Abstract
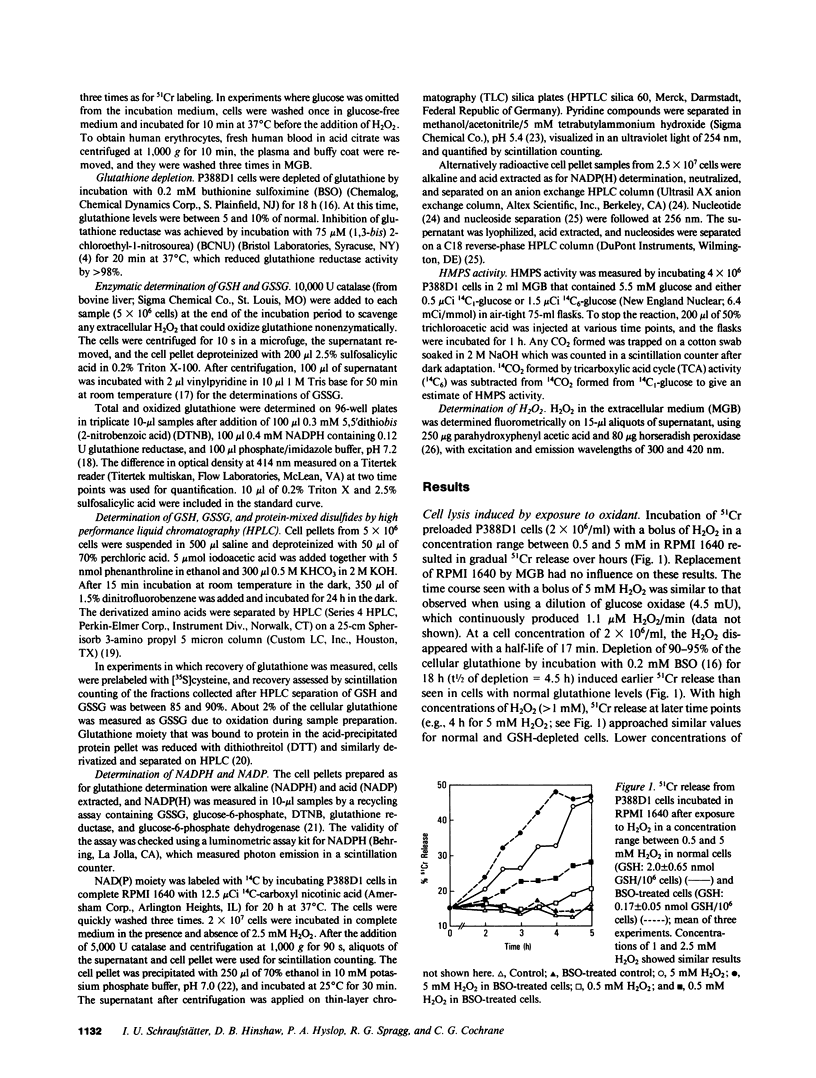
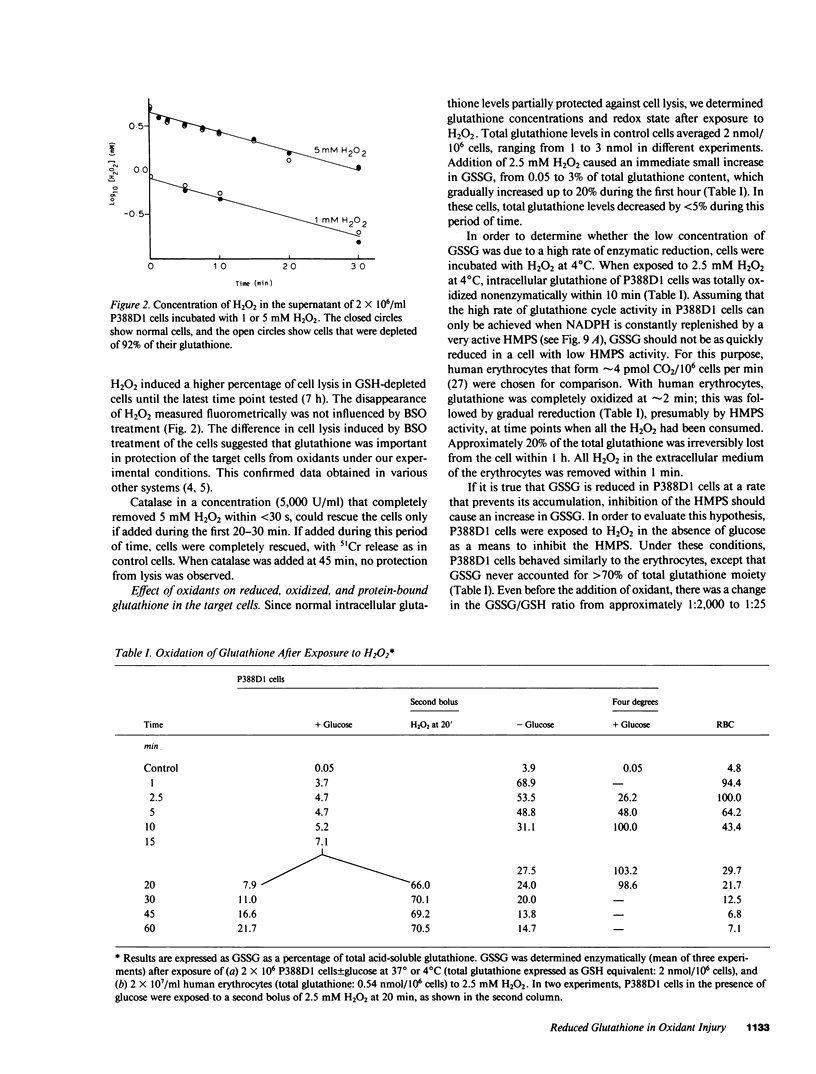
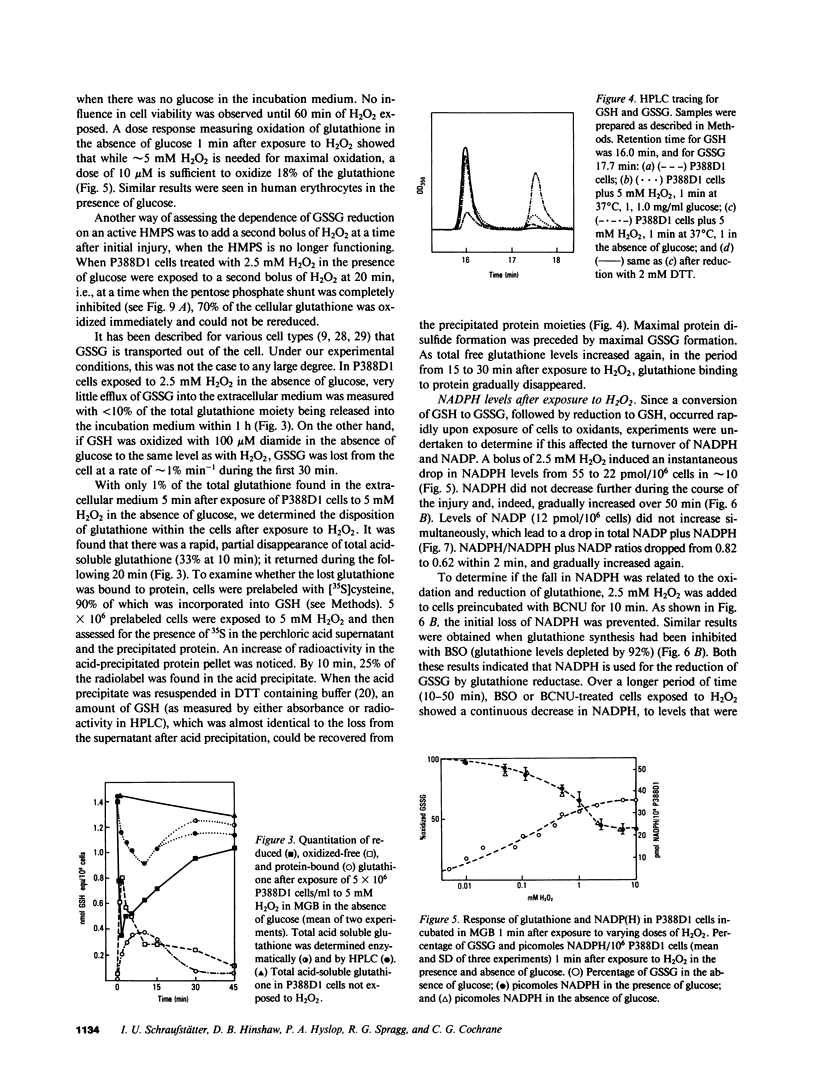
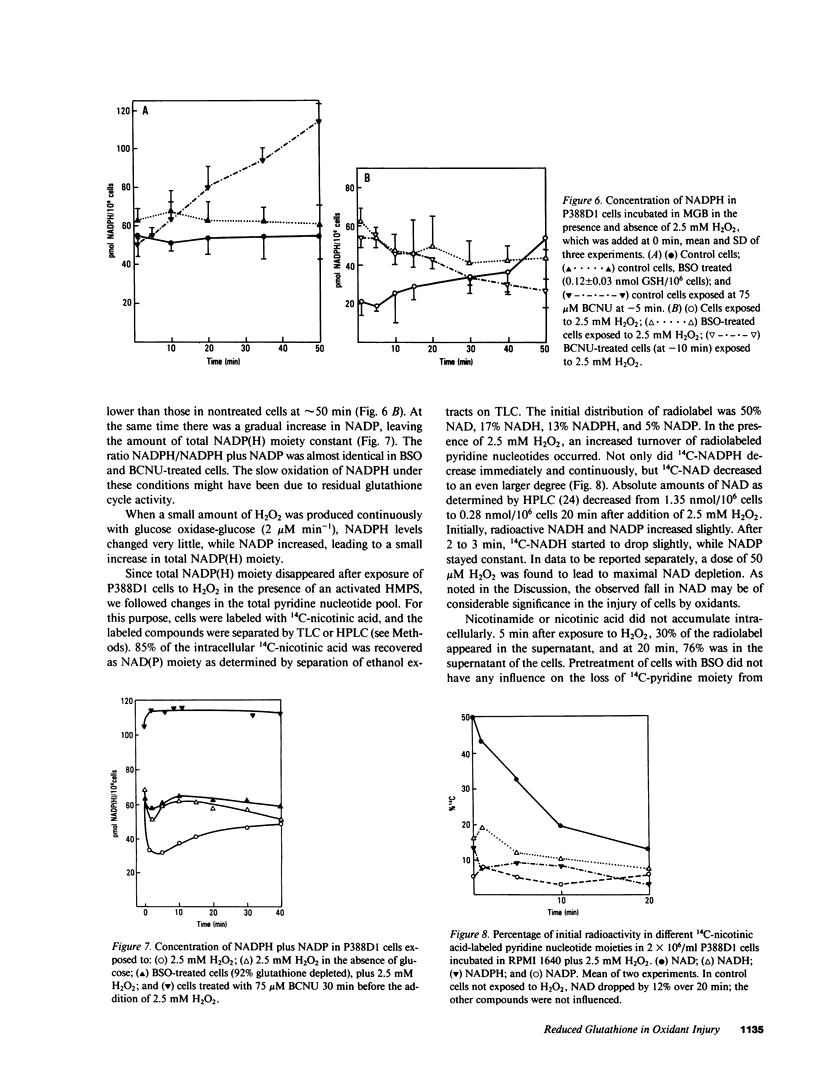
Exposure of target cells to a bolus of H2O2 induced cell lysis after a latent period of several hours, which was prevented only when the H2O2 was removed within the first 30 min of injury by addition of catalase. This indicated that early metabolic events take place that are important in the fate of the cell exposed to oxidants. In this study, we described two early and independent events of H2O2-induced injury in P388D1 macrophagelike tumor cells: activation of the glutathione cycle and depletion of cellular NAD. Glutathione cycle and hexose monophosphate shunt (HMPS) were activated within seconds after the addition of H2O2. High HMPS activity maintained glutathione that was largely reduced. However, when HMPS activity was inhibited--by glucose depletion or by incubation at 4 degrees C--glutathione remained in the oxidized state. Total pyridine nucleotide levels were diminished when cells were exposed to H2O2, and the breakdown product, nicotinamide, was recovered in the extracellular medium. Intracellular NAD levels fell by 80% within 20 min of exposure of cells to H2O2. The loss of NADP(H) and stimulation of the HMPS could be prevented when the glutathione cycle was inhibited by either blocking glutathione synthesis with buthionine sulfoximine (BSO) or by inhibiting glutathione reductase with (1,3-bis) 2 chlorethyl-1-nitrosourea. The loss of NAD developed independently of glutathione cycle and HMPS activity, as it also occurred in BSO-treated cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajmar F., Scharrer B., Hashimoto F., Carson P. E. Interrelation of stromal NAD(P)ase and human erythrocytic 6-phosphogluconic dehydrogenase. Proc Natl Acad Sci U S A. 1968 Feb;59(2):538–545. doi: 10.1073/pnas.59.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. W., Johnson G. J., Cadman S., Kaplan M. E. Membrane polypeptide aggregates in glucose 6-phosphate dehydrogenase-deficient and in vitro aged red blood cells. J Lab Clin Med. 1978 Feb;91(2):321–327. [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F., Griffith O. W., Cohn Z. A. Glutathione depletion sensitizes tumor cells to oxidative cytolysis. J Biol Chem. 1982 Feb 10;257(3):1231–1237. [PubMed] [Google Scholar]
- Baba A., Lee E., Matsuda T., Kihara T., Iwata H. Reversible inhibition of adenylate cyclase activity of rat brain caudate nucleus by oxidized glutathione. Biochem Biophys Res Commun. 1978 Dec 14;85(3):1204–1210. doi: 10.1016/0006-291x(78)90670-8. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Bradley M. O., Erickson L. C. Comparison of the effects of hydrogen peroxide and x-ray irradiation on toxicity, mutation, and DNA damage/repair in mammalian cells (V-79). Biochim Biophys Acta. 1981 Jun 26;654(1):135–141. doi: 10.1016/0005-2787(81)90146-5. [DOI] [PubMed] [Google Scholar]
- Brehe J. E., Burch H. B. Enzymatic assay for glutathione. Anal Biochem. 1976 Jul;74(1):189–197. doi: 10.1016/0003-2697(76)90323-7. [DOI] [PubMed] [Google Scholar]
- Brigelius R. Glutathione oxidation and activation of pentose phosphate cycle during hydroperoxide metabolism. A comparison of livers from fed and fasted rats. Hoppe Seylers Z Physiol Chem. 1983 Aug;364(8):989–996. doi: 10.1515/bchm2.1983.364.2.989. [DOI] [PubMed] [Google Scholar]
- Brigelius R., Muckel C., Akerboom T. P., Sies H. Identification and quantitation of glutathione in hepatic protein mixed disulfides and its relationship to glutathione disulfide. Biochem Pharmacol. 1983 Sep 1;32(17):2529–2534. doi: 10.1016/0006-2952(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Cayama E., Apitz-Castro R. Substrate-dependent, thiol-dependent, inactivation of pig brain nicotinamide adenine dinucleotide glycohydrolase. J Biol Chem. 1973 Sep 25;248(18):6479–6483. [PubMed] [Google Scholar]
- DE LOECKER W. C., PRANKERD T. A. Factors influencing the hexose monophosphate shunt in red cells. Clin Chim Acta. 1961 Sep;6:641–647. doi: 10.1016/0009-8981(61)90108-5. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Eggleston L. V., Krebs H. A. Regulation of the pentose phosphate cycle. Biochem J. 1974 Mar;138(3):425–435. doi: 10.1042/bj1380425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklöw L., Moldéus P., Orrenius S. Oxidation of glutathione during hydroperoxide metabolism. A study using isolated hepatocytes and the glutathione reductase inhibitor 1,3-bis(2-chloroethyl)-1-nitrosourea. Eur J Biochem. 1984 Feb 1;138(3):459–463. doi: 10.1111/j.1432-1033.1984.tb07938.x. [DOI] [PubMed] [Google Scholar]
- Frischer H., Ahmad T. Severe generalized glutathione reductase deficiency after antitumor chemotherapy with BCNU" [1,3-bis(chloroethyl)-1-nitrosourea]. J Lab Clin Med. 1977 May;89(5):1080–1091. [PubMed] [Google Scholar]
- Gilbert H. F. Biological disulfides: the third messenger? Modulation of phosphofructokinase activity by thiol/disulfide exchange. J Biol Chem. 1982 Oct 25;257(20):12086–12091. [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Haest C. W., Kamp D., Deuticke B. Formation of disulfide bonds between glutathione and membrane SH groups in human erythrocytes. Biochim Biophys Acta. 1979 Nov 2;557(2):363–371. doi: 10.1016/0005-2736(79)90334-1. [DOI] [PubMed] [Google Scholar]
- Haest C. W., Kamp D., Deuticke B. Formation of disulfide bonds between glutathione and membrane SH groups in human erythrocytes. Biochim Biophys Acta. 1979 Nov 2;557(2):363–371. doi: 10.1016/0005-2736(79)90334-1. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. T., Jr Thin-layer chromatographic separation of intermediates of the pyridine nucleotide cycle. Anal Biochem. 1983 Apr 1;130(1):185–188. doi: 10.1016/0003-2697(83)90667-x. [DOI] [PubMed] [Google Scholar]
- Hyslop P. A., Sklar L. A. A quantitative fluorimetric assay for the determination of oxidant production by polymorphonuclear leukocytes: its use in the simultaneous fluorimetric assay of cellular activation processes. Anal Biochem. 1984 Aug 15;141(1):280–286. doi: 10.1016/0003-2697(84)90457-3. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Sies H. Cardiac transport of glutathione disulfide and S-conjugate. Studies with isolated perfused rat heart during hydroperoxide metabolism. J Biol Chem. 1984 Mar 25;259(6):3838–3843. [PubMed] [Google Scholar]
- Jørgensen B. M., Rasmussen H. N. Recycling analysis of nicotinamide-adenine dinucleotide phosphates (NADP and NADPH). Anal Biochem. 1979 Nov 1;99(2):297–303. doi: 10.1016/s0003-2697(79)80010-x. [DOI] [PubMed] [Google Scholar]
- Karp M. T., Raunio R. P., Lövgren T. N. Simultaneous extraction and combined bioluminescent assay of NAD+ and NADH. Anal Biochem. 1983 Jan;128(1):175–180. doi: 10.1016/0003-2697(83)90359-7. [DOI] [PubMed] [Google Scholar]
- Kondo T., Dale G. L., Beutler E. Glutathione transport by inside-out vesicles from human erythrocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6359–6362. doi: 10.1073/pnas.77.11.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesko S. A., Drocourt J. L., Yang S. U. Deoxyribonucleic acid-protein and deoxyribonucleic acid interstrand cross-links induced in isolated chromatin by hydrogen peroxide and ferrous ethylenediaminetetraacetate chelates. Biochemistry. 1982 Sep 28;21(20):5010–5015. doi: 10.1021/bi00263a026. [DOI] [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxide-induced loss of pyridine nucleotides and release of calcium from rat liver mitochondria. J Biol Chem. 1980 Oct 10;255(19):9325–9330. [PubMed] [Google Scholar]
- May J. M. The role of glutathione in rat adipocyte pentose phosphate cycle activity. Arch Biochem Biophys. 1981 Mar;207(1):117–127. doi: 10.1016/0003-9861(81)90016-3. [DOI] [PubMed] [Google Scholar]
- McCreanor G. M., Bender D. A. The role of catabolism in controlling tissue concentrations of nicotinamide nucleotide coenzymes. Biochim Biophys Acta. 1983 Sep 13;759(3):222–228. doi: 10.1016/0304-4165(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Meredith M. J. Analysis of protein-glutathione mixed disulfides by high performance liquid chromatography. Anal Biochem. 1983 Jun;131(2):504–509. doi: 10.1016/0003-2697(83)90205-1. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretion of oxygen intermediates: role in effector functions of activated macrophages. Fed Proc. 1982 Apr;41(6):2206–2211. [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermann M. K., McKay M. J., Marsh M. W., Bond J. S. Glutathione disulfide inactivates, destabilizes, and enhances proteolytic susceptibility of fructose-1,6-bisphosphate aldolase. J Biol Chem. 1984 Jul 25;259(14):8886–8891. [PubMed] [Google Scholar]
- Orrenius S., Ormstad K., Thor H., Jewell S. A. Turnover and functions of glutathione studied with isolated hepatic and renal cells. Fed Proc. 1983 Dec;42(15):3177–3188. [PubMed] [Google Scholar]
- Oshino N., Chance B. Properties of glutathione release observed during reduction of organic hydroperoxide, demethylation of aminopyrine and oxidation of some substances in perfused rat liver, and their implications for the physiological function of catalase. Biochem J. 1977 Mar 15;162(3):509–525. doi: 10.1042/bj1620509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogolotti A. L., Jr, Santi D. V. High-pressure liquid chromatography--ultraviolet analysis of intracellular nucleotides. Anal Biochem. 1982 Nov 1;126(2):335–345. doi: 10.1016/0003-2697(82)90524-3. [DOI] [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Richter C., Winterhalter K. H., Baumhüter S., Lötscher H. R., Moser B. ADP-ribosylation in inner membrane of rat liver mitochondria. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3188–3192. doi: 10.1073/pnas.80.11.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R., Farber J. L. Mechanisms of the killing of cultured hepatocytes by hydrogen peroxide. Arch Biochem Biophys. 1984 Feb 1;228(2):450–459. doi: 10.1016/0003-9861(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Schraufstätter I. U., Revak S. D., Cochrane C. G. Proteases and oxidants in experimental pulmonary inflammatory injury. J Clin Invest. 1984 Apr;73(4):1175–1184. doi: 10.1172/JCI111303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto S., Carrera C. J., Kubota M., Wasson D. B., Carson D. A. Mechanism of deoxyadenosine and 2-chlorodeoxyadenosine toxicity to nondividing human lymphocytes. J Clin Invest. 1985 Feb;75(2):377–383. doi: 10.1172/JCI111710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H., Gerstenecker C., Menzel H., Flohé L. Oxidation in the NADP system and release of GSSG from hemoglobin-free perfused rat liver during peroxidatic oxidation of glutathione by hydroperoxides. FEBS Lett. 1972 Oct 15;27(1):171–175. doi: 10.1016/0014-5793(72)80434-4. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Skidmore C. J., Davies M. I., Goodwin P. M., Halldorsson H., Lewis P. J., Shall S., Zia'ee A. A. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem. 1979 Nov 1;101(1):135–142. doi: 10.1111/j.1432-1033.1979.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Smulson M. E., Schein P., Mullins D. W., Jr, Sudhakar S. A putative role for nicotinamide adenine dinucleotide-promoted nuclear protein modification in the antitumor activity of N-methyl-N-nitrosourea. Cancer Res. 1977 Sep;37(9):3006–3012. [PubMed] [Google Scholar]
- Trotta R. J., Sullivan S. G., Stern A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide. Effects of the hexose monophosphate shunt as mediated by glutathione and ascorbate. Biochem J. 1982 May 15;204(2):405–415. doi: 10.1042/bj2040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman S. A., Stossel T. P. Effects of oxygen radical scavengers and antioxidants on phagocyte-induced mutagenesis. J Immunol. 1982 Jun;128(6):2770–2772. [PubMed] [Google Scholar]
- Wielckens K., Schmidt A., George E., Bredehorst R., Hilz H. DNA fragmentation and NAD depletion. Their relation to the turnover of endogenous mono(ADP-ribosyl) and poly(ADP-ribosyl) proteins. J Biol Chem. 1982 Nov 10;257(21):12872–12877. [PubMed] [Google Scholar]
- Yamamoto H., Uchigata Y., Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature. 1981 Nov 19;294(5838):284–286. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]



