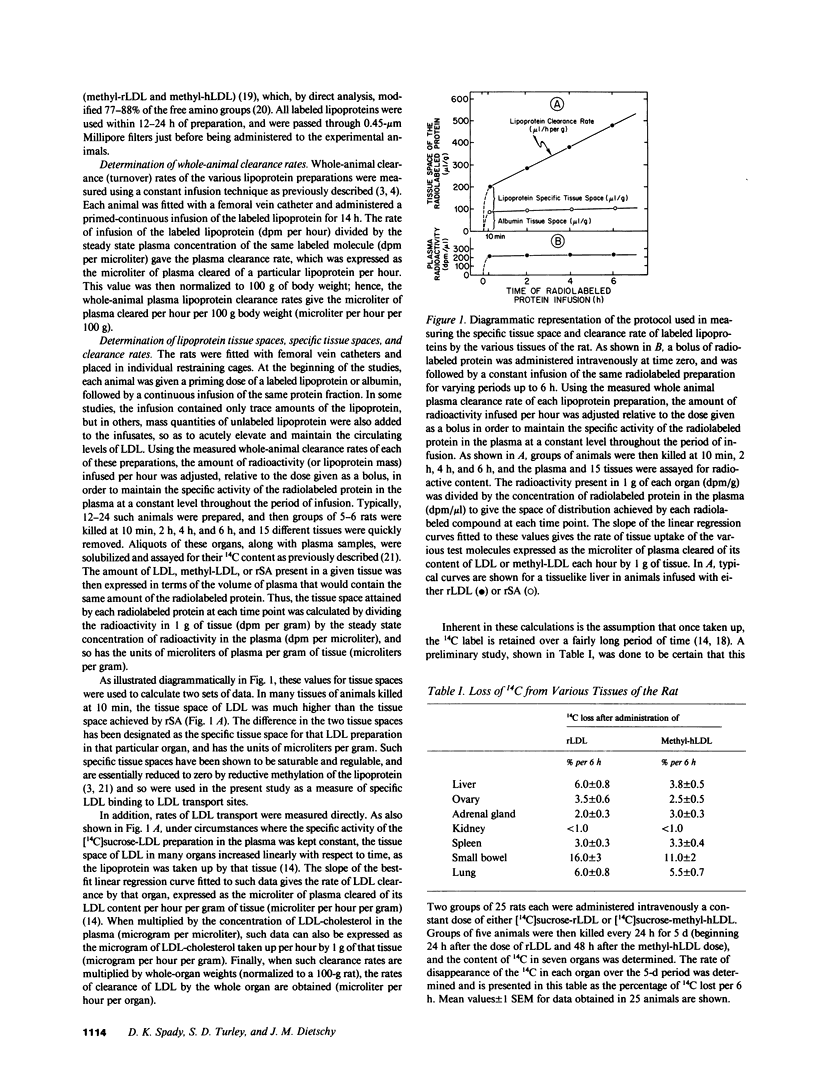
- Carew T. E., Pittman R. C., Steinberg D. Tissue sites of degradation of native and reductively methylated [14C]sucrose-labeled low density lipoprotein in rats. Contribution of receptor-dependent and receptor-independent pathways. J Biol Chem. 1982 Jul 25;257(14):8001–8008. [PubMed] [Google Scholar]
- Dietschy J. M., Kita T., Suckling K. E., Goldstein J. L., Brown M. S. Cholesterol synthesis in vivo and in vitro in the WHHL rabbit, an animal with defective low density lipoprotein receptors. J Lipid Res. 1983 Apr;24(4):469–480. [PubMed] [Google Scholar]
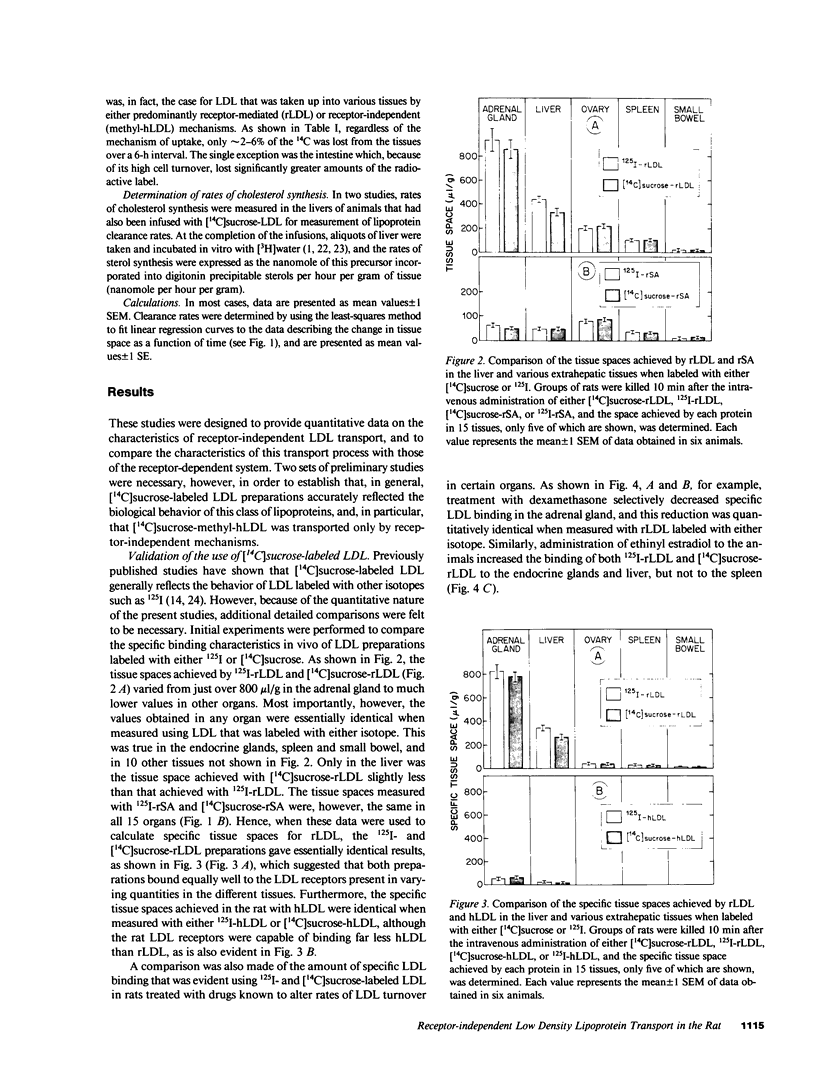
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Harkes L., Van Berkel J. C. Quantitative role of parenchymal and non-parenchymal liver cells in the uptake of [14C]sucrose-labelled low-density lipoprotein in vivo. Biochem J. 1984 Nov 15;224(1):21–27. doi: 10.1042/bj2240021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Disparities in the interaction of rat and human lipoproteins with cultured rat fibroblasts and smooth muscle cells. Requirements for homology for receptor binding activity. J Biol Chem. 1980 Dec 10;255(23):11163–11172. [PubMed] [Google Scholar]
- Jeske D. J., Dietschy J. M. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H]water. J Lipid Res. 1980 Mar;21(3):364–376. [PubMed] [Google Scholar]
- Kesaniemi Y. A., Witztum J. L., Steinbrecher U. P. Receptor-mediated catabolism of low density lipoprotein in man. Quantitation using glucosylated low density lipoprotein. J Clin Invest. 1983 Apr;71(4):950–959. doi: 10.1172/JCI110849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelz H. R., Sherrill B. C., Turley S. D., Dietschy J. M. Correlation of low and high density lipoprotein binding in vivo with rates of lipoprotein degradation in the rat. A comparison of lipoproteins of rat and human origin. J Biol Chem. 1982 Jul 25;257(14):8061–8072. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Melchior G. W., Innerarity T. L., Holcombe K. S. Inhibition of receptor-mediated clearance of lysine and arginine-modified lipoproteins from the plasma of rats and monkeys. Proc Natl Acad Sci U S A. 1980 Jan;77(1):225–229. doi: 10.1073/pnas.77.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Andersen J. M., Dietschy J. M. Sites of tissue binding and uptake in vivo of bacterial lipopolysaccharide-high density lipoprotein complexes: studies in the rat and squirrel monkey. J Clin Invest. 1981 Dec;68(6):1503–1513. doi: 10.1172/JCI110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Attie A. D., Carew T. E., Steinberg D. Tissue sites of catabolism of rat and human low density lipoproteins in rats. Biochim Biophys Acta. 1982 Jan 15;710(1):7–14. doi: 10.1016/0005-2760(82)90183-7. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Attie A. D., Witztum J. L., Watanabe Y., Steinberg D. Receptor-dependent and receptor-independent degradation of low density lipoprotein in normal rabbits and in receptor-deficient mutant rabbits. J Biol Chem. 1982 Jul 25;257(14):7994–8000. [PubMed] [Google Scholar]
- Pittman R. C., Green S. R., Attie A. D., Steinberg D. Radiolabeled sucrose covalently linked to protein. A device for quantifying degradation of plasma proteins catabolized by lysosomal mechanisms. J Biol Chem. 1979 Aug 10;254(15):6876–6879. [PubMed] [Google Scholar]
- Spady D. K., Bilheimer D. W., Dietschy J. M. Rates of receptor-dependent and -independent low density lipoprotein uptake in the hamster. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3499–3503. doi: 10.1073/pnas.80.11.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Dietary saturated triacylglycerols suppress hepatic low density lipoprotein receptor activity in the hamster. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4526–4530. doi: 10.1073/pnas.82.13.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J Lipid Res. 1983 Mar;24(3):303–315. [PubMed] [Google Scholar]
- Spady D. K., Turley S. D., Dietschy J. M. Rates of low density lipoprotein uptake and cholesterol synthesis are regulated independently in the liver. J Lipid Res. 1985 Apr;26(4):465–472. [PubMed] [Google Scholar]
- Stange E. F., Dietschy J. M. Cholesterol synthesis and low density lipoprotein uptake are regulated independently in rat small intestinal epithelium. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5739–5743. doi: 10.1073/pnas.80.18.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher U. P., Witztum J. L., Kesaniemi Y. A., Elam R. L. Comparison of glucosylated low density lipoprotein with methylated or cyclohexanedione-treated low density lipoprotein in the measurement of receptor-independent low density lipoprotein catabolism. J Clin Invest. 1983 Apr;71(4):960–964. doi: 10.1172/JCI110850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tol A., Van 't Hooft F. M., Van Gent T. Discrepancies in the catabolic pathways of rat and human low density lipoproteins as revealed by partial hepatectomy in the rat. Atherosclerosis. 1978 Apr;29(4):449–457. doi: 10.1016/0021-9150(78)90173-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]